Therapeutic Potential of Dental Pulp Stem Cells and Leukocyte- and Platelet-Rich Fibrin for Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Stem Cell Isolation and Culture
2.2. Isolation and Culture of Immature Murine Articular Chondrocytes
2.3. L-PRF Isolation
2.4. L-PRF Conditioned Medium and Exudate
2.5. Chondrogenic Differentiation
2.6. DPSC Conditioned Medium
2.7. Cell Survival and Proliferation Assay
2.8. Reverse Transcriptase Quantitative Polymerase Chain Reaction
2.9. Enzyme-Linked Immunosorbent Assay
2.10. Nitrite Measurements
2.11. Three-Dimensional Culture of iMACs
2.12. Immunocytochemical Staining
2.13. (Immuno)histology
2.13.1. Immunohistochemistry
2.13.2. Histology
2.14. Transmission Electron Microscopy
2.15. Statistical Analysis
3. Results
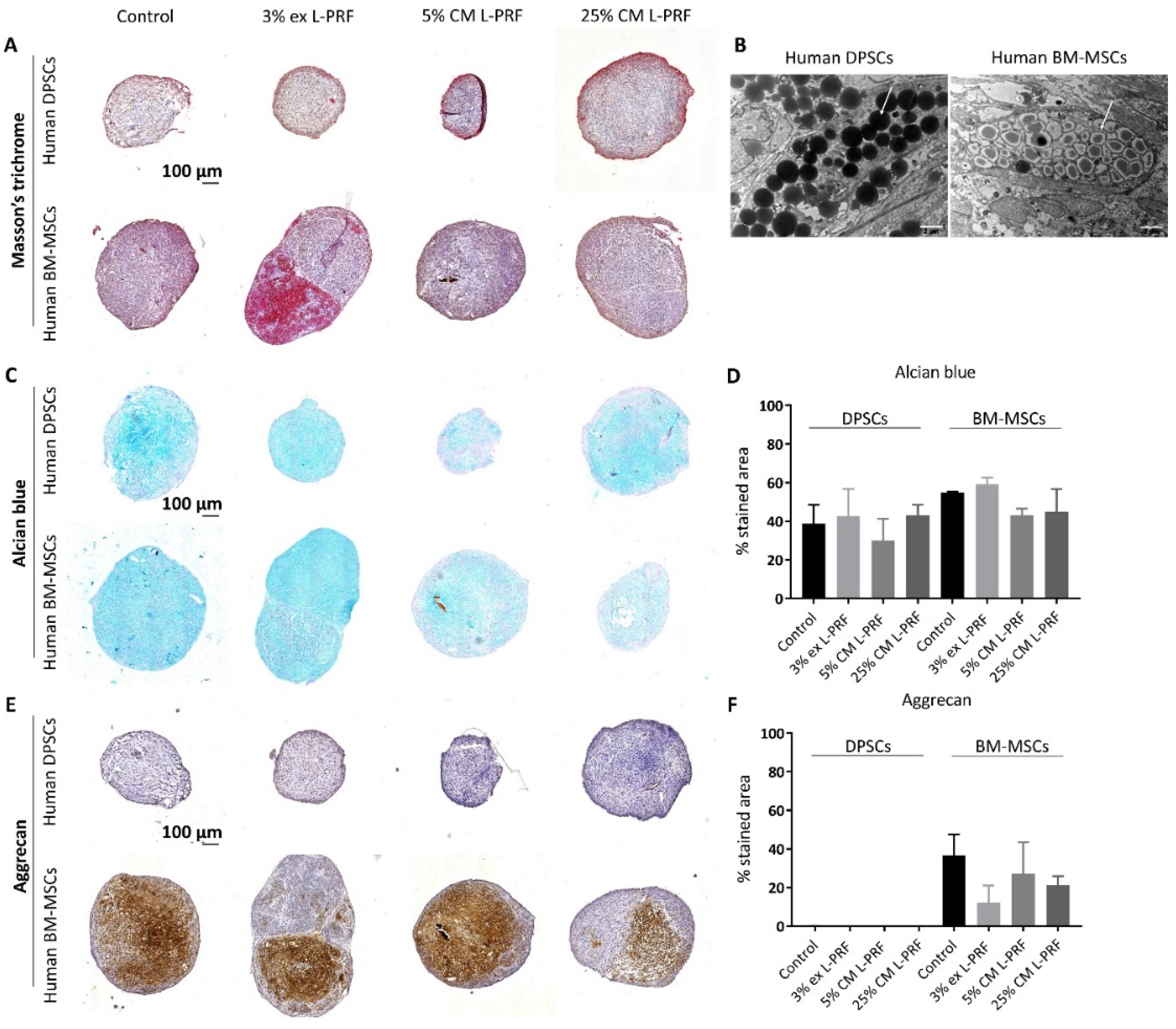
3.1. Differences in Chondrogenic Differentiation Potential Between BM-MSCs and DPSCs and the Effect of Exposure to L-PRF During Chondrogenesis
3.2. Phenotypical and Ultrastructural Characterization of Immature Murine Articular Chondrocytes
