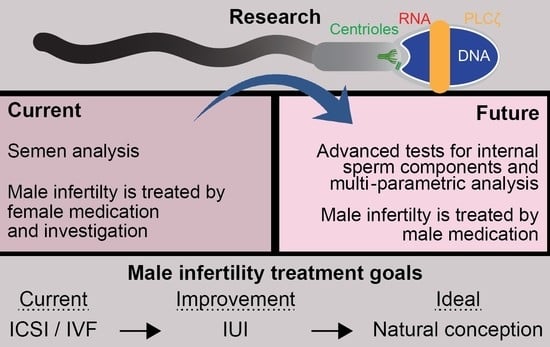
Male Infertility is a Women’s Health Issue—Research and Clinical Evaluation of Male Infertility Is Needed
Abstract
1. Introduction
2. Men Are as Likely as Women to Be Responsible for Infertility
3. Current Infertility Treatments Disproportionality Burden Women
4. There Are Many Barriers to Male Infertility Diagnosis and Treatment
5. Traditional Semen Analysis Only Tests General Sperm Properties, Which Are Not Necessarily Predictive of Fertility
6. There Are Undiagnosed and Potentially Treatable Sperm Defects
7. Advanced Sperm Tests Examine Sperm DNA
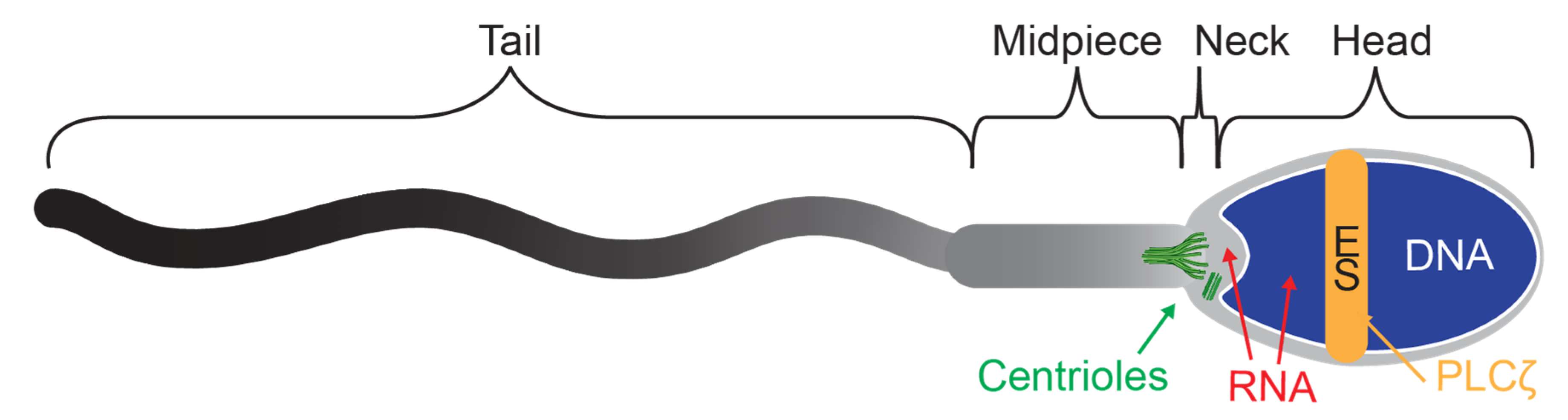
8. Experimental Sperm Tests Aim to Assess Newly Discovered Sperm Components
8.1. RNA—Sperm Transcripts
8.2. Proteins—Sperm Proteome
8.3. Activation Factors
8.4. Centrioles
8.5. Multi-Parametric Analysis
9. The Clinical Benefit of Identifying Male Infertility Due to Internal Sperm Components
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aitken, R.J. Not every sperm is sacred; a perspective on male infertility. Mol. Hum. Reprod. 2018, 24, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Lotti, F.; Maggi, M. Ultrasound of the male genital tract in relation to male reproductive health. Hum. Reprod. Update 2015, 21, 56–83. [Google Scholar] [CrossRef] [PubMed]
- Bieniek, J.M.; Drabovich, A.P.; Lo, K.C. Seminal biomarkers for the evaluation of male infertility. Asian J. Androl. 2016, 18, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Jodar, M.; Soler-Ventura, A.; Oliva, R. Semen proteomics and male infertility. J. Proteom. 2017, 162, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Holdcraft, R.W.; Braun, R.E. Hormonal regulation of spermatogenesis. Int. J. Androl. 2004, 27, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Dohle, G.R.; Smit, M.; Weber, R.F. Androgens and male fertility. World J. Urol. 2003, 21, 341–345. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Liu, B.; Jiang, Y.; Chen, W.; Li, J.; He, Q.; He, Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell Mol. Life Sci. 2019, 76, 2681–2695. [Google Scholar] [CrossRef]
- Procópio, M.S.; de Avelar, G.F.; Costa, G.M.J.; Lacerda, S.; Resende, R.R.; de França, L.R. MicroRNAs in Sertoli cells: Implications for spermatogenesis and fertility. Cell Tissue Res. 2017, 370, 335–346. [Google Scholar] [CrossRef]
- Krausz, C.; Cioppi, F.; Riera-Escamilla, A. Testing for genetic contributions to infertility: Potential clinical impact. Expert Rev. Mol. Diagn. 2018, 18, 331–346. [Google Scholar] [CrossRef]
- Lotti, F.; Maggi, M. Sexual dysfunction and male infertility. Nat. Rev. Urol. 2018, 15, 287–307. [Google Scholar] [CrossRef]
- Chandra, A.; Copen, C.E.; Stephen, E.H. Infertility and impaired fecundity in the United States, 1982-2010: Data from the National Survey of Family Growth. Natl. Health Stat. Rep. 2013, 1–18, 11 p following 19. [Google Scholar]
- Kelley, A.S.; Qin, Y.; Marsh, E.E.; Dupree, J.M. Disparities in accessing infertility care in the United States: Results from the National Health and Nutrition Examination Survey, 2013-16. Fertil. Steril. 2019, 112, 562–568. [Google Scholar] [CrossRef]
