Succinate Activates EMT in Intestinal Epithelial Cells through SUCNR1: A Novel Protagonist in Fistula Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Mice
2.3. Induction of Intestinal Fibrosis by Heterotopic Transplant of Colonic Tissue
2.4. Cell Culture
2.5. Small Interfering (siRNA) Transfection
2.6. RNA Isolation and Real-Time Quantitative PCR (RT-qPCR)
2.7. Protein Extraction and Western Blot Analysis
2.8. Immunofluorescence and Confocal Microscopy
2.9. Immunohistochemical Studies
2.10. Double Immunohistochemistry
2.11. Succinate Quantification
2.12. Statistical Analysis
3. Results
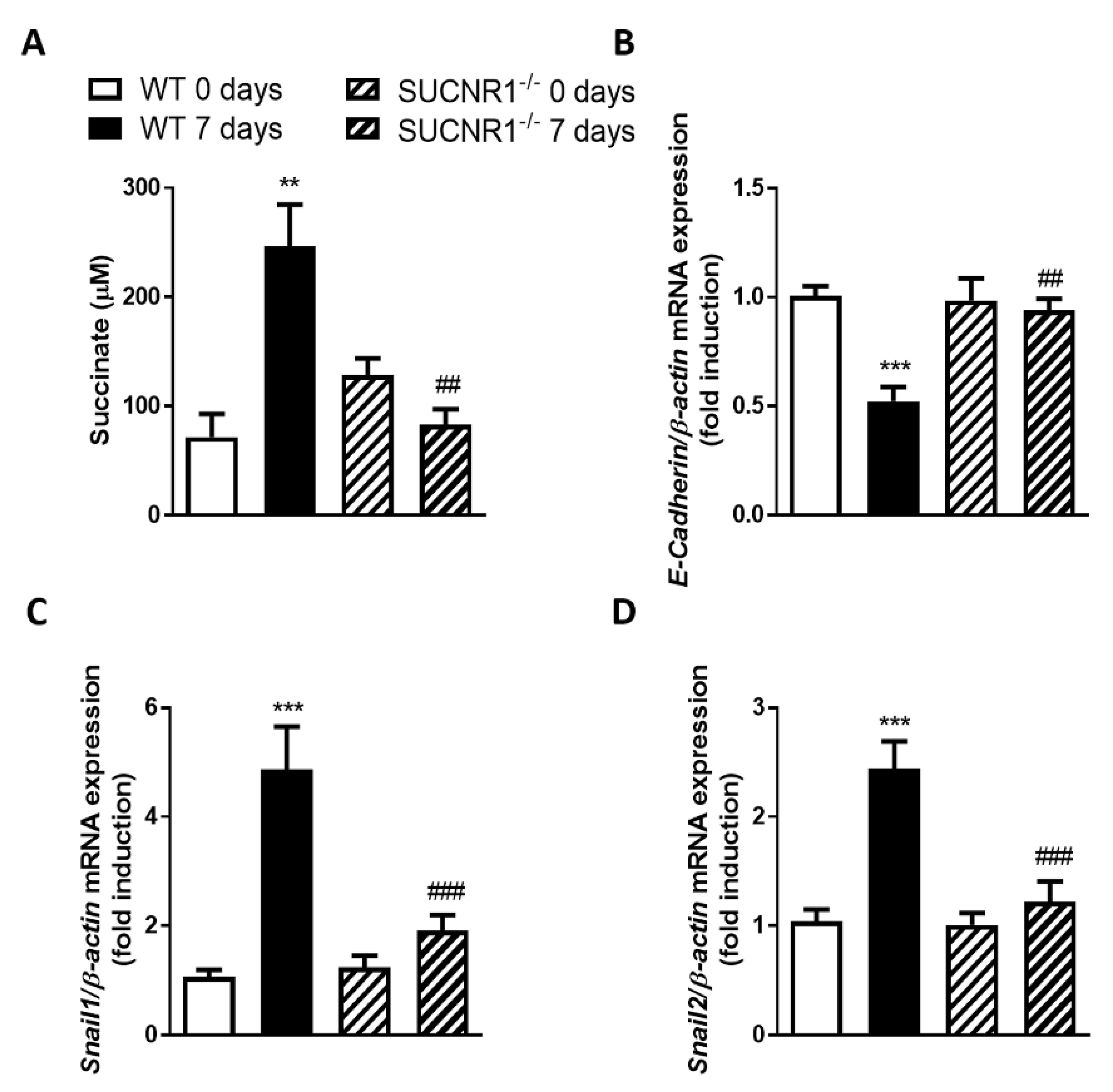
3.1. SUCNR1 Mediates the EMT Associated with Intestinal Fibrosis In Vivo
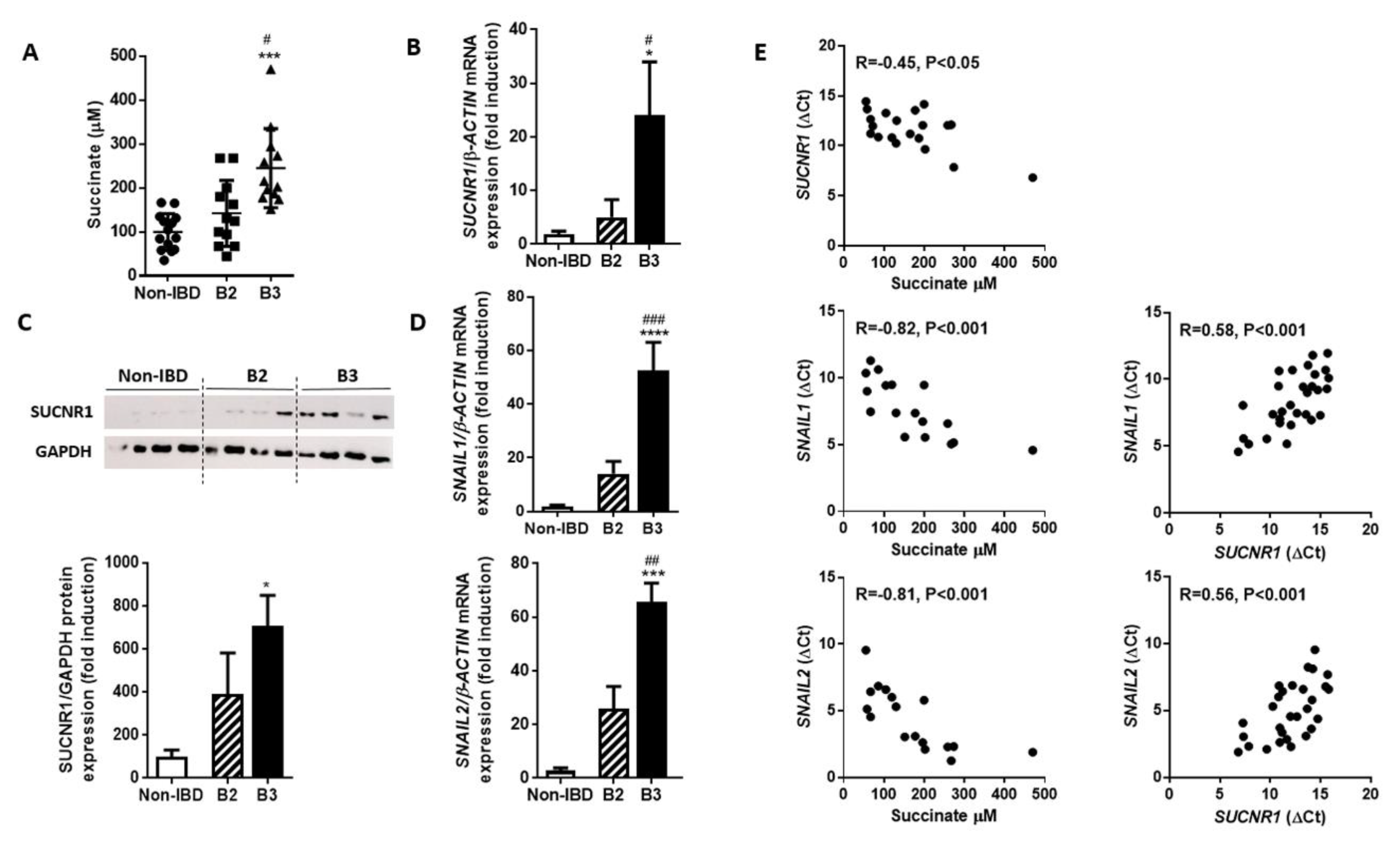
3.2. The intestinal Tissue Surrounding the Fistula Tract of B3-CD Patients Presents Increased Succinate Levels and an Overexpression of SUCNR1 that Correlates Positively with EMT Markers
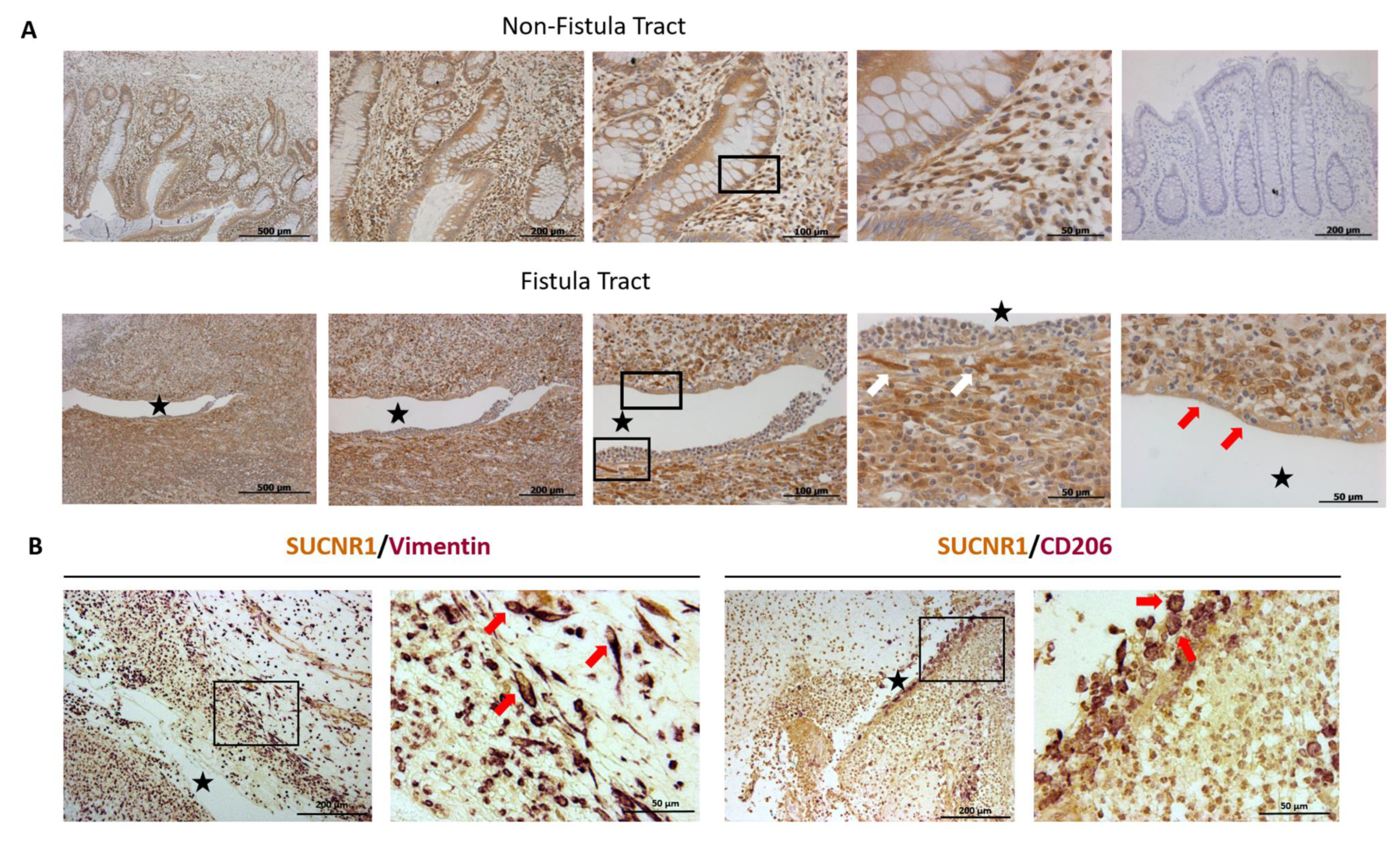
3.3. SUCNR1 Is Expressed Specifically in the Fistula Tract
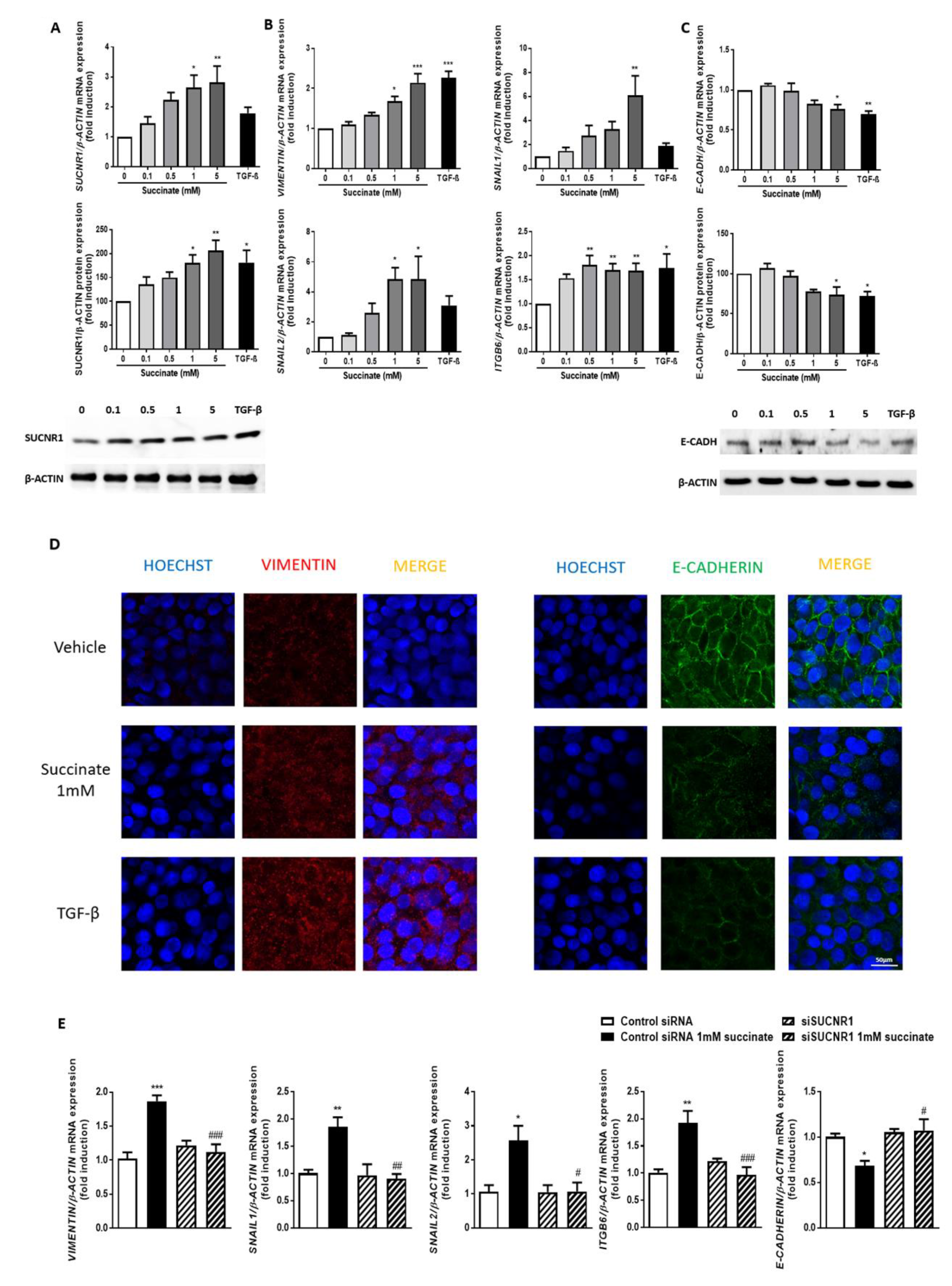
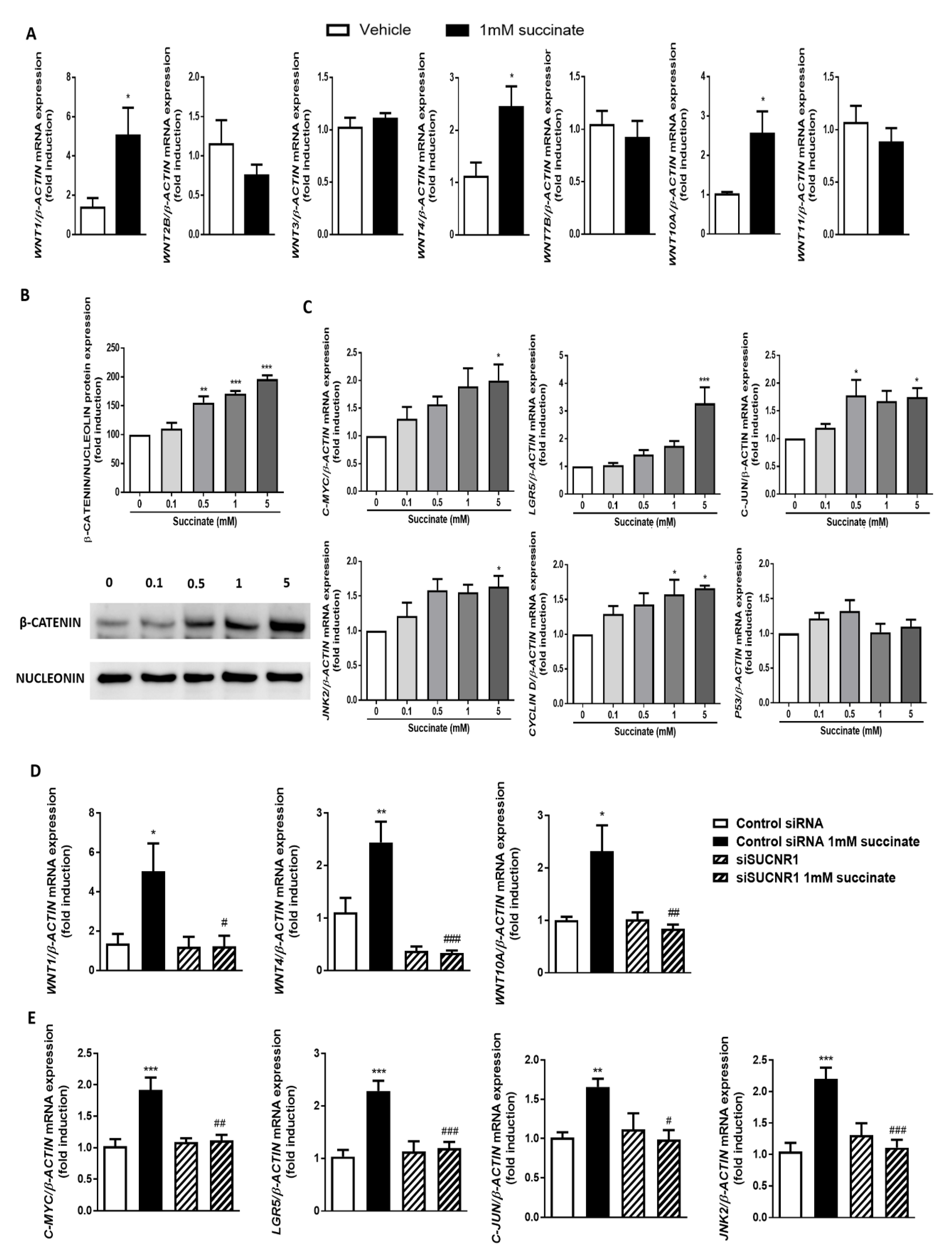
3.4. Succinate Induces EMT through SUCNR1
3.5. SUCNR1 Stimulation by Succinate Activates the Wnt Pathway
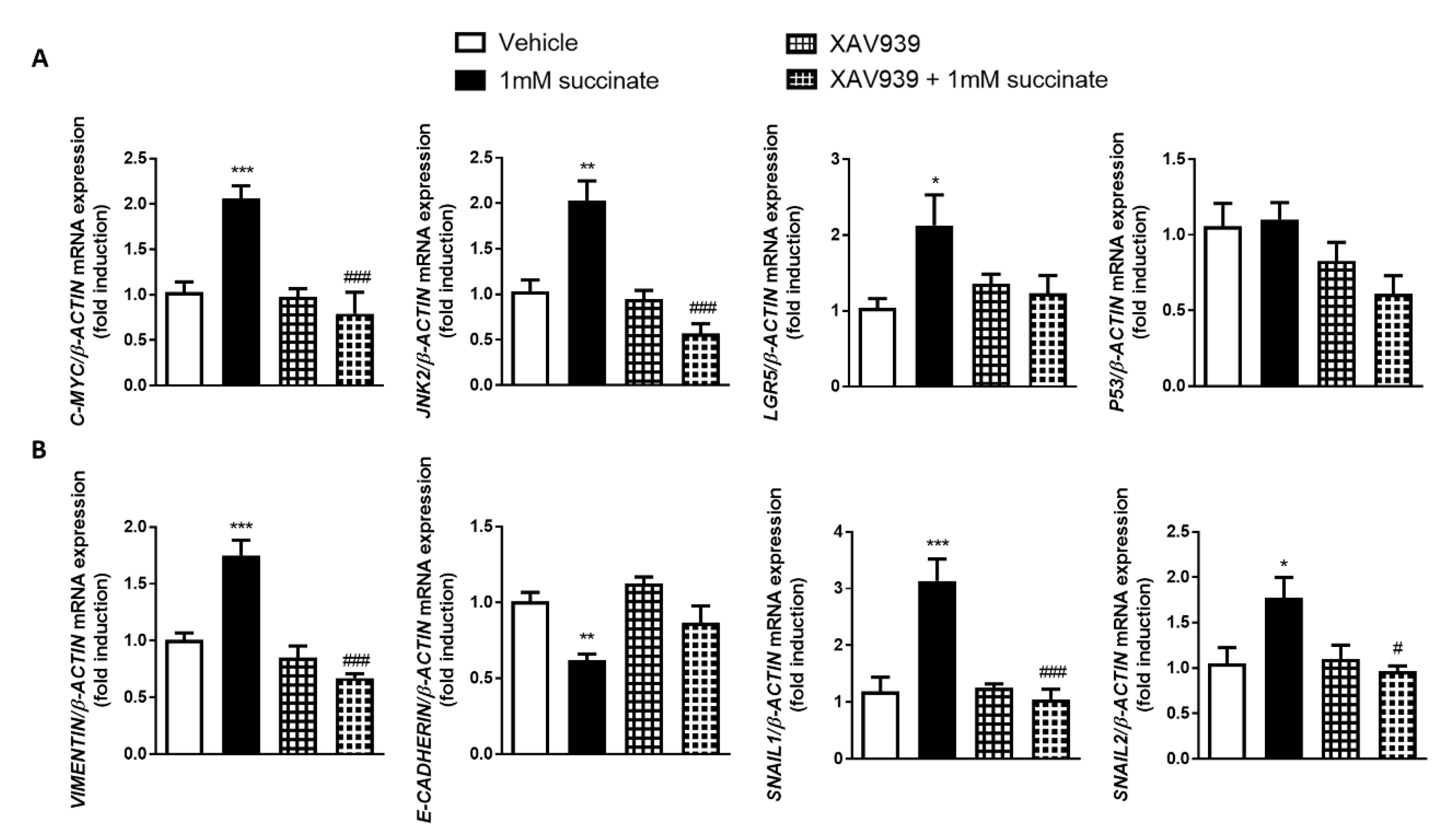
3.6. Wnt Pathway Mediates Succinate-Induced EMT
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peyrin-Biroulet, L.; Loftus, E.V., Jr.; Colombel, J.F.; Sandborn, W.J. The natural history of adult Crohn’s disease in population-based cohorts. Am. J. Gastroenterol. 