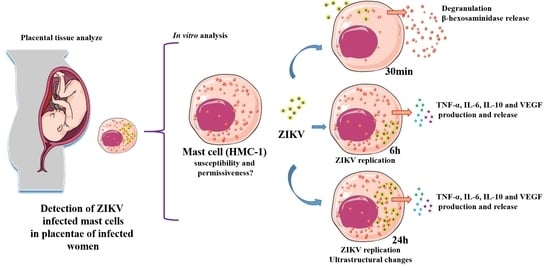
Zika Virus Infects Human Placental Mast Cells and the HMC-1 Cell Line, and Triggers Degranulation, Cytokine Release and Ultrastructural Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Placentae Collection, Patient Clinical History and Ethical Approval
2.2. Histopathology and Histological Detection of Mast Cells in ZIKV Infected Placentae
2.3. Immunofluorescence Assay
2.4. Immunohistochemistry
2.5. Cell Line
2.6. ZIKV Viral Stock Productuion
2.7. ZIKV Infections
2.8. Flow Cytometry Analysis
2.9. Measurement of Mast Cell Degranulation
2.10. ELISA Assays
2.11. Transmission Electron Microscopy Procedure
2.12. Statistical Analysis
3. Results
3.1. Detection of Mast Cells, Histopathology and ZIKV Replication in Placental Infected Tissues
3.2. Infection Rate of ZIKV at Different MOIs
3.3. ZIKV Interaction Induces Degranulation
3.4. ZIKV Led to Release of Cytokines and VEGF
3.5. Ultrastructural Changes Caused by ZIKV Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection—After the Pandemic. N. Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef] [Green Version]
- McCrae, A.W.R.; Kirya, B.G. Yellow fever and Zika virus epizootics and enzootics in Uganda. Trans. R. Soc. Trop. Hyg. 1982, 76, 552–562. [Google Scholar] [CrossRef]
- Gubler, D.; Kuno, D.; Markoff, L. Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Griffin, D.E., Lamb, R.A., Eds.; Lippincott Williams & Wilkins Publishers: Philadelphia, PA, USA, 2007. [Google Scholar]
- Lazear, H.M.; Diamond, M.S. Zika Virus: New Clinical Syndromes and Its Emergence in the Western Hemisphere. J. Virol. 2016, 90, 4864–4875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, F.M.E.; Pietrobon, A.J.; de Mendonça Oliveira, L.; da Silva Oliveira, L.M.; Sato, M.N. Maternal-Fetal Interplay in Zika Virus Infection and Adverse Perinatal Outcomes. Front. Immunol. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Faye, O.; Freire, C.C.M.; Iamarino, A.; Faye, O.; De Oliveira, J.V.C.; Diallo, M.; Zanotto, P.M.A.; Sall, A.A. Molecular Evolution of Zika Virus during Its Emergence in the 20 th Century. PLoS Negleted Trop. Dis. 2014, 8, 1–10. [Google Scholar]
- Abushouk, A.I.; Negida, A.; Ahmed, H. An updated review of Zika virus. J. Clin. Virol. 2016, 84, 53–58. [Google Scholar] [CrossRef]
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus genome organization, expression and replication. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef]
- Eady, R.A.J.; Cowen, T.; Marshall, T.F.; Plummer, V.; Greaves, M.W. Mast cell population density, blood vessel density and histamine content in normal human skin. Br. J. Dermatol. 1979, 100, 623–633. [Google Scholar] [CrossRef]
- Babina, M.; Guhl, S.; Artuc, M.; Trivedi, N.N.; Zuberbier, T. Phenotypic variability in human skin mast cells. Exp. Dermatol. 2016, 434–439. [Google Scholar] [CrossRef]
- Derbala, Y.; Elazzamy, H.; Bilal, M.; Reed, R.; Dambaeva, S.; Dinorah, M.; Garcia, S.; Skariah, A.; Kim, J.K.; Fernandez, E.; et al. Mast cell—Induced immunopathology in recurrent pregnancy losses. Am. J. Reprod. Immunol. 2019, 82, e13128. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, T.; Toyoshima, S.; Sakamoto-sasaki, T. Allergology International Characterization of human decidual mast cells and establishment of a culture system. Allergol. Int. 2018, 67, S18–S24. [Google Scholar] [CrossRef] [PubMed]