- Isidori, A.M.; Pozza, C.; Gianfrilli, D.; Isidori, A. Medical treatment to improve sperm quality. Reprod. Biomed. Online 2006, 12, 704–714. [Google Scholar] [CrossRef]
- Thonneau, P.; Marchand, S.; Tallec, A.; Ferial, M.-L.; Ducot, B.; Lansac, J.; Lopes, P.; Tabaste, J.-M.; Spira, A. Incidence and main causes of infertility in a resident population (1 850 000) of three French regions (1988–1989)*. Hum. Reprod. 1991, 6, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Adamson, G.D.; Baker, V.L. Subfertility: Causes, treatment and outcome. Best Pract. Res. Clin. Obs. Gynaecol. 2003, 17, 169–185. [Google Scholar] [CrossRef]
- Gnoth, C.; Godehardt, E.; Frank-Herrmann, P.; Friol, K.; Tigges, J.; Freundl, G. Definition and prevalence of subfertility and infertility. Hum. Reprod. 2005, 20, 1144–1147. [Google Scholar] [CrossRef]
- Ramasamy, R.; Scovell, J.M.; Kovac, J.R.; Cook, P.J.; Lamb, D.J.; Lipshultz, L.I. Fluorescence in situ hybridization detects increased sperm aneuploidy in men with recurrent pregnancy loss. Fertil. Steril. 2015, 103, 906–909.e901. [Google Scholar] [CrossRef]
- Esquerré-Lamare, C.; Walschaerts, M.; Chansel Debordeaux, L.; Moreau, J.; Bretelle, F.; Isus, F.; Karsenty, G.; Monteil, L.; Perrin, J.; Papaxanthos-Roche, A.; et al. Sperm aneuploidy and DNA fragmentation in unexplained recurrent pregnancy loss: A multicenter case-control study. Basic Clin. 2018, 28, 4. [Google Scholar] [CrossRef]
- Patel, V.; Ginsburg, K.B.; Etnyre, E.; Shun, F.; Loeb, A.; Krawetz, S.A.; Rambhatla, A.A. Practice Patterns for the Treatment of Idiopathic Infertility: Is There a Role for Advanced Semen Testing? AME Med. J. 2019, 4, 4. [Google Scholar] [CrossRef]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Hjelmstedt, A.; Andersson, L.; Skoog-Svanberg, A.; Bergh, T.; Boivin, J.; Collins, A. Gender differences in psychological reactions to infertility among couples seeking IVF-and ICSI-treatment. Acta Obstet. Gynecol. Scand. 1999, 78, 42–49. [Google Scholar] [CrossRef]
- Diagnostic evaluation of the infertile female: A committee opinion. Fertil. Steril. 2015, 103, e44–e50. [CrossRef]
- Laumann, E.O.; Paik, A.; Rosen, R.C. Sexual dysfunction in the United States: Prevalence and predictors. JAMA 1999, 281, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Tjon-Kon-Fat, R.I.; Bensdorp, A.J.; Scholten, I.; Repping, S.; van Wely, M.; Mol, B.W.J.; van der Veen, F. IUI and IVF for unexplained subfertility: Where did we go wrong? Hum. Reprod. 2016, 31, 2665–2667. [Google Scholar] [CrossRef][Green Version]
- Kumar, P.; Sait, S.F.; Sharma, A.; Kumar, M. Ovarian hyperstimulation syndrome. J. Hum. Reprod. Sci. 2011, 4, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Hennebicq, S.; Blagosklonov, O.; Eustache, F.; Papaxanthos, A.; Drouineaud, V.; Guillemain, C.; Mirallie, S.; Delepine, B.; Rives, N.; Berthaut, I.; et al. Donor sperm insemination after failed intra-couple intracytoplasmic sperm injection. Syst. Biol. Reprod. Med. 2018, 64, 130–137. [Google Scholar] [CrossRef]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Van Steirteghem, A. Celebrating ICSI’s twentieth anniversary and the birth of more than 2.5 million children—The ‘how, why, when and where’. Hum. Reprod. 2012, 27, 1–2. [Google Scholar] [CrossRef][Green Version]