3.3. Effect of Secreted Factors of DPSCs and L-PRF on Healthy Chondrocyte Survival and Proliferation and Viability of TNF-α- and IL-1β-Stimulated iMACs
3.4. Effect of Secreted Factors of DPSCs and L-PRF on OA-related mRNA Expression of Unstimulated and TNF-α- and IL-1β-Stimulated iMACs
3.5. IL-6 and PGE2 Release Are Increased After Supplementation of Cytokines Combined With L-PRF CM
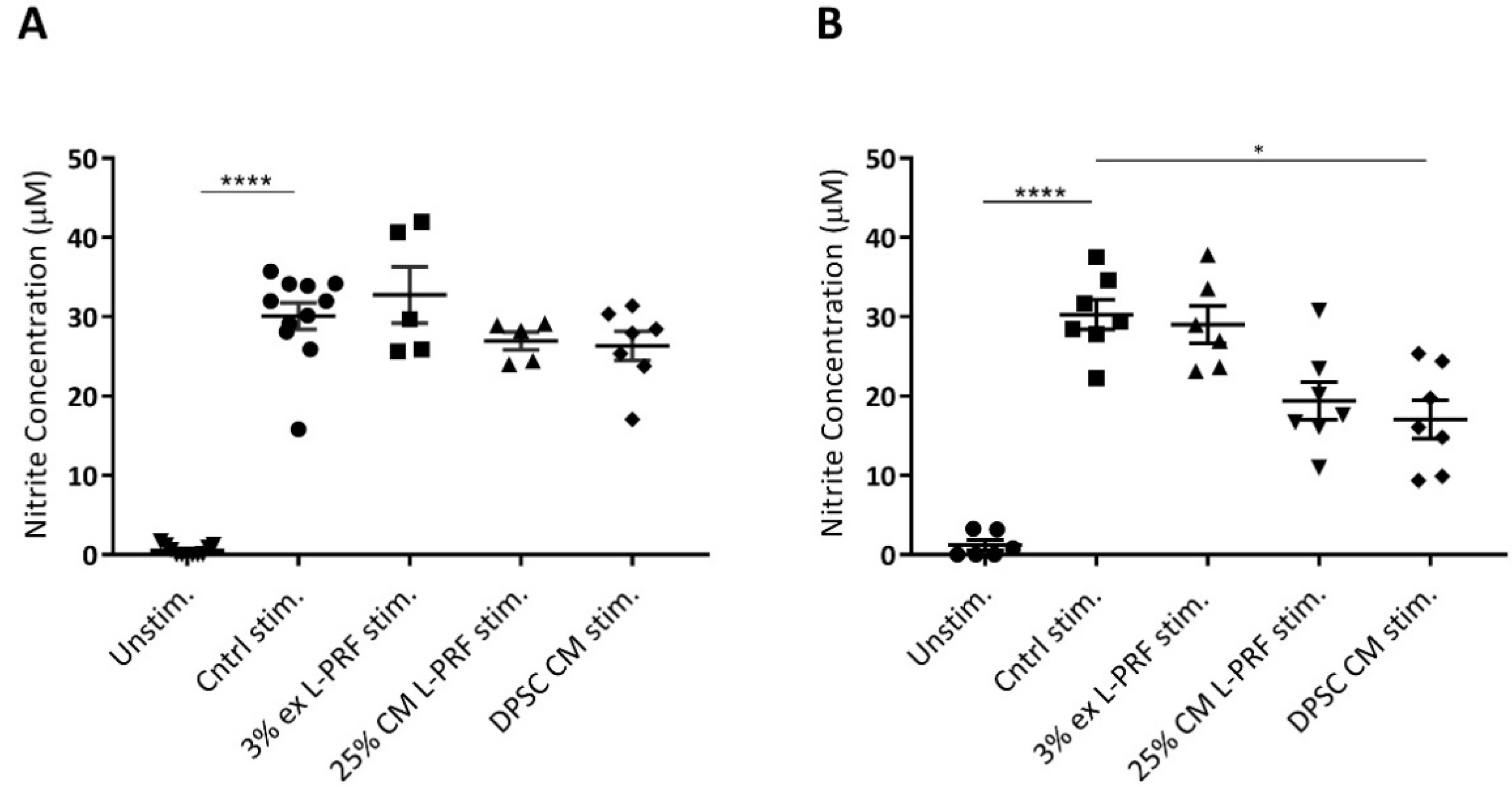
3.6. Nitrite Levels Are Increased Upon Cytokine Stimulation and Decreased by DPSC CM
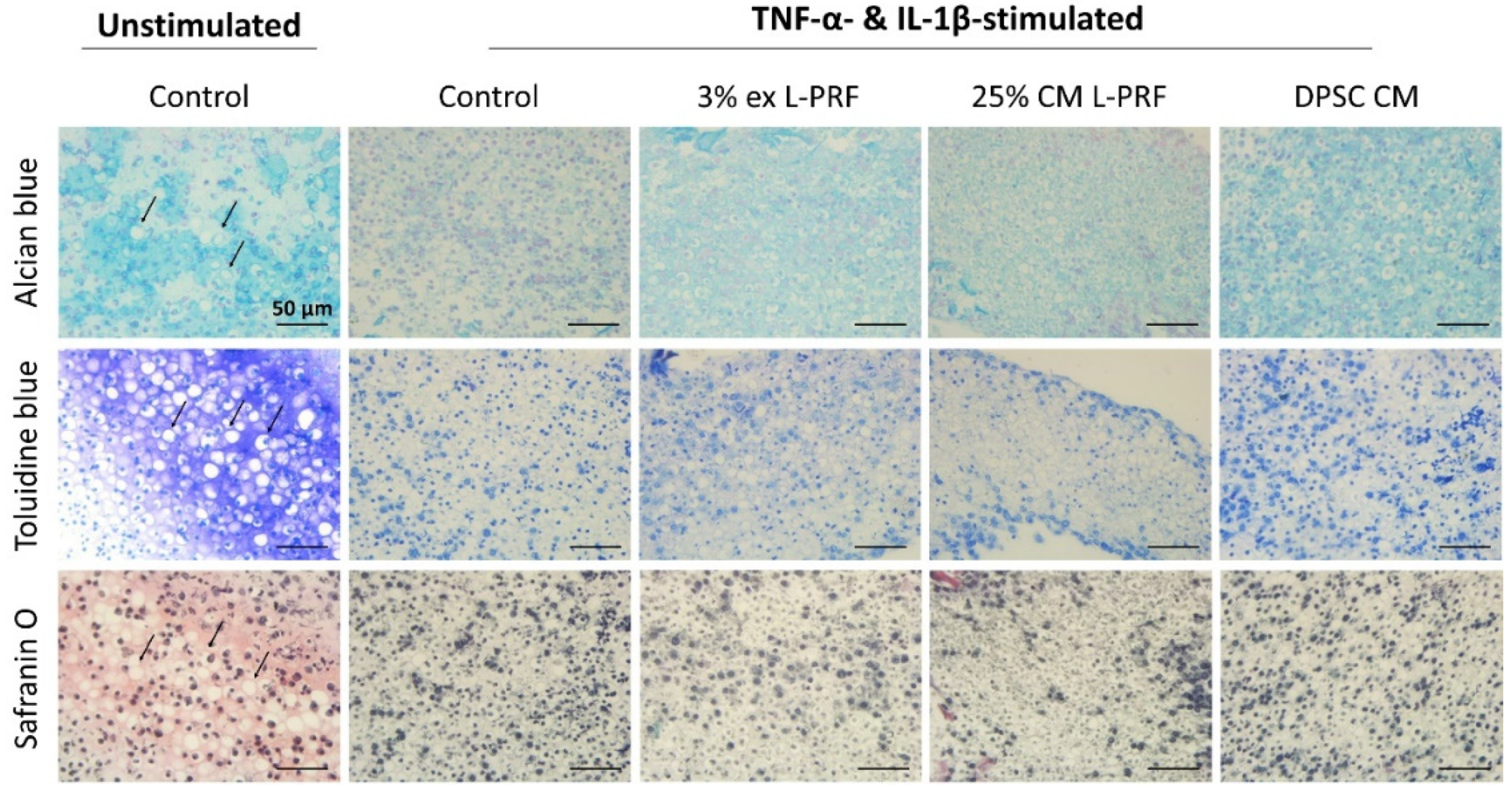
3.7. Cartilage-Specific ECM Production of iMACs in 3D Culture After Exposure to L-PRF ex, L-PRF CM and DPSC CM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage. Sports Heal. Multidiscip. Approach 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Monaco, M.L.; Merckx, G.; Ratajczak, J.; Gervois, P.; Hilkens, P.; Clegg, P.D.; Bronckaers, A.; Vandeweerd, J.-M.; Lambrichts, I. Stem Cells for Cartilage Repair: Preclinical Studies and Insights in Translational Animal Models and Outcome Measures. Stem Cells Int. 2018, 2018, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Solheim, E.; Krokeide, A.M.; Melteig, P.; Larsen, A.; Strand, T.; Brittberg, M. Symptoms and function in patients with articular cartilage lesions in 1000 knee arthroscopies. Knee Surg. Sports Traumatol. Arthrosc. 2014, 24, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Widuchowski, W.; Widuchowski, J.; Koczy, B.; Szyluk, K. Untreated Asymptomatic Deep Cartilage Lesions Associated with Anterior Cruciate Ligament Injury. Am. J. Sports Med. 2009, 37, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Widuchowski, W.; Widuchowski, J.; Faltus, R.; Lukasik, P.; Kwiatkowski, G.; Szyluk, K.; Koczy, B. Long-term clinical and radiological assessment of untreated severe cartilage damage in the knee: A natural history study. Scand. J. Med. Sci. Sports 2011, 21, 106–110. [Google Scholar] [CrossRef]
- Brooks, P.M. Impact of osteoarthritis on individuals and society: How much disability? Social consequences and health economic implications. Curr. Opin. Rheumatol. 2002, 14, 573–577. [Google Scholar] [CrossRef]
- Litwic, A.; Edwards, M.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Available online: https://www.who.int/chp/topics/rheumatic/en/ (accessed on 10 March 2020).