2010, 105, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, G.; Stangl, P.C.; Vogelsang, H.; Schober, E.; Herbst, F.; Gasche, C. Significant association of strictures and internal fistula formation in Crohn’s disease. Virchows. Arch. 2000, 437, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Pariente, B.; Hu, S.; Bettenworth, D.; Speca, S.; Desreumaux, P.; Meuwis, M.A.; Danese, S.; Rieder, F.; Louis, E. Treatments for Crohn’s disease-associated bowel damage: A systematic review. Clin. Gastroenterol. Hepatol. 2019, 17, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Scharl, M.; Bruckner, R.S.; Rogler, G. The two sides of the coin: Similarities and differences in the pathomechanisms of fistulas and stricture formations in irritable bowel disease. United Eur. Gastroenterol. J. 2016, 4, 506–514. [Google Scholar] [CrossRef]
- Rieder, F.; Fiocchi, C.; Rogler, G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology 2017, 152, 340–350. [Google Scholar] [CrossRef]
- Scharl, M.; Rogler, G. Pathophysiology of fistula formation in Crohn’s disease. World J. Gastrointest. Pathophysiol. 2014, 5, 205–212. [Google Scholar] [CrossRef]
- Bataille, F.; Rohrmeier, C.; Bates, R.; Weber, A.; Rieder, F.; Brenmoehl, J.; Strauch, U.; Farkas, S.; Furst, A.; Hofstadter, F.; et al. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohn’s disease. Inflamm. Bowel. Dis. 2008, 14, 1514–1527. [Google Scholar] [CrossRef]
- Scharl, M.; Huber, N.; Lang, S.; Furst, A.; Jehle, E.; Rogler, G. Hallmarks of epithelial to mesenchymal transition are detectable in Crohn’s disease associated intestinal fibrosis. Clin. Transl. Med. 2015, 4, 1. [Google Scholar] [CrossRef]
- Larue, L.; Bellacosa, A. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 2005, 24, 7443–7454. [Google Scholar] [CrossRef]