- Barratt, C.L.R.; De Jonge, C.J.; Sharpe, R.M. ‘Man Up’: The importance and strategy for placing male reproductive health centre stage in the political and research agenda. Hum. Reprod. (Oxf. Engl.) 2018, 33, 541–545. [Google Scholar] [CrossRef]
- Marzano, G.; Chiriaco, M.S.; Primiceri, E.; Dell’Aquila, M.E.; Ramalho-Santos, J.; Zara, V.; Ferramosca, A.; Maruccio, G. Sperm selection in assisted reproduction: A review of established methods and cutting-edge possibilities. Biotechnol. Adv. 2019, 107498. [Google Scholar] [CrossRef]
- Allen, V.M.; Wilson, R.D.; Cheung, A. Pregnancy outcomes after assisted reproductive technology. J. Obs. Gynaecol. Can. 2006, 28, 220–233. [Google Scholar] [CrossRef]
- Hu, L.; Du, J.; Lv, H.; Zhao, J.; Chen, M.; Wang, Y.; Wu, F.; Liu, F.; Chen, X.; Zhang, J.; et al. Influencing factors of pregnancy loss and survival probability of clinical pregnancies conceived through assisted reproductive technology. Reprod. Biol. Endocrinol. RBE 2018, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Liberman, R.F.; Getz, K.D.; Heinke, D.; Luke, B.; Stern, J.E.; Declercq, E.R.; Chen, X.; Lin, A.E.; Anderka, M. Assisted Reproductive Technology and Birth Defects: Effects of Subfertility and Multiple Births. Birth Defects Res. 2017, 109, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Adamson, G.D.; de Mouzon, J.; Chambers, G.M.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; Banker, M.; Dyer, S. International Committee for Monitoring Assisted Reproductive Technology: World report on assisted reproductive technology, 2011. Fertil. Steril. 2018, 110, 1067–1080. [Google Scholar] [CrossRef]
- Katz, P.; Showstack, J.; Smith, J.F.; Nachtigall, R.D.; Millstein, S.G.; Wing, H.; Eisenberg, M.L.; Pasch, L.A.; Croughan, M.S.; Adler, N. Costs of infertility treatment: Results from an 18-month prospective cohort study. Fertil. Steril. 2011, 95, 915–921. [Google Scholar] [CrossRef]
- Le Ray, C.; Pelage, L.; Seco, A.; Bouvier-Colle, M.H.; Chantry, A.A.; Deneux-Tharaux, C. Risk of severe maternal morbidity associated with in vitro fertilisation: A population-based study. BJOG 2019, 126, 1033–1041. [Google Scholar] [CrossRef]
- Mehta, A.; Nangia, A.K.; Dupree, J.M.; Smith, J.F. Limitations and barriers in access to care for male factor infertility. Fertil. Steril. 2016, 105, 1128–1137. [Google Scholar] [CrossRef]
- Nangia, A.K.; Likosky, D.S.; Wang, D. Distribution of male infertility specialists in relation to the male population and assisted reproductive technology centers in the United States. Fertil. Steril. 2010, 94, 599–609. [Google Scholar] [CrossRef]