- Guanche, H.; Gutiérrez, F.; Ramirez, M.; Ruiz, A.; Pérez, C.R.; González, A. Clinical relevance of Zika symptoms in the context of a Zika Dengue epidemic. J. Infect. Public Health 2020, 13, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Paixão, E.S.; Barreto, F.; Teixeira, G.; Costa, C.N.; Rodrigues, L.C. History, Epidemiology, and Clinical Manifestations of Zika: A Systematic Review. Am. J. Public Health 2016, 106, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Munjal, A.; Khandia, R.; Dhama, K.; Sachan, S. Advances in Developing Therapies to Combat Zika Virus: Current Knowledge and Future Perspectives. Front. Microbiol. 2017, 8, 1–19. [Google Scholar] [CrossRef]
- Syenina, A.; Jagaraj, C.J.; Aman, S.A.; Sridharan, A.; St John, A.L. Dengue vascular leakage is augmented by mast cell degranulation mediated by immunoglobulin Fcγ receptors. Elife 2015, 4, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuta, T.; Murao, L.A.; Thi, N.; Lan, P.; Huy, N.T.; Thi, V.; Huong, Q.; Thuy, T.T.; Tham, V.D.; Thi, C.; et al. Association of Mast Cell-Derived VEGF and Proteases in Dengue Shock Syndrome. PLoS Neglected Trop. Dis. 2012, 6. [Google Scholar] [CrossRef] [Green Version]
- Troupin, A.; Shirley, D.; Londono-renteria, B.; Watson, A.M.; Mchale, C.; Hall, A.; Klimstra, W.B.; Gomez, G.; Colpitts, T.M.; Troupin, A.; et al. A Role for Human Skin Mast Cells in Dengue Virus Infection and Systemic Spread. J. Immunol. 2016, 197, 4382–4391. [Google Scholar] [CrossRef] [Green Version]
- Londono-renteria, B.; Marinez-angarita, J.C.; Troupin, A.; Colpitts, T.M. Role of Mast Cells in Dengue Virus Pathogenesis 1 2 3. DNA Cell Boil. 2017, 36, 423–427. [Google Scholar] [CrossRef]
- Nilsson, G.; Blom, T.; Kjellenf, M.K.L.; Butterfieldj, J.H.; Sundstrom, C.; Nilsson, K.; Hellman, L. Phenotypic Characterization of the Human Mast-Cell Line HMC-1. Scand. J. Immunol. 1994, 489–498. [Google Scholar] [CrossRef]
- Cochrane, D.E.; Carraway, R.E.; Harrington, K.; Laudano, M.; Rawlings, S.; Feldberg, R.S. HMC-1 human mast cells synthesize neurotensin (NT) precursor, secrete bioactive NT-like peptide (s) and express NT receptor NTS1. Inflamm. Res. 2011, 60, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, M.; Befus, A.D. Interferon- c regulates chemokine expression and release in the human mast cell line HMC 1: Role of nitric oxide. Immunology 2007, 123, 209–217. [Google Scholar] [PubMed]
- Brown, M.G.; King, C.A.; Sherren, C.; Marshall, J.S.; Anderson, R. A dominant role for FcɣRII in antibody-enhanced dengue virus infection of human mast cells and associated CCL5 release Abstract: Dengue virus is a major mosquito- borne human pathogen with four known serotypes. The presence of antidengue virus antibod. J. Leukoc. Biol. 2006, 80, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, K.; de Souza, C.F.; de Souza, L.J.; de Souza, T.L.; dos Santos, F.B.; Nunes, P.C.G.; de Azeredo, E.L.; Salomão, N.G.; Trindade, G.F.; Basílio-de-Oliveira, C.A.; et al. Placental Histopathology and clinical presentation of severe congenital Zika syndrome in a human immunodeficiency virus-exposed uninfected infant. Front. Immunol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stan, S.M.; Duffy, M.R. Genetic and Serologic Properties of Zika Virus Associated with an Epidemic, Yap State. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Wang, C.; Fang-Hoover, J.; Harris, E.; Pereira, L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Rabelo, K.; Souza, L.J.; Salomão, N.G.; Oliveira, E.R.A.; de Sentinelli, L.P.; Lacerda, M.S.; Saraquino, P.B.; Rosman, F.C.; Basílio-de-Oliveira, R.; Carvalho, J.J.; et al. Placental inflammation and fetal injury in a rare Zika case associated with Guillain-Barré Syndrome and abortion. Front. Microbiol. 