- Shook, D.; Keller, R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech. Dev. 2003, 120, 1351–1383. [Google Scholar] [CrossRef]
- Kalluri, R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Investig. 2009, 119, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Novoa, J.M.; Nieto, M.A. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol. Med. 2009, 1, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shen, J.; Ran, Z. Epithelial-mesenchymal transition in Crohn’s disease. Mucosal Immunol. 2018, 11, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Macias-Ceja, D.C.; Ortiz-Masia, D.; Salvador, P.; Gisbert-Ferrandiz, L.; Hernandez, C.; Hausmann, M.; Rogler, G.; Esplugues, J.V.; Hinojosa, J.; Alos, R.; et al. Succinate receptor mediates intestinal inflammation and fibrosis. Mucosal Immunol. 2019, 12, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Toma, I.; Kang, J.J.; Sipos, A.; Vargas, S.; Bansal, E.; Hanner, F.; Meer, E.; Peti-Peterdi, J. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J. Clin. Investig. 2008, 118, 2526–2534. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, J.; Xuan, J.; Jung, Y.H.; Cha, H.S.; Kim, K.H. Global metabolite profiling of synovial fluid for the specific diagnosis of rheumatoid arthritis from other inflammatory arthritis. PLoS ONE 2014, 9, e97501. [Google Scholar] [CrossRef]
- Zhao, T.; Mu, X.; You, Q. Succinate: An initiator in tumorigenesis and progression. Oncotarget 2017, 8, 53819–53828. [Google Scholar] [CrossRef]