- Jequier, A.M. Clinical andrology—still a major problem in the treatment of infertility. Hum. Reprod. 2004, 19, 1245–1249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Glander, H.J.; Haidl, G.; Köhn, F.M.; Ochsendorf, F.; Paasch, U.; Schuppe, H.C. Andrology. JDDG: J. Dtsch. Dermatol. Ges. 2007, 5, 924–933. [Google Scholar] [CrossRef]
- De Jonge, C.; Barratt, C.L.R. The present crisis in male reproductive health: An urgent need for a political, social, and research roadmap. Andrology 2019, 7. [Google Scholar] [CrossRef]
- Tiegs, A.W.; Landis, J.; Garrido, N.; Scott, R.; Hotaling, J. Total motile sperm count trend over time across two continents: Evaluation of semen analyses from 119,972 infertile men. Fertil. Steril. 2018, 110, e27. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Jorgensen, N.; Andersson, A.M.; Juul, A.; Main, K.M.; Jensen, T.K.; Toppari, J. Populations, decreasing fertility, and reproductive health. Lancet 2019, 393, 1500–1501. [Google Scholar] [CrossRef]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg Michael, L.; Lathi Ruth, B.; Baker Valerie, L.; Westphal Lynn, M.; Milki Amin, A.; Nangia Ajay, K. Frequency of the Male Infertility Evaluation: Data from the National Survey of Family Growth. J. Urol. 2013, 189, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Ledger, W.L. Demographics of infertility. Reprod. Biomed. Online 2009, 18 (Suppl. 2), 11–14. [Google Scholar] [CrossRef]
- Hughes, E.G.; Grantmyre, J.; Zini, A. An integrated approach to male-factor subfertility: Bridging the gap between fertility specialists trained in urology and gynaecology. J. Obs. Gynaecol. Can. 2015, 37, 258–265. [Google Scholar] [CrossRef]
- Canale, D.; Caietti, L. Infertile male patients are patients, not numbers. Hum. Reprod. 1996, 11, 2807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cousineau, T.M.; Domar, A.D. Psychological impact of infertility. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Hasanpoor-Azghdy, S.B.; Simbar, M.; Vedadhir, A. The emotional-psychological consequences of infertility among infertile women seeking treatment: Results of a qualitative study. Iran. J. Reprod. Med. 2014, 12, 131–138. [Google Scholar]
- Inhorn, M.C. “The Worms Are Weak”: Male Infertility and Patriarchal Paradoxes in Egypt. Men Masc. 2003, 5, 236–256. [Google Scholar] [CrossRef]
- Bechoua, S.; Hamamah, S.; Scalici, E. Male infertility: An obstacle to sexuality? Andrology 2016, 4, 395–403. [Google Scholar] [CrossRef]
- Barlow, P.W. Why so many sperm cells? Not only a possible means of mitigating the hazards inherent to human reproduction but also an indicator of an exaptation. Commun. Integr. Biol. 2016, 9, e1204499. [Google Scholar] [CrossRef]
- Reynaud, K.; Schuss, Z.; Rouach, N.; Holcman, D. Why so many sperm cells? Commun. Integr. Biol. 2015, 8, e1017156. [Google Scholar] [CrossRef]
