Progesterone through Progesterone Receptor B Isoform Promotes Rodent Embryonic Oligodendrogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. OPC Culture
2.3. Immunofluorescence
2.4. RNA Isolation and PCR Experiments
2.5. Analysis of Potential Progesterone Response Elements
2.6. Western Blot
2.7. siRNA-Mediated PR-B Silencing
2.8. Statistical Analysis
3. Results
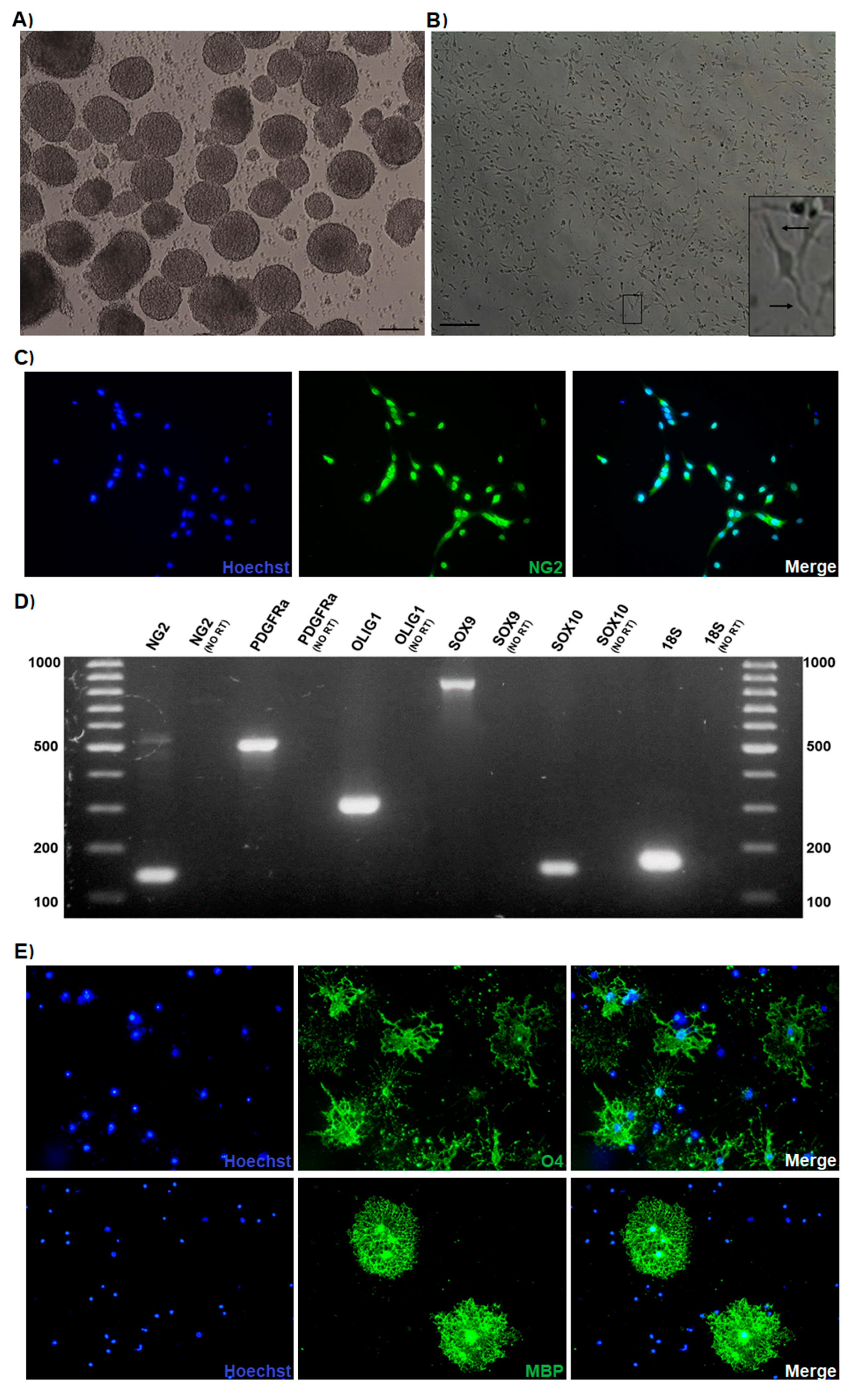
3.1. Oligodendrocyte Progenitor Cultures Derived from the Mouse Embryonic Spinal Cord
3.2. Progesterone Promotes the Proliferation and Differentiation of Mouse Embryonic OPC and Increases Its Potential of Myelination through the PR
3.3. Progesterone Upregulates the Expression of Oligodendroglial Genes
3.4. The Mouse Embryonic OPC Express the PR-A and PR-B Isoforms and the Oligodendrogenic Actions of Progesterone are Mediated by the PR-B
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kinney, H.C.; Volpe, J.J. Volpe’s Neurology of the Newborn; Elsevier: Amsterdam, The Netherlands, 2018; pp. 176–188. [Google Scholar]
- Downes, N.J.; Mullins, P. The Development of Myelin in the Brain of the Juvenile Rat. Toxicol. Pathol. 2013, 42, 913–922. [Google Scholar] [CrossRef] [Green Version]
- Bergles, D.E.; Richardson, W.D. Oligodendrocyte Development and Plasticity. Cold Spring Harb. Perspect. Boil. 2015, 8, a020453. [Google Scholar] [CrossRef] [PubMed]
- Rowitch, D.H.; Kriegstein, A.R. Developmental genetics of vertebrate glial–cell specification. Nature 2010, 468, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Sim, F.J.; McClain, C.R.; Schanz, S.J.; Protack, T.L.; Windrem, M.S.; A Goldman, S. CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nat. Biotechnol. 2011, 29, 934–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orentas, D.M.; E Hayes, J.; Dyer, K.L.; Miller, R.H. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development 1999, 126, 2419–2429. [Google Scholar] [PubMed]
- A Goldman, S.; Kuypers, N.J. How to make an oligodendrocyte. Development 2015, 142, 3983–3995. [Google Scholar] [CrossRef] [Green Version]
- Vallstedt, A.; Klos, J.M.; Ericson, J. Multiple Dorsoventral Origins of Oligodendrocyte Generation in the Spinal Cord and Hindbrain. Neuron 2005, 45, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Finzsch, M.; Stolt, C.C.; Lommes, P.; Wegner, M. Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor α expression. Development 2008, 135, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Reiprich, S.; Cantone, M.; Weider, M.; Baroti, T.; Wittstatt, J.; Schmitt, C.; Küspert, M.; Vera, J.; Wegner, M. Transcription factor Sox10 regulates oligodendroglial Sox9 levels via microRNAs. Glia 2017, 65, 1089–1102. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Niu, J.; Munji, R.; Davalos, D.; Chang, J.; Zhang, J.; Tien, A.-C.; Kuo, C.J.; Chan, J.R.; Daneman, R.; et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 2016, 351, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Baron, W.; Metz, B.; Bansal, R.; Hoekstra, D.; De Vries, H. PDGF and FGF-2 Signaling in Oligodendrocyte Progenitor Cells: Regulation of Proliferation and Differentiation by Multiple Intracellular Signaling Pathways. Mol. Cell. Neurosci. 2000, 15, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Sarliève, L.L.; Rodriguez-Pena, A.; Langley, K. Expression of thyroid hormone receptor isoforms in the oligodendrocyte lineage. Neurochem. Res. 2004, 29, 903–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Petratos, S. Thyroid Hormone Signaling in Oligodendrocytes: From Extracellular Transport to Intracellular Signal. Mol. Neurobiol. 2016, 53, 6568–6583. [Google Scholar] [CrossRef] [PubMed]
