Ginsenoside Rg1 Acts as a Selective Glucocorticoid Receptor Agonist with Anti-Inflammatory Action without Affecting Tissue Regeneration in Zebrafish Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Lines and Maintenance
2.2. Fish Embryo Acute Toxicity Test (FET) for Rg1
2.3. Tail Fin Amputation and Drug Treatments
2.4. Visualization and Quantification of Macrophages and Neutrophils
2.5. Regeneration of Amputated Larvae Tail Fin
2.6. Quantitative PCR (qPCR) Analysis
2.7. Statistical Analysis
3. Results
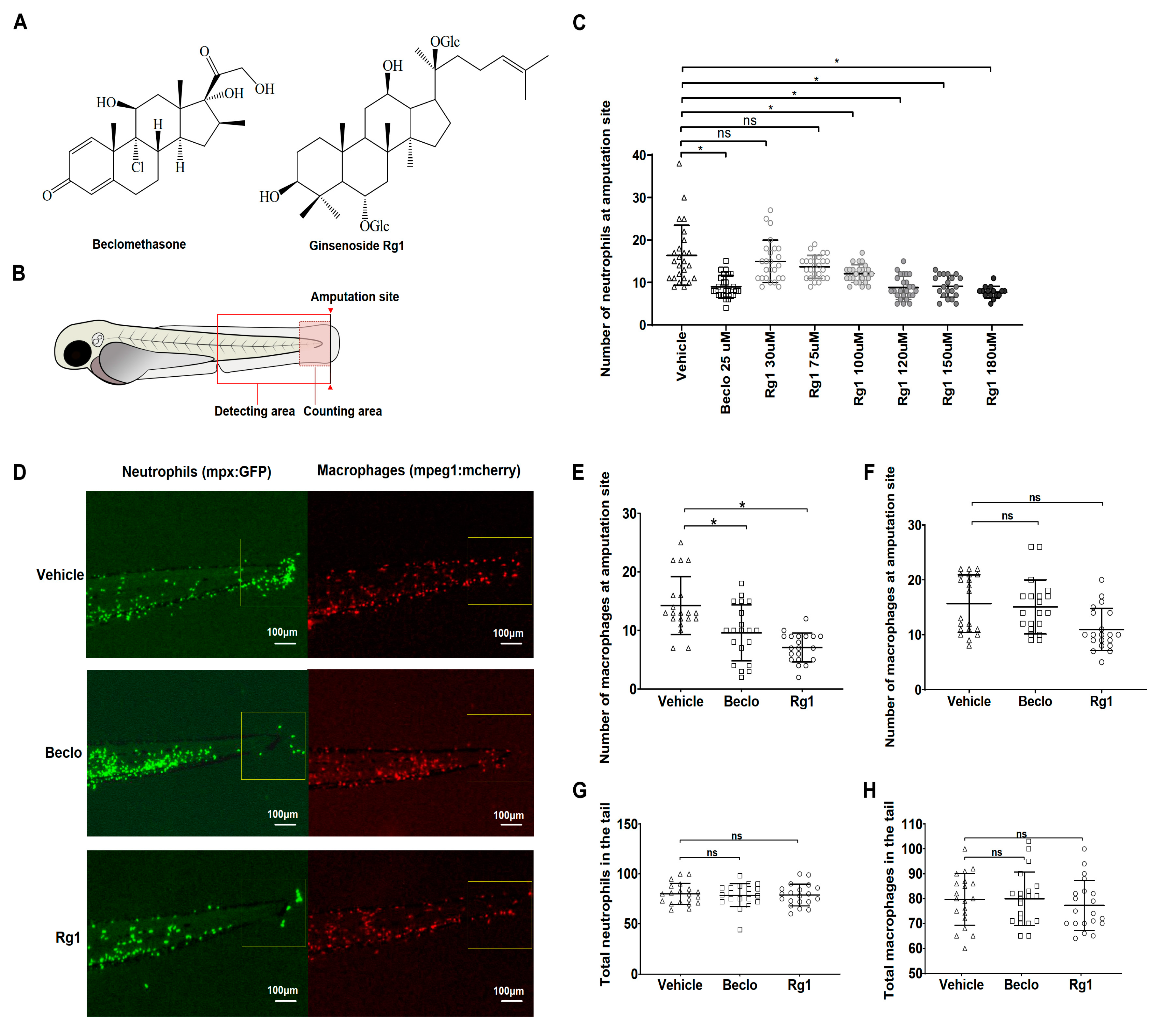
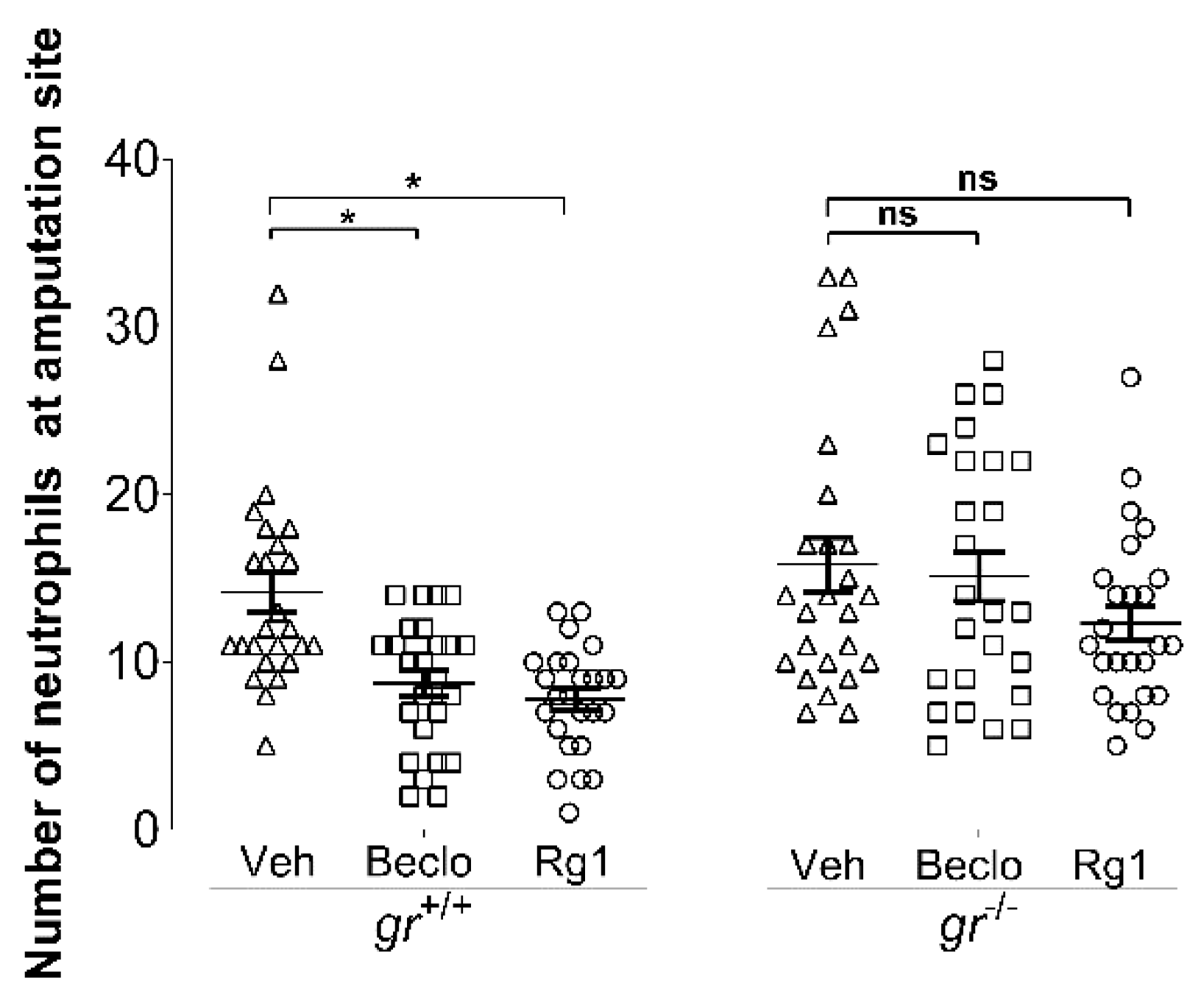
3.1. Ginsenoside Rg1 Has Anti-Inflammatory Effects that Are Mediated by the Gr
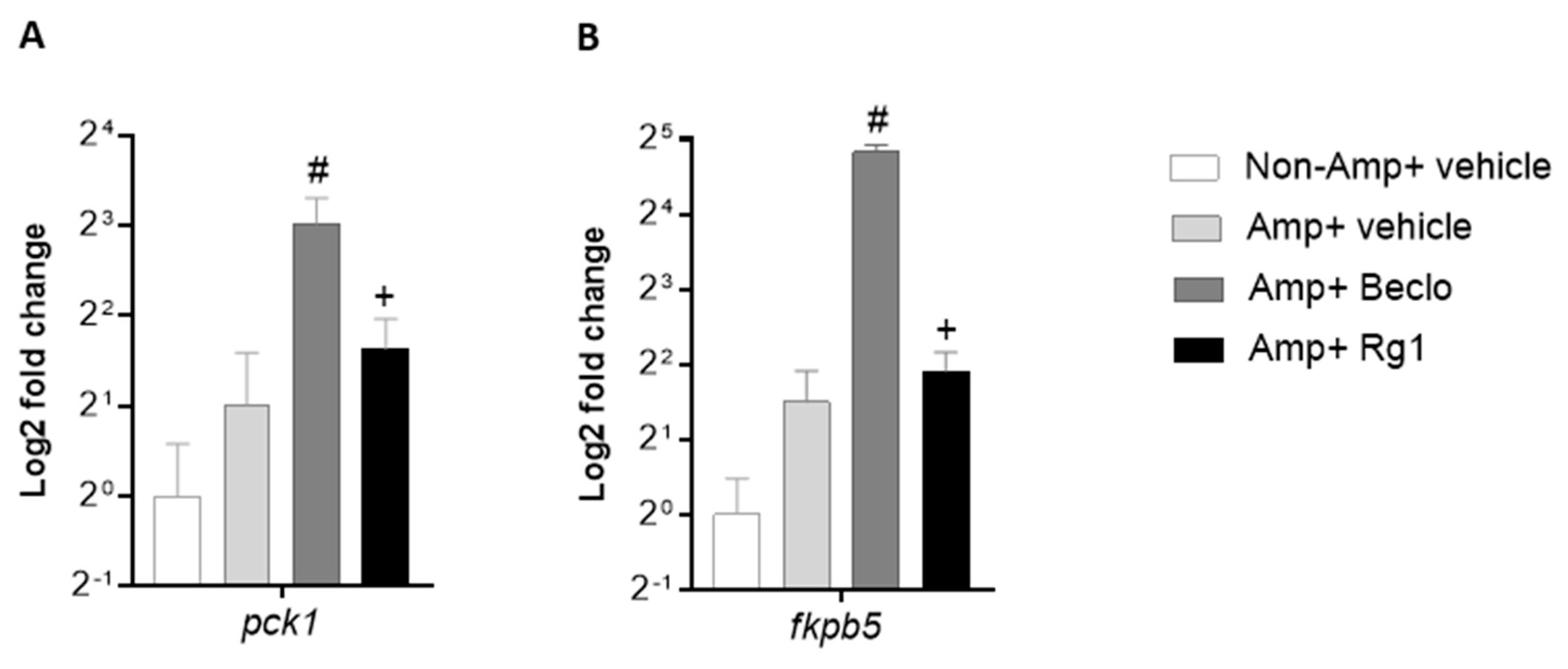
3.2. Rg1 And Beclomethasone Differentially Regulate Gene Expression
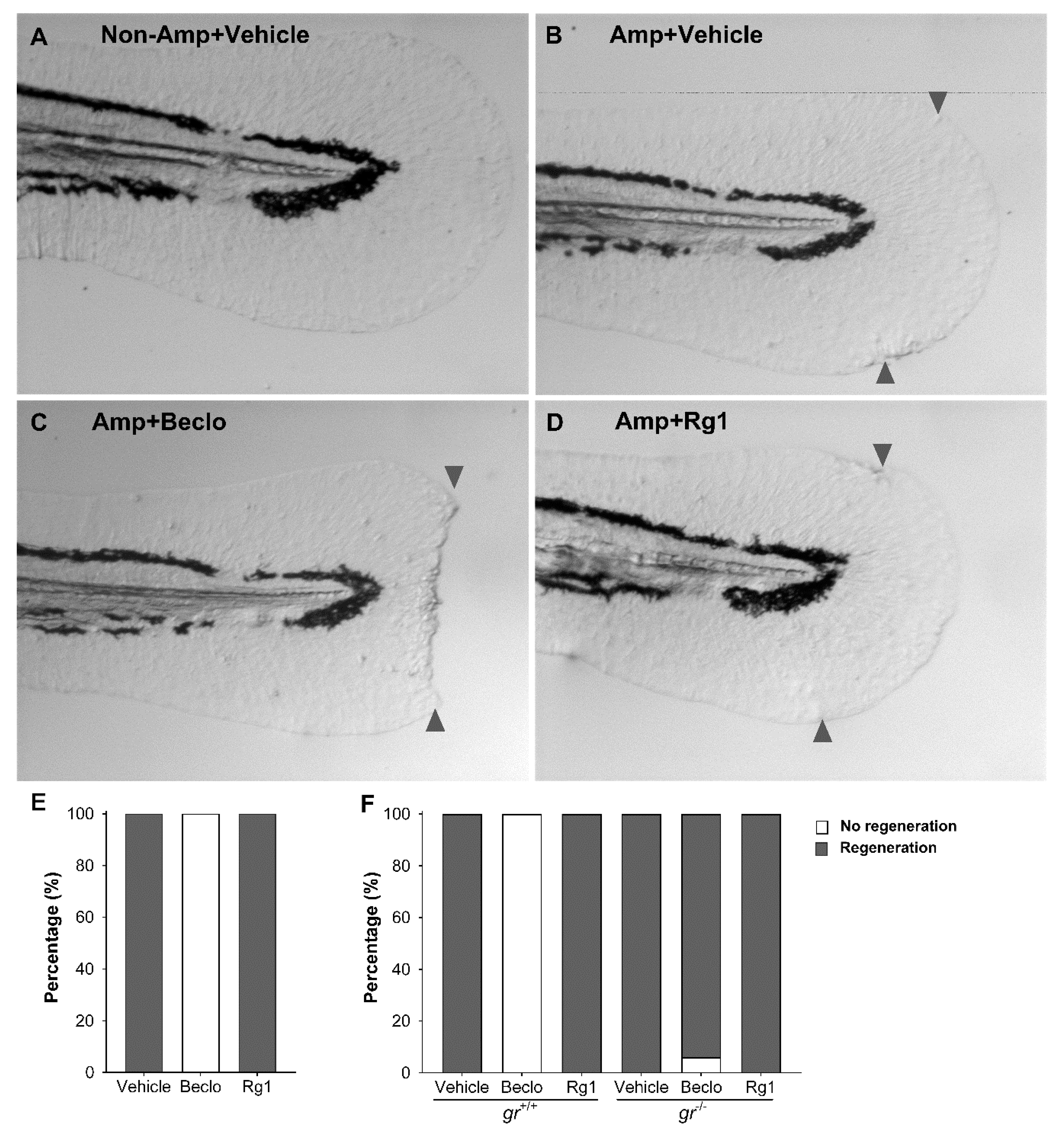
3.3. Rg1 Does Not Inhibit Tissue Regeneration, Unlike Beclomethasone