- Wang, C.; Swerdloff, R.S. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil. Steril. 2014, 102, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.G.; Noonan, E.; von Eckardstein, S.; Auger, J.; Baker, H.W.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Filimberti, E.; Degl’Innocenti, S.; Borsotti, M.; Quercioli, M.; Piomboni, P.; Natali, I.; Fino, M.G.; Caglieresi, C.; Criscuoli, L.; Gandini, L.; et al. High variability in results of semen analysis in andrology laboratories in Tuscany (Italy): The experience of an external quality control (EQC) programme. Andrology 2013, 1, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Olds-Clarke, P. Unresolved issues in mammalian fertilization. Int. Rev. Cytol. 2003, 232, 129–184. [Google Scholar] [CrossRef]
- Mortimer, D. The functional anatomy of the human spermatozoon: Relating ultrastructure and function. Mol. Hum. Reprod. 2018, 24, 567–592. [Google Scholar] [CrossRef]
- Sharma, R.; Said, T.; Agarwal, A. Sperm DNA damage and its clinical relevance in assessing reproductive outcome. Asian J. 2004, 6, 139–148. [Google Scholar]
- Lewis, S.E.M.; Agbaje, I.; Alvarez, J. Sperm DNA Tests as Useful Adjuncts to Semen Analysis. Syst. Biol. Reprod. Med. 2008, 54, 111–125. [Google Scholar] [CrossRef]
- Gil, M.; Sar-Shalom, V.; Melendez Sivira, Y.; Carreras, R.; Checa, M.A. Sperm selection using magnetic activated cell sorting (MACS) in assisted reproduction: A systematic review and meta-analysis. J. Assist. Reprod. Genet. 2013, 30, 479–485. [Google Scholar] [CrossRef]
- Herrero, M.B.; Lusignan, M.F.; Son, W.Y.; Sabbah, M.; Buckett, W.; Chan, P. ICSI outcomes using testicular spermatozoa in non-azoospermic couples with recurrent ICSI failure and no previous live births. Andrology 2019, 7, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.; AlMalki, A.; AlBadr, M.; Burjaq, H.; Majzoub, A.; AlSaid, S.; Elbardisi, H. ICSI outcome in patients with high DNA fragmentation: Testicular versus ejaculated spermatozoa. Andrologia 2018, 50. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K. Oxidative stress and male infertility--a clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Panner Selvam, M.K.; Arafa, M.; Okada, H.; Homa, S.; Killeen, A.; Balaban, B.; Saleh, R.; Armagan, A.; Roychoudhury, S.; et al. Multi-center evaluation of oxidation-reduction potential by the MiOXSYS in males with abnormal semen. Asian J. 2019. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef]
- Tandon, S. Sperm DNA fragmentation testing: Where we stand in 2017. Transl. Urol. 2017, 6, S697–S698. [Google Scholar] [CrossRef]
- Shamsi, M.B.; Kumar, R.; Malhotra, N.; Singh, N.; Mittal, S.; Upadhyay, A.D.; Dada, R. Chromosomal aberrations, Yq microdeletion, and sperm DNA fragmentation in infertile men opting for assisted reproduction. Mol. Reprod. Dev. 2012, 79, 637–650. [Google Scholar] [CrossRef]
- Smith, R.P.; Coward, R.M.; Lipshultz, L.I. The office visit. Urol. Clin. N. Am. 2014, 41, 19–37. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A.; Esteves, S.C.; Ko, E.; Ramasamy, R.; Zini, A. Clinical utility of sperm DNA fragmentation testing: Practice recommendations based on clinical scenarios. Transl. Urol. 2016, 5, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Kathrins, M. MOXI trial-is it time to stop routinely recommending antioxidant therapy to infertile men? Fertil. Steril. 2020, 113, 542. [Google Scholar] [CrossRef]