- Barres, B.; Burne, J.; Höltmann, B.; Thoenen, H.; Sendtner, M.; Raff, M. Ciliary Neurotrophic Factor Enhances the Rate of Oligodendrocyte Generation. Mol. Cell. Neurosci. 1996, 8, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Buttery, P.C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol. Cell. Neurosci. 1999, 14, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, T.M.P.; De Faria, J.P.; Bippes, C.A.; Maia, J.; Da Silva, J.L.; Relvas, J.B.; Grãos, M. Modulation of oligodendrocyte differentiation and maturation by combined biochemical and mechanical cues. Sci. Rep. 2016, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Snaidero, N.; Simons, M. Myelination at a glance. J. Cell Sci. 2014, 127, 2999–3004. [Google Scholar] [CrossRef] [Green Version]
- Labombarda, F.; Jure, I.; De Nicola, A.F. Progesterone effects on the oligodendrocyte linage: All roads lead to the progesterone receptor. Neural Regen. Res. 2019, 14, 2029–2034. [Google Scholar] [CrossRef]
- Morel, Y.; Roucher, F.; Plotton, I.; Goursaud, C.; Tardy, V.; Mallet, D. Evolution of steroids during pregnancy: Maternal, placental and fetal synthesis. Ann. d’Endocrinol. 2016, 77, 82–89. [Google Scholar] [CrossRef]
- Rego, J.L.D.; Seong, J.Y.; Burel, D.; Leprince, J.; Luu-The, V.; Tsutsui, K.; Tonon, M.-C.; Pelletier, G.; Vaudry, H. Neurosteroid biosynthesis: Enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front. Neuroendocr. 2009, 30, 259–301. [Google Scholar] [CrossRef]
- Snyder, A.M.; Hull, E.M. Perinatal progesterone affects learning in rats. Psychoneuroendocrinology 1980, 5, 113–119. [Google Scholar] [CrossRef]
- Schumacher, M.; Mattern, C.; Ghoumari, A.; Oudinet, J.; Liere, P.; Labombarda, F.; Sitruk-Ware, R.; De Nicola, A.; Guennoun, R. Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Prog. Neurobiol. 2014, 113, 6–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ogle, T.F. Signal transducer and activator of transcription 3 is expressed in the decidualized mesometrium of pregnancy and associates with the progesterone receptor through protein-protein interactions. Boil. Reprod. 2002, 67, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, B.A.; Bona, B.J.; Edwards, D.P.; Nordeen, S.K. The constitution of a progesterone response element. Mol. Endocrinol. 1993, 7, 515–527. [Google Scholar]
- Valadez-Cosmes, P.; Vázquez-Martínez, E.R.; Cerbón, M.; Camacho-Arroyo, I. Membrane progesterone receptors in reproduction and cancer. Mol. Cell. Endocrinol. 2016, 434, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Boonyaratanakornkit, V.; Bi, Y.; Rudd, M.; Edwards, D. The role and mechanism of progesterone receptor activation of extra-nuclear signaling pathways in regulating gene transcription and cell cycle progression. Steroids 2008, 73, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.; Horwitz, K.B. Progesterone receptors, their isoforms and progesterone regulated transcription. Mol. Cell. Endocrinol. 2011, 357, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Kastner, P.; Krust, A.; Turcotte, B.; Stropp, U.; Tora, L.; Gronemeyer, H.; Chambon, P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990, 9, 1603–1614. [Google Scholar] [CrossRef]