- Alshami, A.M. Knee osteoarthritis related pain: A narrative review of diagnosis and treatment. Int. J. Heal. Sci. 2014, 8, 85–104. [Google Scholar] [CrossRef]
- Negoro, T.; Takagaki, Y.; Okura, H.; Matsuyama, A. Trends in clinical trials for articular cartilage repair by cell therapy. NPJ Regen. Med. 2018, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Pareek, A.; Carey, J.L.; Reardon, P.J.; Peterson, L.; Stuart, M.J.; Krych, A.J. Long-Term Outcomes after Autologous Chondrocyte Implantation. Cartilage 2016, 7, 298–308. [Google Scholar] [CrossRef] [Green Version]
- Niemeyer, P.; Albrecht, D.; Andereya, S.; Angele, P.; Ateschrang, A.; Aurich, M.; Baumann, M.; Bosch, U.; Erggelet, C.; Fickert, S.; et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: A guideline by the working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU). Knee 2016, 23, 426–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aae, T.F.; Randsborg, P.-H.; Lurås, H.; Årøen, A.; Lian Øystein, B. Microfracture is more cost-effective than autologous chondrocyte implantation: A review of level 1 and level 2 studies with 5 year follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2017, 26, 1044–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.; Gronthos, S. Perivascular Niche of Postnatal Mesenchymal Stem Cells in Human Bone Marrow and Dental Pulp. J. Bone Miner. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibarretxe, G.; Crende, O.; Aurrekoetxea, M.; García-Murga, V.; Etxaniz, J.; Unda, F. Neural Crest Stem Cells from Dental Tissues: A New Hope for Dental and Neural Regeneration. Stem Cells Int. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ledesma-Martínez, E.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2015, 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hilkens, P.; Gervois, P.; Fanton, Y.; Vanormelingen, J.; Martens, W.; Struys, T.; Politis, C.; Lambrichts, I.; Bronckaers, A. Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell and Tissue Research 2013, 353, 65–78. [Google Scholar] [CrossRef]
- Ahmed, N.; Murakami, M.; Hirose, Y.; Nakashima, M. Therapeutic Potential of Dental Pulp Stem Cell Secretome for Alzheimer’s Disease Treatment: An In Vitro Study. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Hossein-Khannazer, N.; Hashemi, S.M.; Namaki, S.; Ghanbarian, H.; Sattari, M.; Khojasteh, A. Study of the immunomodulatory effects of osteogenic differentiated human dental pulp stem cells. Life Sci. 2019, 216, 111–118. [Google Scholar] [CrossRef]
- Bronckaers, A.; Hilkens, P.; Fanton, Y.; Struys, T.; Gervois, P.; Politis, C.; Martens, W.; Lambrichts, I. Angiogenic Properties of Human Dental Pulp Stem Cells. PLoS ONE 2013, 8, e71104. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Jiang, C.-M.; An, S.; Cheng, Q.; Huang, Y.-F.; Wang, Y.-T.; Gou, Y.; Xiao, L.; Yu, W.-J.; Wang, J. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 2013, 20, 25–34. [Google Scholar] [CrossRef]
- Ishikawa, J.; Takahashi, N.; Matsumoto, T.; Yoshioka, Y.; Yamamoto, N.; Nishikawa, M.; Hibi, H.; Ishigro, N.; Ueda, M.; Furukawa, K.; et al. Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental rheumatoid arthritis. Bone 2016, 83, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wang, J.; Lu, J.; Zou, D.; Sun, H.; Dong, Y.; Yu, H.; Zhang, L.; Yang, T.; Zhang, X.; et al. The effect of co-culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials 2012, 33, 7699–7711. [Google Scholar] [CrossRef] [PubMed]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.; Cole, B.J. The Role of Growth Factors in Cartilage Repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampson, S.; Gerhardt, M.; Mandelbaum, B. Platelet rich plasma injection grafts for musculoskeletal injuries: A review. Curr. Rev. Musculoskelet. Med. 2008, 1, 165–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrenfest, D.M.D.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.I.; Whitney, K.; Evans, T.; Laprade, R.F. Platelet-Rich Plasma and Cartilage Repair. Curr. Rev. Musculoskelet. Med. 2018, 11, 573–582. [Google Scholar] [CrossRef]
- Moussa, M.; Lajeunesse, D.; Hilal, G.; El Atat, O.; Haykal, G.; Serhal, R.; Chalhoub, A.; Khalil, C.; Alaaeddine, N. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp. Cell Res. 2017, 352, 146–156. [Google Scholar] [CrossRef]
- Akeda, K.; An, H.; Okuma, M.; Attawia, M.; Miyamoto, K.; Thonar, E.-M.; Lenz, M.; Sah, R.; Masuda, K. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthr. Cartil. 2006, 14, 1272–1280. [Google Scholar] [CrossRef] [Green Version]