- Dawiskiba, T.; Deja, S.; Mulak, A.; Zabek, A.; Jawien, E.; Pawelka, D.; Banasik, M.; Mastalerz-Migas, A.; Balcerzak, W.; Kaliszewski, K.; et al. Serum and urine metabolomic fingerprinting in diagnostics of inflammatory bowel diseases. World J. Gastroenterol. 2014, 20, 163–174. [Google Scholar] [CrossRef]
- de Castro Fonseca, M.; Aguiar, C.J.; da Rocha Franco, J.A.; Gingold, R.N.; Leite, M.F. GPR91: Expanding the frontiers of Krebs cycle intermediates. Cell Commun. Signal. 2016, 14, 3. [Google Scholar] [CrossRef]
- Cosin-Roger, J.; Ortiz-Masia, D.; Calatayud, S.; Hernandez, C.; Esplugues, J.V.; Barrachina, M.D. The activation of Wnt signaling by a STAT6-dependent macrophage phenotype promotes mucosal repair in murine IBD. Mucosal Immunol. 2016, 9, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Cosin-Roger, J.; Ortiz-Masia, D.; Calatayud, S.; Hernandez, C.; Alvarez, A.; Hinojosa, J.; Esplugues, J.V.; Barrachina, M.D. M2 macrophages activate WNT signaling pathway in epithelial cells: Relevance in ulcerative colitis. PLoS ONE 2013, 8, e78128. [Google Scholar] [CrossRef]
- Van den Bossche, J.; O’Neill, L.A.; Menon, D. Macrophage immunometabolism: Where are we (going)? Trends Immunol. 2017, 38, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Bernard, K.; Logsdon, N.J.; Benavides, G.A.; Sanders, Y.; Zhang, J.; Darley-Usmar, V.M.; Thannickal, V.J. Glutaminolysis is required for transforming growth factor-beta1-induced myofibroblast differentiation and activation. J. Biol. Chem. 2018, 293, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Serena, C.; Ceperuelo-Mallafre, V.; Keiran, N.; Queipo-Ortuno, M.I.; Bernal, R.; Gomez-Huelgas, R.; Urpi-Sarda, M.; Sabater, M.; Perez-Brocal, V.; Andres-Lacueva, C.; et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018, 12, 1642–1657. [Google Scholar] [CrossRef]