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Quicke, K.M.; Bowen, J.R.; Johnson, E.L.; Schinazi, R.F.; Chakraborty, R.; Suthar, M.S.; Quicke, K.M.; Bowen, J.R.; Johnson, E.L.; Mcdonald, C.E.; et al. Zika Virus Infects Human Placental Macrophages. Cell Host Microbe 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, A.Z.; Yu, W.; Hill, D.A.; Reyes, C.A.; Schwartz, D.A. Placental Pathology of Zika Virus: Viral Infection of the Placenta Induces Villous Stromal Macrophage (Hofbauer Cell) Proliferation and Hyperplasia. Arch. Pathol. Lab. Med. 2017, 141, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Zulu, M.Z.; Martinez, O.; Gordon, S.; Gray, M. The Elusive Role of Placental Macrophages: The Hofbauer Cell. J. Innate Immun. 2019, 11, 447–456. [Google Scholar] [CrossRef]
- Woidacki, K.; Jensen, F.; Zenclussen, A.C. Mast cells as novel mediators of reproductive processes. Front. Immunol. 2013, 4, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faas, M.M.; Vos, P. De Innate immune cells in the placental bed in healthy pregnancy and preeclampsia. Placenta 2018, 69, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Kanegasaki, S.; Jin, F.; Deng, Y.; You, Z.; Chang, J.-H.; Kim, D.Y.; Timilshina, M.; Kim, J.-R.; Lee, Y.J.; et al. A common signaling pathway leading to degranulation in mast cells and its regulation by CCR1-ligand. Basic Transl. Allergy Immunol. 2019, 1, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Fukuishi, N.; Murakami, S.; Ohno, A.; Matsui, N.; Fukutsuji, K.; Itoh, K.; Akagi, M.; Alerts, E. Does β -Hexosaminidase Function Only as a Degranulation Indicator in Mast Cells? The Primary Role of β -Hexosaminidase in Mast Cell Granules. J. Immunol. Does 2014, 193, 1886–1894. [Google Scholar] [CrossRef] [Green Version]
- Schemann, M.; Kugler, E.M.; Buhner, S.; Eastwood, C.; Donovan, J.; Jiang, W.; Grundy, D. The Mast Cell Degranulator Compound 48/80 Directly Activates Neurons. PLoS ONE 2012, 7, e52104. [Google Scholar] [CrossRef] [Green Version]
- Van Der Schaar, H.M.; Rust, M.J.; Chen, C.; Van Der Ende-Metselaar, H.; Wilschut, J.; Zhuang, X.; Smit, J.M. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog. 2008, 4. [Google Scholar] [CrossRef] [Green Version]
- Arciuch, L.; Bielecki, D.; Borzym, M.; Południewski, G.; Arciszewki, K.; Rózański, A.; Zwierz, K. Isoenzymes of N-acetyl-beta-hexosaminidase in complicated pregnancy. Acta Biochim. Pol. 1999, 46, 977–983. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2015, 12, 49–62. [Google Scholar] [CrossRef]
- Cop, N.; Ebo, D.G.; Bridts, C.H.; Elst, J.; Hagendorens, M.M.; Mertens, C.; Faber, M.A.; De Clerck, L.S.; Sabato, V. Influence of IL-6, IL-33, and TNF- a on Human Mast Cell Activation: Lessons from Single Cell Analysis by Flow Cytometry. Int. Clin. Cytom. Soc. 2018, 411, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Boil. 2014, 6. [Google Scholar] [CrossRef]
- Mchale, C.; Mohammed, Z.; Deppen, J.; Gomez, G. BBA—General Subjects Interleukin-6 potentiates Fc ε RI-induced PGD 2 biosynthesis and induces VEGF from human in situ-matured skin mast cells. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1069–1078. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Kam, Y.W.; Leite, J.A.; Lum, F.M.; Tan, J.J.L.; Lee, B.; Judice, C.C.; De Toledo Teixeira, D.A.; Andreata-Santos, R.; Vinolo, M.A.; Angerami, R.; et al. Specific biomarkers associated with neurological complications and congenital central nervous system abnormalities from Zika virus-infected patients in Brazil. J. Infect. Dis. 2017, 216, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Polukort, S.H.; Rovatti, J.; Carlson, L.; Thompson, C.; Ser-Dolansky, J.; Kinney, S.R.M.; Schneider, S.S.; Mathias, C. IL-10 enhances IgE-mediated mast cell responses and is essential for the development of experimental food allergy in IL-10-deficient mice. J Immunol. 2017, 196, 4865–4876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagliari, C.; Fernandes, E.R.; Guedes, F.; Alves, C.; Sotto, M.N. Role of mast cells as IL10 producing cells in paracoccidioidomycosis skin lesions. Mycopathologia 2006, 162, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Haase, K.; Gillrie, M.R.; Hajal, C.; Kamm, R.D. Pericytes Contribute to Dysfunction in a Human 3D Model of Placental Microvasculature through VEGF-Ang-Tie2 Signaling. Adv. Sci. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Sherif, N.A.; Ghozy, S.; Zayan, A.H.; Elkady, A.H. Mast cell mediators in relation to dengue severity: A systematic review and meta-analysis. Rev. Med. Virol. 2019, 2084, 1–12. [Google Scholar] [CrossRef]
- Fish-Low, C.-Y.; Abubakar, S.; Othman, F.; Chee, H. Ultrastructural aspects of sylvatic dengue virus infection in Vero cell. Malays. J. Pathol. 2019, 41, 41–46. [Google Scholar]
- Tadkalkar, N.; Prasad, S.; Gangodkar, S.; Ghosh, K. Dengue Virus NS1 Exposure Affects von Willebrand Factor Profile and Platelet Adhesion Properties of Cultured Vascular Endothelial Cells. Indian J. Hematol. Blood Transfus. 2018, 35, 502–505. [Google Scholar] [CrossRef]





| Genome Position | Region | Sequence | |
|---|---|---|---|
| 835–857 | M/E | sense | TTGGTCATGATACTGCTGATTGC |
| 911–890 | M/E | reverse | CCTTCCACAAAGTCCCTATTGC |
| 860–886 | M/E | probe | FAM-CGGCATACAGCATCAGGTGCATAGGAG-NFQ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabelo, K.; Gonçalves, A.J.d.S.; Souza, L.J.d.; Sales, A.P.; Lima, S.M.B.d.; Trindade, G.F.; Ciambarella, B.T.; Amorim Tasmo, N.R.; Diaz, B.L.; Carvalho, J.J.d.; et al. Zika Virus Infects Human Placental Mast Cells and the HMC-1 Cell Line, and Triggers Degranulation, Cytokine Release and Ultrastructural Changes. Cells 2020, 9, 975. https://doi.org/10.3390/cells9040975
Rabelo K, Gonçalves AJdS, Souza LJd, Sales AP, Lima SMBd, Trindade GF, Ciambarella BT, Amorim Tasmo NR, Diaz BL, Carvalho JJd, et al. Zika Virus Infects Human Placental Mast Cells and the HMC-1 Cell Line, and Triggers Degranulation, Cytokine Release and Ultrastructural Changes. Cells. 2020; 9(4):975. https://doi.org/10.3390/cells9040975
Chicago/Turabian StyleRabelo, Kíssila, Antônio José da Silva Gonçalves, Luiz José de Souza, Anna Paula Sales, Sheila Maria Barbosa de Lima, Gisela Freitas Trindade, Bianca Torres Ciambarella, Natália Recardo Amorim Tasmo, Bruno Lourenço Diaz, Jorge José de Carvalho, and et al. 2020. "Zika Virus Infects Human Placental Mast Cells and the HMC-1 Cell Line, and Triggers Degranulation, Cytokine Release and Ultrastructural Changes" Cells 9, no. 4: 975. https://doi.org/10.3390/cells9040975
APA StyleRabelo, K., Gonçalves, A. J. d. S., Souza, L. J. d., Sales, A. P., Lima, S. M. B. d., Trindade, G. F., Ciambarella, B. T., Amorim Tasmo, N. R., Diaz, B. L., Carvalho, J. J. d., Duarte, M. P. d. O., & Paes, M. V. (2020). Zika Virus Infects Human Placental Mast Cells and the HMC-1 Cell Line, and Triggers Degranulation, Cytokine Release and Ultrastructural Changes. Cells, 9(4), 975. https://doi.org/10.3390/cells9040975