- Prakash, S.; Thakur, A. Platelet Concentrates: Past, Present and Future. J. Maxillofac. Oral Surg. 2011, 10, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, J.; Vangansewinkel, T.; Gervois, P.; Merckx, G.; Hilkens, P.; Quirynen, M.; Lambrichts, I.; Bronckaers, A. Angiogenic Properties of ‘Leukocyte- and Platelet-Rich Fibrin’. Sci. Rep. 2018, 8, 14632. [Google Scholar] [CrossRef] [Green Version]
- De Melo, B.A.G.; Luzo Ângela, C.M.; Lana, J.F.S.D. Centrifugation Conditions in the L-PRP Preparation Affect Soluble Factors Release and Mesenchymal Stem Cell Proliferation in Fibrin Nanofibers. Molecules 2019, 24, 2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabiri, A.; Esfandiari, E.; Esmaeili, A.; Hashemibeni, B.; Pourazar, A.; Mardani, M. Platelet-rich plasma application in chondrogenesis. Adv. Biomed. Res. 2014, 3, 138. [Google Scholar] [CrossRef] [PubMed]
- Barbon, S.; Stocco, E.; Macchi, V.; Contran, M.; Grandi, C.; Borean, A.; Parnigotto, P.P.; Porzionato, A.; De Caro, R. Platelet-Rich Fibrin Scaffolds for Cartilage and Tendon Regenerative Medicine: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, C.-S.; Ho, H.-O.; Liang, Y.-C.; Ko, P.-H.; Sheu, M.-T.; Chen, C.-H. Incorporation of exudates of human platelet-rich fibrin gel in biodegradable fibrin scaffolds for tissue engineering of cartilage. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 948–955. [Google Scholar] [CrossRef]
- El Raouf, M.A.; Wang, X.; Miusi, S.; Chai, J.; Abdel-Aal, A.B.M.; Helmy, M.M.N.; Ghanaati, S.; Choukroun, J.; Choukroun, E.; Zhang, Y.; et al. Injectable-platelet rich fibrin using the low speed centrifugation concept improves cartilage regeneration when compared to platelet-rich plasma. Platelets 2017, 30, 213–221. [Google Scholar] [CrossRef]
- Wong, C.-C.; Chen, C.-H.; Chan, W.P.; Chiu, L.-H.; Ho, W.-P.; Hsieh, F.-J.; Chen, Y.-T.; Yang, T.-L. Single-Stage Cartilage Repair Using Platelet-Rich Fibrin Scaffolds With Autologous Cartilaginous Grafts. Am. J. Sports Med. 2017, 45, 3128–3142. [Google Scholar] [CrossRef]
- De Souza, F.G.; Fernandes, B.; Rebelatto, C.L.K.; De Aguiar, A.M.; Fracaro, L.; Brofman, P.R.S. Proliferation and differentiation of stem cells in contact with eluate from fibrin-rich plasma membrane. Rev. Bras. de Ortop. (Engl. Ed.) 2018, 53, 45–52. [Google Scholar] [CrossRef]
- Gosset, M.; Berenbaum, F.; Thirion, S.; Jacques, C. Primary culture and phenotyping of murine chondrocytes. Nat. Protoc. 2008, 3, 1253–1260. [Google Scholar] [CrossRef]
- Gervois, P.; Struys, T.; Hilkens, P.; Bronckaers, A.; Ratajczak, J.; Politis, C.; Brone, B.; Lambrichts, I.; Martens, W. Neurogenic Maturation of Human Dental Pulp Stem Cells Following Neurosphere Generation Induces Morphological and Electrophysiological Characteristics of Functional Neurons. Stem Cells Dev. 2015, 24, 296–311. [Google Scholar] [CrossRef] [Green Version]
- Bretschneider, A.; Burns, W.; Morrison, A. Pop-Off Technique—he Ultrastructure of Paraffin-Embedded Sections. Am J Clin Pathol 1981, 76, 450–453. [Google Scholar] [CrossRef]
- Branly, T.; Contentin, R.; Desancé, M.; Jacquel, T.; Bertoni, L.; Leroy, S.; Mallein-Gerin, F.; Denoix, J.-M.; Audigié, F.; Demoor, M.; et al. Improvement of the Chondrocyte-Specific Phenotype upon Equine Bone Marrow Mesenchymal Stem Cell Differentiation: Influence of Culture Time, Transforming Growth Factors and Type I Collagen siRNAs on the Differentiation Index. Int. J. Mol. Sci. 2018, 19, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mata, M.; Milian, L.; Oliver, M.; Zurriaga, J.; Sancho-Tello, M.; De Llano, J.J.M.; Carda, C. In Vivo Articular Cartilage Regeneration Using Human Dental Pulp Stem Cells Cultured in an Alginate Scaffold: A Preliminary Study. Stem Cells Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, C.L.; Janebodin, K.; Yuan, A.E.; Dennis, J.E.; Reyes, M.; Kim, D.-H. Enhanced Chondrogenic Differentiation of Dental Pulp Stem Cells Using Nanopatterned PEG-GelMA-HA Hydrogels. Tissue Eng. Part A 2014, 20, 2817–2829. [Google Scholar] [CrossRef] [PubMed]