- Meier, R.; Lutz, C.; Cosin-Roger, J.; Fagagnini, S.; Bollmann, G.; Hunerwadel, A.; Mamie, C.; Lang, S.; Tchouboukov, A.; Weber, F.E.; et al. Decreased fibrogenesis after treatment with pirfenidone in a newly developed mouse model of intestinal fibrosis. Inflamm. Bowel. Dis. 2016, 22, 569–582. [Google Scholar] [CrossRef]
- Aspuria, P.P.; Lunt, S.Y.; Varemo, L.; Vergnes, L.; Gozo, M.; Beach, J.A.; Salumbides, B.; Reue, K.; Wiedemeyer, W.R.; Nielsen, J.; et al. Succinate dehydrogenase inhibition leads to epithelial-mesenchymal transition and reprogrammed carbon metabolism. Cancer Metab. 2014, 2, 21. [Google Scholar] [CrossRef]
- Sciacovelli, M.; Frezza, C. Metabolic reprogramming and epithelial-to-mesenchymal transition in cancer. FEBS J 2017, 284, 3132–3144. [Google Scholar] [CrossRef]
- Heuberger, J.; Birchmeier, W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, a002915. [Google Scholar] [CrossRef]
- Littlewood-Evans, A.; Sarret, S.; Apfel, V.; Loesle, P.; Dawson, J.; Zhang, J.; Muller, A.; Tigani, B.; Kneuer, R.; Patel, S.; et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J. Exp. Med. 2016, 213, 1655–1662. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, J.Y.; Liu, J.Q.; Yang, J.; Liu, Y.; Wang, C.; Ma, X.N.; Liu, B.L.; Xin, G.Z.; Liu, L.F. Succinate/NLRP3 inflammasome induces synovial fibroblast activation: Therapeutical effects of clematichinenoside AR on arthritis. Front. Immunol. 2016, 7, 532. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Masia, D.; Cosin-Roger, J.; Calatayud, S.; Hernandez, C.; Alos, R.; Hinojosa, J.; Apostolova, N.; Alvarez, A.; Barrachina, M.D. Hypoxic macrophages impair autophagy in epithelial cells through Wnt1: Relevance in IBD. Mucosal Immunol. 2014, 7, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, X.; Peng, Y.; Wu, M.; Zhang, P.; Xie, R.; Wu, Y.; Yan, Q.; Liu, S.; Wang, J. HIF-1alpha promotes epithelial-mesenchymal transition and metastasis through direct regulation of ZEB1 in Colorectal cancer. PLoS ONE 2015, 10, e0129603. [Google Scholar]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Baysal, B.E.; Ferrell, R.E.; Willett-Brozick, J.E.; Lawrence, E.C.; Myssiorek, D.; Bosch, A.; van der Mey, A.; Taschner, P.E.; Rubinstein, W.S.; Myers, E.N.; et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 2000, 287, 848–851. [Google Scholar] [CrossRef]
- Xekouki, P.; Pacak, K.; Almeida, M.; Wassif, C.A.; Rustin, P.; Nesterova, M.; de la Luz Sierra, M.; Matro, J.; Ball, E.; Azevedo, M.; et al. Succinate dehydrogenase (SDH) D subunit (SDHD) inactivation in a growth-hormone-producing pituitary tumor: A new association for SDH? J. Clin. Endocrinol. Metab. 2012, 97, E357–E366. [Google Scholar] [CrossRef]
- Hobert, J.A.; Mester, J.L.; Moline, J.; Eng, C. Elevated plasma succinate in PTEN, SDHB, and SDHD mutation-positive individuals. Genet. Med. 2012, 14, 616–619. [Google Scholar] [CrossRef]
- Mu, X.; Zhao, T.; Xu, C.; Shi, W.; Geng, B.; Shen, J.; Zhang, C.; Pan, J.; Yang, J.; Hu, S.; et al. Oncometabolite succinate promotes angiogenesis by upregulating VEGF expression through GPR91-mediated STAT3 and ERK activation. Oncotarget 2017, 8, 13174–13185. [Google Scholar] [CrossRef]