- Mesiano, S.; Wang, Y.; Norwitz, E.R. Progesterone receptors in the human pregnancy uterus: Do they hold the key to birth timing? Reprod. Sci. 2011, 18, 6–19. [Google Scholar] [CrossRef]
- Labombarda, F.; González, S.L.; Deniselle, M.C.G.; Guennoun, R.; Schumacher, M.; De Nicola, A. Cellular Basis for Progesterone Neuroprotection in the Injured Spinal Cord. J. Neurotrauma 2002, 19, 343–355. [Google Scholar] [CrossRef]
- Guerra-Araiza, C.; Villamar-Cruz, O.; González-Arenas, A.; Chavira, R.; Camacho-Arroyo, I. Changes in progestrone receptor isoforms content in the rat brain during the oestrous cycle and after oestradiol and progesterone treatments. J. Neuroendocrinol. 2003, 15, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Arroyo, I.; Hansberg-Pastor, V.; Gutiérrez-Rodríguez, A.; Chávez-Jiménez, J.; González-Morán, M.G. Expression of sex hormone receptors in the brain of male and female newly hatched chicks. Anim. Reprod. Sci. 2018, 188, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Arroyo, I.; Guerra-Araiza, C.; Cerbón, M.A. Progesterone receptor isoforms are differentially regulated by sex steroids in the rat forebrain. NeuroReport 1998, 9, 3993–3996. [Google Scholar] [CrossRef]
- Bellance, C.; Khan, J.A.; Meduri, G.; Guiochon-Mantel, A.; Lombès, M.; Loosfelt, H. Progesterone receptor isoforms PRA and PRB differentially contribute to breast cancer cell migration through interaction with focal adhesion kinase complexes. Mol. Boil. Cell 2013, 24, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- A Lamb, C.; Fabris, V.T.; Jacobsen, B.M.; Molinolo, A.; Lanari, C. Biological and clinical impact of imbalanced progesterone receptor isoform ratios in breast cancer. Endocr.-Relat. Cancer 2018, 25, R605–R624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilicic, M.; Zakar, T.; Paul, J. Modulation of Progesterone Receptor Isoform Expression in Pregnant Human Myometrium. BioMed Res. Int. 2017, 2017, 4589214. [Google Scholar] [CrossRef] [PubMed]
- Richer, J.K.; Jacobsen, B.; Manning, N.G.; Abel, M.G.; Wolf, D.M.; Horwitz, K.B. Differential Gene Regulation by the Two Progesterone Receptor Isoforms in Human Breast Cancer Cells. J. Boil. Chem. 2001, 277, 5209–5218. [Google Scholar] [CrossRef] [Green Version]
- Ghoumari, A.M.; Baulieu, E.; Schumacher, M. Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience 2005, 135, 47–58. [Google Scholar] [CrossRef]
- Ghoumari, A.M.; Ibanez, C.; El-Etr, M.; Leclerc, P.; Eychenne, B.; O’Malley, B.W.; Baulieu, E.-E.; Schumacher, M. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J. Neurochem. 2003, 86, 848–859. [Google Scholar] [CrossRef]
- Gago, N.; Akwa, Y.; Sananès, N.; Guennoun, R.; Baulieu, E.E.; El-Etr, M.; Schumacher, M. Progesterone and the oligodendroglial lineage: Stage-dependent biosynthesis and metabolism. Glia 2001, 36, 295–308. [Google Scholar] [CrossRef]