- Westin, C.; Trinca, R.B.; Zuliani, C.; Coimbra, I.B.; Moraes, Â.M. Differentiation of dental pulp stem cells into chondrocytes upon culture on porous chitosan-xanthan scaffolds in the presence of kartogenin. Mater. Sci. Eng. C 2017, 80, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Christiansen-Weber, T.; Noskov, A.; Cardiff, D.; Garitaonandia, I.; Dillberger, A.; Semechkin, A.; Gonzalez, R.; Kern, R. Supplementation of specific carbohydrates results in enhanced deposition of chondrogenic-specific matrix during mesenchymal stem cell differentiation. J. Tissue Eng. Regen. Med. 2018, 12, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Pingguan-Murphy, B.; Abas, W.A.B.W.; Azmi, M.A.N.; Omar, S.Z.; Chua, K.H.; Safwani, W.K.Z.W. Impact of low oxygen tension on stemness, proliferation and differentiation potential of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2014, 448, 218–224. [Google Scholar] [CrossRef]
- Rizk, A.; Rabie, A.B.M. Human dental pulp stem cells expressing transforming growth factor β3 transgene for cartilage-like tissue engineering. Cytotherapy 2013, 15, 712–725. [Google Scholar] [CrossRef]
- Khan, H.; Mafi, P.; Mafi, R.; Khan, W.S. The effects of ageing on differentiation and characterisation of human mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2016, 13, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef]
- Longoni, A.; Utomo, L.; Van Hooijdonk, I.; Bittermann, G.; Vetter, V.C.; Spanjer, E.C.K.; Ross, J.; Rosenberg, A.; Gawlitta, D. The chondrogenic differentiation potential of dental pulp stem cells. Eur. Cells Mater. 2020, 39, 121–135. [Google Scholar] [CrossRef]
- Pinto, N.R.; Ubilla, M.; Zamora, Y.; Del Rio, V.; Ehrenfest, D.M.D.; Quirynen, M. Leucocyte- and platelet-rich fibrin (L-PRF) as a regenerative medicine strategy for the treatment of refractory leg ulcers: A prospective cohort study. Platelets 2017, 29, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Mardani, M.; Kabiri, A.; Esfandiari, E.; Esmaeili, A.; Pourazar, A.; Ansar, M.; Hashemibeni, B. The Effect of Platelet Rich Plasma on Chondrogenic Differentiation of Human Adipose Derived Stem Cells in Transwell Culture. Iran. J. Basic Med. Sci. 2013, 16, 1163–1169. [Google Scholar] [PubMed]
- Drengk, A.; Zapf, A.; Stürmer, E.K.; Stürmer, K.M.; Frosch, K.-H. Influence of Platelet-Rich Plasma on Chondrogenic Differentiation and Proliferation of Chondrocytes and Mesenchymal Stem Cells. Cells Tissues Organs 2009, 189, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Tummala, P.; King, A.; Lee, B.; Kraus, M.; Tse, V.; Jacobs, C.R. Buffered Platelet-Rich Plasma Enhances Mesenchymal Stem Cell Proliferation and Chondrogenic Differentiation. Tissue Eng. Part C Methods 2009, 15, 431–435. [Google Scholar] [CrossRef]
- Liou, J.-J.; Rothrauff, B.; Alexander, P.G.; Tuan, R.S. Effect of Platelet-Rich Plasma on Chondrogenic Differentiation of Adipose- and Bone Marrow-Derived Mesenchymal Stem Cells. Tissue Eng. Part A 2018, 24, 1432–1443. [Google Scholar] [CrossRef]
- Qian, Y.; Han, Q.; Chen, W.; Song, J.; Zhao, X.; Ouyang, Y.; Yuan, W.-E.; Fan, C. Platelet-Rich Plasma Derived Growth Factors Contribute to Stem Cell Differentiation in Musculoskeletal Regeneration. Front. Chem. 2017, 5, 89. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Bielecki, T.; Ehrenfest, D.M.D.; Everts, P.A.; Wiczkowski, A. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: New perspectives. Curr. Pharm. Biotechnol. 2012, 13, 1153–1162. [Google Scholar] [CrossRef]
- Ozer, K.; Colak, O. Leucocyte- and platelet-rich fibrin as a rescue therapy for small-to-medium-sized complex wounds of the lower extremities. Burn. Trauma 2019, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- Ehrenfest, D.M.D.; De Peppo, G.M.; Doglioli, P.; Sammartino, G. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): A gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors 2009, 27, 63–69. [Google Scholar] [CrossRef]
- Kubo, S.; Cooper, G.M.; Matsumoto, T.; Phillippi, J.A.; Corsi, K.A.; Usas, A.; Li, G.; Fu, F.H.; Huard, J. Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle-derived stem cells. Arthritis Rheum. 2009, 60, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.-M. Epidermal Growth Factor Negatively Regulates Chondrogenesis of Mesenchymal Cells by Modulating the Protein Kinase C-alpha, Erk-1, and p38 MAPK Signaling Pathways. J. Boil. Chem. 2000, 275, 12353–12359. [Google Scholar] [CrossRef] [Green Version]