- Johnson, G.D.; Mackie, P.; Jodar, M.; Moskovtsev, S.; Krawetz, S.A. Chromatin and extracellular vesicle associated sperm RNAs. Nucleic Acids Res. 2015, 43, 6847–6859. [Google Scholar] [CrossRef]
- Johnson, G.D.; Lalancette, C.; Linnemann, A.K.; Leduc, F.; Boissonneault, G.; Krawetz, S.A. The sperm nucleus: Chromatin, RNA, and the nuclear matrix. Reproduction 2011, 141, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Lalancette, C.; Miller, D.; Li, Y.; Krawetz, S.A. Paternal contributions: New functional insights for spermatozoal RNA. J. Cell Biochem. 2008, 104, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Swanson, G.M.; Estill, M.; Diamond, M.P.; Legro, R.S.; Coutifaris, C.; Barnhart, K.T.; Huang, H.; Hansen, K.R.; Trussell, J.C.; Coward, R.M.; et al. Human chromatin remodeler cofactor, RNA interactor, eraser and writer sperm RNAs responding to obesity. Epigenetics 2019, 15, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Estill, M.S.; Hauser, R.; Krawetz, S.A. RNA element discovery from germ cell to blastocyst. Nucleic Acids Res. 2019, 47, 2263–2275. [Google Scholar] [CrossRef]
- Jodar, M.; Sendler, E.; Moskovtsev, S.I.; Librach, C.L.; Goodrich, R.; Swanson, S.; Hauser, R.; Diamond, M.P.; Krawetz, S.A. Absence of sperm RNA elements correlates with idiopathic male infertility. Sci. Transl. Med. 2015, 7, 295re296. [Google Scholar] [CrossRef]
- Platts, A.E.; Dix, D.J.; Chemes, H.E.; Thompson, K.E.; Goodrich, R.; Rockett, J.C.; Rawe, V.Y.; Quintana, S.; Diamond, M.P.; Strader, L.F.; et al. Success and failure in human spermatogenesis as revealed by teratozoospermic RNAs. Hum. Mol. Genet. 2007, 16, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Burl, R.B.; Clough, S.; Sendler, E.; Estill, M.; Krawetz, S.A. Sperm RNA elements as markers of health. Syst. Biol. Reprod. Med. 2018, 64, 25–38. [Google Scholar] [CrossRef]
- Estill, M.; Hauser, R.; Nassan, F.L.; Moss, A.; Krawetz, S.A. The effects of di-butyl phthalate exposure from medications on human sperm RNA among men. Sci. Rep. 2019, 9, 12397. [Google Scholar] [CrossRef] [PubMed]
- Panner Selvam, M.K.; Agarwal, A. Update on the proteomics of male infertility: A systematic review. Arab. J. Urol. 2018, 16, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Hu, H.; Wang, Z.; Chen, X.; Yang, F.; Zhu, Z.; Fang, P.; Dai, J.; Wang, L.; Shi, H.; et al. Proteomic characteristics of spermatozoa in normozoospermic patients with infertility. J. Proteom. 2012, 75, 5426–5436. [Google Scholar] [CrossRef] [PubMed]
- Azpiazu, R.; Amaral, A.; Castillo, J.; Estanyol, J.M.; Guimera, M.; Ballesca, J.L.; Balasch, J.; Oliva, R. High-throughput sperm differential proteomics suggests that epigenetic alterations contribute to failed assisted reproduction. Hum. Reprod. 2014, 29, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Legare, C.; Droit, A.; Fournier, F.; Bourassa, S.; Force, A.; Cloutier, F.; Tremblay, R.; Sullivan, R. Investigation of male infertility using quantitative comparative proteomics. J. Proteome Res. 2014, 13, 5403–5414. [Google Scholar] [CrossRef] [PubMed]