- Schneider, C.; O’Leary, C.E.; von Moltke, J.; Liang, H.E.; Ang, Q.Y.; Turnbaugh, P.J.; Radhakrishnan, S.; Pellizzon, M.; Ma, A.; Locksley, R.M. A metabolite-triggered tuft cell-ILC2 circuit drives small intestinal remodeling. Cell 2018, 174, 271–284. [Google Scholar] [CrossRef]
- Siegmund, B.; Feakins, R.M.; Barmias, G.; Ludvig, J.C.; Teixeira, F.V.; Rogler, G.; Scharl, M. Results of the fifth scientific workshop of the ECCO (II): Pathophysiology of perianal fistulizing disease. J. Crohns Colitis 2016, 10, 377–386. [Google Scholar] [CrossRef]
- Rivera-Nieves, J.; Bamias, G.; Vidrich, A.; Marini, M.; Pizarro, T.T.; McDuffie, M.J.; Moskaluk, C.A.; Cohn, S.M.; Cominelli, F. Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology 2003, 124, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, R.S.; Nissim-Eliraz, E.; Marsiano, N.; Nir, E.; Shemesh, H.; Leutenegger, M.; Gottier, C.; Lang, S.; Spalinger, M.R.; Leibl, S.; et al. Transplantation of human intestine into the mouse: A novel platform for study of inflammatory enterocutaneous fistulas. J. Crohns Colitis 2019, 13, 798–806. [Google Scholar] [CrossRef] [PubMed]
| B2-CD Patients | B3-CD Patients | Non-IBD Patients | |
|---|---|---|---|
| Number of Patients | 19 | 16 | 10 |
| Age | |||
| Median | 43 | 43 | 54 |
| Interval | [22–77] | [15–61] | [28–76] |
| Gender | |||
| Male | 6 | 5 | 4 |
| Female | 13 | 11 | 6 |
| Localization | |||
| Terminal Ileum | 9 | 7 | 4 |
| Cecum | 6 | 6 | - |
| Colon | 4 | 3 | 6 |
| Treatment | B2-CD Patients | B3-CD Patients |
|---|---|---|
| Azathioprine | 2 | 1 |
| Azathioprine + 5-aminosalicylic acid | 1 | 0 |
| Infliximab | 6 | 5 |