- Gervois, P.; Ratajczak, J.; Wolfs, E.; Vangansewinkel, T.; Dillen, Y.; Merckx, G.; Bronckaers, A.; Lambrichts, I. Preconditioning of Human Dental Pulp Stem Cells with Leukocyte- and Platelet-Rich Fibrin-Derived Factors Does Not Enhance Their Neuroregenerative Effect. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Wehling, N.; Palmer, G.D.; Pilapil, C.; Liu, F.; Wells, J.W.; Müller, P.E.; Evans, C.H.; Porter, R.M. Interleukin-1β and tumor necrosis factor α inhibit chondrogenesis by human mesenchymal stem cells through NF-κB-dependent pathways. Arthritis Rheum. 2009, 60, 801–812. [Google Scholar] [CrossRef]
- Liu, W.; Sun, Y.; He, Y.; Zhang, H.; Zheng, Y.; Yao, Y.; Zhang, Z. IL-1β impedes the chondrogenic differentiation of synovial fluid mesenchymal stem cells in the human temporomandibular joint. Int. J. Mol. Med. 2016, 39, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Zayed, M.; Schumacher, J.; Misk, N.A.; Dhar, M. Effects of pro-inflammatory cytokines on chondrogenesis of equine mesenchymal stromal cells derived from bone marrow or synovial fluid. Veter- J. 2016, 217, 26–32. [Google Scholar] [CrossRef]
- Danisovic, L.; Varga, I.; Polak, S. Growth factors and chondrogenic differentiation of mesenchymal stem cells. Tissue Cell 2012, 44, 69–73. [Google Scholar] [CrossRef]
- Yang, A.; Lu, Y.; Xing, J.; Li, Z.; Yin, X.; Dou, C.; Dong, S.; Luo, F.; Xie, Z.; Hou, T.; et al. IL-8 Enhances Therapeutic Effects of BMSCs on Bone Regeneration via CXCR2-Mediated PI3k/Akt Signaling Pathway. Cell. Physiol. Biochem. 2018, 48, 361–370. [Google Scholar] [CrossRef]
- Stokes, D.G.; Liu, G.; Coimbra, I.B.; Piera-Velazquez, S.; Crowl, R.M.; A Jimenez, S. Assessment of the gene expression profile of differentiated and dedifferentiated human fetal chondrocytes by microarray analysis. Arthritis Rheum. 2002, 46, 404–419. [Google Scholar] [CrossRef] [Green Version]
- Srirangan, S.; Choy, E.H. The Role of Interleukin 6 in the Pathophysiology of Rheumatoid Arthritis. Ther. Adv. Musculoskelet. Dis. 2010, 2, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Ogata, A.; Kato, Y.; Higa, S.; Yoshizaki, K. IL-6 inhibitor for the treatment of rheumatoid arthritis: A comprehensive review. Mod. Rheumatol. 2019, 29, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef] [Green Version]
- Vuolteenaho, K.; Koskinen-Kolasa, A.; Laavola, M.; Nieminen, R.; Moilanen, T.; Moilanen, E. High synovial fluid interleukin-6 levels are associated with increased matrix metalloproteinase levels and severe radiographic changes in osteoarthritis patients. Osteoarthr. Cartil. 2017, 25, S92–S93. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, L.; Jiang, H.; Zhou, J.; Tang, Y. Inhibition of interleukin-6 function attenuates the central sensitization and pain behavior induced by osteoarthritis. Eur. J. Pharmacol. 2017, 811, 260–267. [Google Scholar] [CrossRef]
- Van De Loo, F.A.; Kuiper, S.; Van Enckevort, F.H.; Arntz, O.J.; Berg, W.B.V.D. Interleukin-6 reduces cartilage destruction during experimental arthritis. A study in interleukin-6-deficient mice. Am. J. Pathol. 1997, 151, 177–191. [Google Scholar]
- De Hooge, A.S.; Van De Loo, F.A.; Bennink, M.B.; Arntz, O.J.; De Hooge, P.; Berg, W.B.V.D.; Vandenberg, W. Male IL-6 gene knock out mice developed more advanced osteoarthritis upon aging. Osteoarthr. Cartil. 2005, 13, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Nagao, M.; Hamilton, J.L.; Kc, R.; Berendsen, A.D.; Duan, X.; Cheong, C.; Li, X.; Im, H.-J.; Olsen, B.R. Vascular Endothelial Growth Factor in Cartilage Development and Osteoarthritis. Sci. Rep. 2017, 7, 13027. [Google Scholar] [CrossRef]
- Enomoto, H.; Inoki, I.; Komiya, K.; Shiomi, T.; Ikeda, E.; Obata, K.-I.; Matsumoto, H.; Toyama, Y.; Okada, Y. Vascular Endothelial Growth Factor Isoforms and Their Receptors Are Expressed in Human Osteoarthritic Cartilage. Am. J. Pathol. 2003, 162, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Pufe, T.; Harde, V.; Petersen, W.; Goldring, M.B.; Tillmann, B.; Mentlein, R. Vascular endothelial growth factor(VEGF) induces matrix metalloproteinase expression in immortalized chondrocytes. J. Pathol. 2004, 202, 367–374. [Google Scholar] [CrossRef]
- Klooster, A.R.; Bernier, S.M. Tumor necrosis factor alpha and epidermal growth factor act additively to inhibit matrix gene expression by chondrocyte. Arthritis Res. Ther. 2004, 7, R127–R138. [Google Scholar] [CrossRef] [Green Version]
- Namba, A.; Aida, Y.; Suzuki, N.; Watanabe, Y.; Kawato, T.; Motohashi, M.; Maeno, M.; Matsumura, H.; Matsumoto, M. Effects of IL-6 and Soluble IL-6 Receptor on the Expression of Cartilage Matrix Proteins in Human Chondrocytes. Connect. Tissue Res. 2007, 48, 263–270. [Google Scholar] [CrossRef]