- Panner Selvam, M.K.; Agarwal, A.; Pushparaj, P.N.; Baskaran, S.; Bendou, H. Sperm Proteome Analysis and Identification of Fertility-Associated Biomarkers in Unexplained Male Infertility. Genes 2019, 10, 522. [Google Scholar] [CrossRef]
- Amdani, S.N.; Jones, C.; Coward, K. Phospholipase C zeta (PLCzeta): Oocyte activation and clinical links to male factor infertility. Adv. Biol. Regul. 2013, 53, 292–308. [Google Scholar] [CrossRef]
- Amdani, S.N.; Yeste, M.; Jones, C.; Coward, K. Phospholipase C zeta (PLCzeta) and male infertility: Clinical update and topical developments. Adv. Biol. Regul. 2016, 61, 58–67. [Google Scholar] [CrossRef]
- Chithiwala, Z.H.; Lee, H.C.; Hill, D.L.; Jellerette-Nolan, T.; Fissore, R.; Grow, D.; Dumesic, D.A. Phospholipase C-zeta deficiency as a cause for repetitive oocyte fertilization failure during ovarian stimulation for in vitro fertilization with ICSI: A case report. J. Assist. Reprod. Genet. 2015, 32, 1415–1419. [Google Scholar] [CrossRef]
- Sanusi, R.; Yu, Y.; Nomikos, M.; Lai, F.A.; Swann, K. Rescue of failed oocyte activation after ICSI in a mouse model of male factor infertility by recombinant phospholipase Czeta. Mol. Hum. Reprod. 2015, 21, 783–791. [Google Scholar] [CrossRef]
- Fishman, E.L.; Jo, K.; Nguyen, Q.P.H.; Kong, D.; Royfman, R.; Cekic, A.R.; Khanal, S.; Miller, A.L.; Simerly, C.; Schatten, G.; et al. A novel atypical sperm centriole is functional during human fertilization. Nat. Commun. 2018, 9, 2210. [Google Scholar] [CrossRef] [PubMed]
- Avidor-Reiss, T. Rapid Evolution of Sperm Produces Diverse Centriole Structures that Reveal the Most Rudimentary Structure Needed for Function. Cells 2018, 7, 67. [Google Scholar] [CrossRef]
- Chemes, H.E.; Rawe, V.Y. The making of abnormal spermatozoa: Cellular and molecular mechanisms underlying pathological spermiogenesis. Cell Tissue Res. 2010, 341, 349–357. [Google Scholar] [CrossRef]
- Schatten, G. The centrosome and its mode of inheritance: The reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev. Biol. 1994, 165, 299–335. [Google Scholar] [CrossRef]
- Avidor-Reiss, T.; Mazur, M.; Fishman, E.L.; Sindhwani, P. The Role of Sperm Centrioles in Human Reproduction—The Known and the Unknown. Front. Cell Dev. Biol. 2019, 7. [Google Scholar] [CrossRef]
- Poulton, J.; Kennedy, S.; Oakeshott, P.; Wells, D. Preventing transmission of maternally inherited mitochondrial DNA diseases. BMJ 2009, 338, b94. [Google Scholar] [CrossRef]
- Schatten, G.; Stearns, T. Sperm Centrosomes: Kiss Your Asterless Goodbye, for Fertility’s Sake. Curr. Biol. 2015, 25, R1178–R1181. [Google Scholar] [CrossRef][Green Version]
- Khatun, A.; Rahman, M.S.; Pang, M.-G. Clinical assessment of the male fertility. Obs. Gynecol. Sci. 2018, 61, 179–191. [Google Scholar] [CrossRef]
- Oehninger, S.; Ombelet, W. Limits of current male fertility testing. Fertil. Steril. 2019, 111, 835–841. [Google Scholar] [CrossRef]