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, J.E.; Barbul, A. Understanding the role of immune regulation in wound healing. Am. J. Surg. 2004, 187, 11S–16S. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Oates, A.C.; Crowhurst, M.O.; Ward, A.C.; Layton, J.E. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood 2001, 98, 3087–3096. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Martin, K.R.; Ohayon, D.; Witko-Sarsat, V. Promoting apoptosis of neutrophils and phagocytosis by macrophages: Novel strategies in the resolution of inflammation. Swiss Med. Wkly. 2015, 145, w14056. [Google Scholar] [CrossRef]
- De Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef]
- Gordon, S. The macrophage. BioEssays 1995, 17, 977–986. [Google Scholar] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Sacca, R.; Cuff, C.A.; Ruddle, N.H. Mediators of inflammation. Curr. Opin. Immunol. 1997, 9, 851–857. [Google Scholar] [CrossRef]
- Homo-Delarche, F.; Fitzpatrick, F.; Christeff, N.; Nunez, E.A.; Bach, J.F.; Dardenne, M. Sex steroids, glucocorticoids, stress and autoimmunity. J. Steroid Biochem. Mol. Biol. 1991, 40, 619–637. [Google Scholar] [CrossRef]
- Hall, B.M.; Hodgkinson, S.J.; Quin, J. Corticosteroids in autoimmune diseases. Aust. Prescr. 1999, 22, 9–11. [Google Scholar]
- Trombetta, A.C.; Meroni, M.; Cutolo, M. Steroids and Autoimmunity. Front. Horm. Res. 2017, 48, 121–132. [Google Scholar]
- Ashwell, J.D.; Lu, F.W.M.; Vacchio, M.S. Glucocorticoids in T Cell Development and Function. Annu. Rev. Immunol. 2000, 18, 309–345. [Google Scholar] [CrossRef]
- Ratman, D.; Vanden Berghe, W.; Dejager, L.; Libert, C.; Tavernier, J.; Beck, I.M.; De Bosscher, K. How glucocorticoid receptors modulate the activity of other transcription factors: A scope beyond tethering. Mol. Cell Endocrinol. 2013, 380, 41–54. [Google Scholar] [CrossRef]
- Reichardt, H.M.; Tuckermann, J.P.; Gottlicher, M.; Vujic, M.; Weih, F.; Angel, P.; Herrlich, P.; Schutz, G. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. Embo J. 2001, 20, 7168–7173. [Google Scholar] [CrossRef]