- Zanotti, S.; Canalis, E. Interleukin 6 mediates selected effects of Notch in chondrocytes. Osteoarthr. Cartil. 2013, 21, 1766–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cawston, T.E.; Curry, V.A.; Summers, C.A.; Clark, I.M.; Riley, G.P.; Life, P.F.; Spaull, J.R.; Goldring, M.B.; Koshy, P.J.; Rowan, A.D.; et al. The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheum 1998, 41, 1760–1771. [Google Scholar] [CrossRef]
- Rowan, A.D.; Koshy, P.J.T.; Shingleton, W.; Degnan, B.A.; Heath, J.K.; Vernallis, A.B.; Spaull, J.R.; Life, P.F.; Hudson, K.; Cawston, T.E. Synergistic effects of glycoprotein 130 binding cytokines in combination with interleukin-1 on cartilage collagen breakdown. Arthritis Rheum. 2001, 44, 1620–1632. [Google Scholar] [CrossRef]
- Xu, Y.-K.; Ke, Y.; Wang, B.; Lin, J.-H. The role of MCP-1-CCR2 ligand-receptor axis in chondrocyte degradation and disease progress in knee osteoarthritis. Boil. Res. 2015, 48, 64. [Google Scholar] [CrossRef] [Green Version]
- Alaaeddine, N.; Olee, T.; Hashimoto, S.; Creighton-Achermann, L.; Lotz, M. Production of the chemokine RANTES by articular chondrocytes and role in cartilage degradation. Arthritis Rheum. 2001, 44, 1633–1643. [Google Scholar] [CrossRef]
- Merz, D.; Liu, R.; Johnson, K.A.; Terkeltaub, R. IL-8/CXCL8 and Growth-Related Oncogene α/CXCL1 Induce Chondrocyte Hypertrophic Differentiation. J. Immunol. 2003, 171, 4406–4415. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Kumar, V.; Rattan, V.; Jha, V.; Bhattacharyya, S. Secretome Cues Modulate the Neurogenic Potential of Bone Marrow and Dental Stem Cells. Mol. Neurobiol. 2016, 54, 4672–4682. [Google Scholar] [CrossRef]
- Man, R.C.; Sulaiman, N.; Idrus, R.; Ariffin, S.H.Z.; Wahab, R.M.A.; Yazid, M.D. Insights into the Effects of the Dental Stem Cell Secretome on Nerve Regeneration: Towards Cell-Free Treatment. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef]
- Tran-Hung, L.; Laurent, P.; Camps, J.; About, I. Quantification of angiogenic growth factors released by human dental cells after injury. Arch. Oral Boil. 2008, 53, 9–13. [Google Scholar] [CrossRef]
- Narcisi, R.; Quarto, R.; Ulivi, V.; Muraglia, A.; Molfetta, L.; Giannoni, P. TGFβ-1 administration during Ex vivo expansion of human articular chondrocytes in a serum-free medium redirects the cell phenotype toward hypertrophy. J. Cell. Physiol. 2012, 227, 3282–3290. [Google Scholar] [CrossRef]
- Lozito, T.P.; Tuan, R.S. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J. Cell. Physiol. 2010, 226, 385–396. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1803, 55–71. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, M. Mesenchymal Stem Cell Therapy for Osteoarthritis: The Critical Role of the Cell Secretome. Front. Bioeng. Biotechnol. 2019, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.I.; Argyle, D.J.; Clements, D.N. In vitro models for the study of osteoarthritis. Veter- J. 2016, 209, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Haltmayer, E.; Ribitsch, I.; Gabner, S.; Rosser, J.; Gueltekin, S.; Peham, J.; Giese, U.; Dolezal, M.; Egerbacher, M.; Jenner, F. Co-culture of osteochondral explants and synovial membrane as in vitro model for osteoarthritis. PLoS ONE 2019, 14, e0214709. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Monaco, M.; Gervois, P.; Beaumont, J.; Clegg, P.; Bronckaers, A.; Vandeweerd, J.-M.; Lambrichts, I. Therapeutic Potential of Dental Pulp Stem Cells and Leukocyte- and Platelet-Rich Fibrin for Osteoarthritis. Cells 2020, 9, 980. https://doi.org/10.3390/cells9040980
Lo Monaco M, Gervois P, Beaumont J, Clegg P, Bronckaers A, Vandeweerd J-M, Lambrichts I. Therapeutic Potential of Dental Pulp Stem Cells and Leukocyte- and Platelet-Rich Fibrin for Osteoarthritis. Cells. 2020; 9(4):980. https://doi.org/10.3390/cells9040980
Chicago/Turabian StyleLo Monaco, Melissa, Pascal Gervois, Joel Beaumont, Peter Clegg, Annelies Bronckaers, Jean-Michel Vandeweerd, and Ivo Lambrichts. 2020. "Therapeutic Potential of Dental Pulp Stem Cells and Leukocyte- and Platelet-Rich Fibrin for Osteoarthritis" Cells 9, no. 4: 980. https://doi.org/10.3390/cells9040980
APA StyleLo Monaco, M., Gervois, P., Beaumont, J., Clegg, P., Bronckaers, A., Vandeweerd, J.-M., & Lambrichts, I. (2020). Therapeutic Potential of Dental Pulp Stem Cells and Leukocyte- and Platelet-Rich Fibrin for Osteoarthritis. Cells, 9(4), 980. https://doi.org/10.3390/cells9040980