- Thurston, L.; Abbara, A.; Dhillo, W.S. Investigation and management of subfertility. J. Clin. Pathol. 2019, 72, 579–587. [Google Scholar] [CrossRef]
- Santi, D.; De Vincentis, S.; Magnani, E.; Spaggiari, G. Impairment of sperm DNA methylation in male infertility: A meta-analytic study. Andrology 2017, 5, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Panner Selvam, M.K.; Agarwal, A. A systematic review on sperm DNA fragmentation in male factor infertility: Laboratory assessment. Arab. J. Urol. 2018, 16, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Men’s Health 2019, 37, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, H. Laboratory semen assessment and prediction of fertility: Still utopia? Reprod. Domest. Anim. = Zuchthyg. 2003, 38, 312–318. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, H. Can we increase the estimated value of semen assessment? Reprod. Domest. Anim. = Zuchthyg. 2006, 41 (Suppl. 2), 2–10. [Google Scholar] [CrossRef]
- Amann, R.P.; Hammerstedt, R.H. In vitro evaluation of sperm quality: An opinion. J. Androl. 1993, 14, 397–406. [Google Scholar]
- Ramón, M.; Martínez-Pastor, F. Implementation of novel statistical procedures and other advanced approaches to improve analysis of CASA data. Reprod. Fertil. Dev. 2018, 30, 860–866. [Google Scholar] [CrossRef]
- Jarow, J.; Sigman, M.; Kolettis, P. The Optimal Evaluation of the Infertile Male: Best Practice Statement; Reviewed and Validity Confirmed 2011; American Urological Association: Linthicum, MD, USA, 2011. [Google Scholar]
- Yatsenko, A.N.; Roy, A.; Chen, R.; Ma, L.; Murthy, L.J.; Yan, W.; Lamb, D.J.; Matzuk, M.M. Non-invasive genetic diagnosis of male infertility using spermatozoal RNA: KLHL10 mutations in oligozoospermic patients impair homodimerization. Hum. Mol. Genet. 2006, 15, 3411–3419. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, K.A.; Rambhatla, A.; Schon, S.; Agarwal, A.; Krawetz, S.A.; Dupree, J.M.; Avidor-Reiss, T. Male Infertility is a Women’s Health Issue—Research and Clinical Evaluation of Male Infertility Is Needed. Cells 2020, 9, 990. https://doi.org/10.3390/cells9040990
Turner KA, Rambhatla A, Schon S, Agarwal A, Krawetz SA, Dupree JM, Avidor-Reiss T. Male Infertility is a Women’s Health Issue—Research and Clinical Evaluation of Male Infertility Is Needed. Cells. 2020; 9(4):990. https://doi.org/10.3390/cells9040990
Chicago/Turabian StyleTurner, Katerina A., Amarnath Rambhatla, Samantha Schon, Ashok Agarwal, Stephen A. Krawetz, James M. Dupree, and Tomer Avidor-Reiss. 2020. "Male Infertility is a Women’s Health Issue—Research and Clinical Evaluation of Male Infertility Is Needed" Cells 9, no. 4: 990. https://doi.org/10.3390/cells9040990
APA StyleTurner, K. A., Rambhatla, A., Schon, S., Agarwal, A., Krawetz, S. A., Dupree, J. M., & Avidor-Reiss, T. (2020). Male Infertility is a Women’s Health Issue—Research and Clinical Evaluation of Male Infertility Is Needed. Cells, 9(4), 990. https://doi.org/10.3390/cells9040990