- Labombarda, F.; González, S.L.; Lima, A.; Roig, P.; Guennoun, R.; Schumacher, M.; De Nicola, A. Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury. Glia 2009, 57, 884–897. [Google Scholar] [CrossRef] [PubMed]
- Jure, I.; De Nicola, A.F.; Labombarda, F. Progesterone effects on oligodendrocyte differentiation in injured spinal cord. Brain Res. 2019, 1708, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Fornes, O.; Stigliani, A.; Gheorghe, M.; Castro-Mondragon, J.A.; Van Der Lee, R.; Bessy, A.; Chèneby, J.; Kulkarni, S.R.; Tan, G.; et al. JASPAR 2018: Update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 2018, 46, D1284. [Google Scholar] [CrossRef] [PubMed]
- Wingender, E.; Schoeps, T.; Haubrock, M.; Krull, M.; Dönitz, J. TFClass: Expanding the classification of human transcription factors to their mammalian orthologs. Nucleic Acids Res. 2017, 46, D343–D347. [Google Scholar] [CrossRef] [Green Version]
- Podvinec, M.; Kaufmann, M.R.; Handschin, C.; Meyer, U.A. NUBIScan, an in silico approach for prediction of nuclear receptor response elements. Mol. Endocrinol. 2002, 16, 1269–1279. [Google Scholar] [CrossRef]
- Ahlenius, H.; Kokaia, Z. Isolation and Generation of Neurosphere Cultures from Embryonic and Adult Mouse Brain. In Mouse Cell Culture; Humana Press: Totowa, NJ, USA, 2010; pp. 241–252. [Google Scholar]
- Pedraza, C.E.; Monk, R.; Lei, J.; Hao, Q.; Macklin, W.B. Production, characterization, and efficient transfection of highly pure oligodendrocyte precursor cultures from mouse embryonic neural progenitors. Glia 2008, 56, 1339–1352. [Google Scholar] [CrossRef] [Green Version]
- Pedraza, C.E.; Taylor, C.; Pereira, A.; Seng, M.; Tham, C.-S.; Izrael, M.; Webb, M. Induction of Oligodendrocyte Differentiation and In Vitro Myelination by Inhibition of Rho-Associated Kinase. ASN Neuro 2014, 6. [Google Scholar] [CrossRef]
- Cao, J.; Spielmann, M.; Qiu, X.; Huang, X.; Ibrahim, D.M.; Hill, A.J.; Zhang, F.; Mundlos, S.; Christiansen, L.; Steemers, F.J.; et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 2019, 566, 496–502. [Google Scholar] [CrossRef]
- González-Orozco, J.C.; Camacho-Arroyo, I. Progesterone Actions During Central Nervous System Development. Front. Mol. Neurosci. 2019, 13, 503. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, M.; Guennoun, R.; Mercier, G.; Désarnaud, F.; Lacor, P.; Benavides, J.; Ferzaz, B.; Robert, F.; Baulieu, E.E. Progesterone synthesis and myelin formation in peripheral nerves. Brain Res. Rev. 2001, 37, 343–359. [Google Scholar] [CrossRef]
- Labombarda, F.; Garcia-Ovejero, D. Give progesterone a chance. Neural Regen. Res. 2014, 9, 1422–1424. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Jessell, T.M. Diversity and Pattern in the Developing Spinal Cord. Science 1996, 274, 1115–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glasgow, S.M.; Carlson, J.C.; Zhu, W.; Chaboub, L.S.; Kang, P.; Lee, H.K.; Clovis, Y.M.; E Lozzi, B.; McEvilly, R.J.; Rosenfeld, M.G.; et al. Glia-specific enhancers and chromatin structure regulate NFIA expression and glioma tumorigenesis. Nat. Neurosci. 2017, 20, 1520–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, T.K.; Karsten, S.L.; Geschwind, D.H.; Kornblum, H. Cell Lineage and Regional Identity of Cultured Spinal Cord Neural Stem Cells and Comparison to Brain-Derived Neural Stem Cells. PLoS ONE 2009, 4, e4213. [Google Scholar] [CrossRef] [Green Version]