| Adalimumab | 1 | 3 |
| Adalimumab + Azathioprine | 4 | 2 |
| Adalimumab + Methotrexate | 1 | 0 |
| Ustekinumab | 0 | 3 |
| Ustekinumab + Infliximab | 1 | 0 |
| Vedolizumab | 1 | 0 |
| Certolizumab | 2 | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Masiá, D.; Gisbert-Ferrándiz, L.; Bauset, C.; Coll, S.; Mamie, C.; Scharl, M.; Esplugues, J.V.; Alós, R.; Navarro, F.; Cosín-Roger, J.; et al. Succinate Activates EMT in Intestinal Epithelial Cells through SUCNR1: A Novel Protagonist in Fistula Development. Cells 2020, 9, 1104. https://doi.org/10.3390/cells9051104
Ortiz-Masiá D, Gisbert-Ferrándiz L, Bauset C, Coll S, Mamie C, Scharl M, Esplugues JV, Alós R, Navarro F, Cosín-Roger J, et al. Succinate Activates EMT in Intestinal Epithelial Cells through SUCNR1: A Novel Protagonist in Fistula Development. Cells. 2020; 9(5):1104. https://doi.org/10.3390/cells9051104
Chicago/Turabian StyleOrtiz-Masiá, Dolores, Laura Gisbert-Ferrándiz, Cristina Bauset, Sandra Coll, Céline Mamie, Michael Scharl, Juan V. Esplugues, Rafael Alós, Francisco Navarro, Jesús Cosín-Roger, and et al. 2020. "Succinate Activates EMT in Intestinal Epithelial Cells through SUCNR1: A Novel Protagonist in Fistula Development" Cells 9, no. 5: 1104. https://doi.org/10.3390/cells9051104
APA StyleOrtiz-Masiá, D., Gisbert-Ferrándiz, L., Bauset, C., Coll, S., Mamie, C., Scharl, M., Esplugues, J. V., Alós, R., Navarro, F., Cosín-Roger, J., Barrachina, M. D., & Calatayud, S. (2020). Succinate Activates EMT in Intestinal Epithelial Cells through SUCNR1: A Novel Protagonist in Fistula Development. Cells, 9(5), 1104. https://doi.org/10.3390/cells9051104