- Temple, S.; Raff, M.C. Clonal analysis of oligodendrocyte development in culture: Evidence for a developmental clock that counts cell divisions. Cell 1986, 44, 773–779. [Google Scholar] [CrossRef]
- Garay, L.; Deniselle, M.G.; Brocca, M.E.; Lima, A.; Roig, P.; De Nicola, A. Progesterone down-regulates spinal cord inflammatory mediators and increases myelination in experimental autoimmune encephalomyelitis. Neuroscience 2012, 226, 40–50. [Google Scholar] [CrossRef]
- Jung-Testas, I.; Renoir, J.; Gasc, J.; Baulieu, E. Estrogen-inducible progesterone receptor in primary cultures of rat glial cells. Exp. Cell Res. 1991, 193, 12–19. [Google Scholar] [CrossRef]
- Labombarda, F.; Guennoun, R.; Gonzalez, S.; Roig, P.; Lima, A.; Schumacher, M.; De Nicola, A.F. Immunocytochemical evidence for a progesterone receptor in neurons and glial cells of the rat spinal cord. Neurosci. Lett. 2000, 288, 29–32. [Google Scholar] [CrossRef]
- Compagnone, N.A.; Mellon, S.H. Neurosteroids: Biosynthesis and Function of These Novel Neuromodulators. Front. Neuroendocr. 2000, 21, 1–56. [Google Scholar] [CrossRef]
- Wagner, C.K.; Quadros-Mennella, P. Progesterone from maternal circulation binds to progestin receptors in fetal brain. Dev. Neurobiol. 2016, 77, 767–774. [Google Scholar] [CrossRef]
- Nguyen, P.N.; Billiards, S.S.; Walker, D.W.; Hirst, J.J. Changes in 5α-pregnane steroids and neurosteroidogenic enzyme expression in the perinatal sheep. Pediatr. Res. 2003, 53, 956–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiles, J.; Jernigan, T.L. The Basics of Brain Development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef] [Green Version]
- Shakeri, M.; Boustani, M.R.; Pak, N.; Panahi, F.; Salehpour, F.; Lotfinia, I.; Meshkini, A.; Daghighi, S.; Vahedi, P.; Khani, M.; et al. Effect of progesterone administration on prognosis of patients with diffuse axonal injury due to severe head trauma. Clin. Neurol. Neurosurg. 2013, 115, 2019–2022. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Denier, C.; Oudinet, J.-P.; Adams, D.; Guennoun, R. Progesterone neuroprotection: The background of clinical trial failure. J. Steroid Biochem. Mol. Boil. 2016, 160, 53–66. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Orozco, J.C.; Moral-Morales, A.D.; Camacho-Arroyo, I. Progesterone through Progesterone Receptor B Isoform Promotes Rodent Embryonic Oligodendrogenesis. Cells 2020, 9, 960. https://doi.org/10.3390/cells9040960
González-Orozco JC, Moral-Morales AD, Camacho-Arroyo I. Progesterone through Progesterone Receptor B Isoform Promotes Rodent Embryonic Oligodendrogenesis. Cells. 2020; 9(4):960. https://doi.org/10.3390/cells9040960
Chicago/Turabian StyleGonzález-Orozco, Juan Carlos, Aylin Del Moral-Morales, and Ignacio Camacho-Arroyo. 2020. "Progesterone through Progesterone Receptor B Isoform Promotes Rodent Embryonic Oligodendrogenesis" Cells 9, no. 4: 960. https://doi.org/10.3390/cells9040960