- Mathew, L.K.; Sengupta, S.; Kawakami, A.; Andreasen, E.A.; Löhr, C.V.; Loynes, C.A.; Renshaw, S.A.; Peterson, R.T.; Tanguay, R.L. Unraveling tissue regeneration pathways using chemical genetics. J. Biol. Chem. 2007, 282, 35202–35210. [Google Scholar] [CrossRef]
- Kang, S.; Min, H. Ginseng, the “Immunity Boost”: The Effects of Panax ginseng on Immune System. J. Ginseng Res. 2012, 36, 354–368. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Huang, X.; Liu, S.; Guo, C.; Xie, Y.; Meijer, A.H.; Wang, M. The Difference between White and Red Ginseng: Variations in Ginsenosides and Immunomodulation. Planta Med. 2018, 84, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.W.; Pon, Y.L.; Wong, R.N.S.; Wong, A.S.T. Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells. J. Biol. Chem. 2006, 281, 36280–36288. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Chung, E.; Lee, K.Y.; Lee, Y.H.; Huh, B.; Lee, S.K. Ginsenoside-Rg1, one of the major active molecules from Panax ginseng, is a functional ligand of glucocorticoid receptor. Mol. Cell. Endocrinol. 1997, 133, 135–140. [Google Scholar] [CrossRef]
- Leung, K.W.; Cheng, Y.-K.; Mak, N.K.; Chan, K.K.C.; Fan, T.P.D.; Wong, R.N.S. Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Lett. 2006, 580, 3211–3216. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cheng, B.; Zhu, X.; Ling, C.; Alerts, E. Ginsenoside Rg1, a Novel Glucocorticoid Receptor Agonist of Plant Origin, Maintains Glucocorticoid Efficacy with Reduced Side Effects. J. Immunol. 2011, 187, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef]
- Schaaf, M.J.M.; Chatzopoulou, A.; Spaink, H.P. The zebrafish as a model system for glucocorticoid receptor research. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 75–82. [Google Scholar] [CrossRef]
- Alsop, D.; Vijayan, M.M. Molecular programming of the corticosteroid stress axis during zebrafish development. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 49–54. [Google Scholar] [CrossRef]
- Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. The use of the zebrafish model in stress research. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1432–1451. [Google Scholar] [CrossRef]
- Trede, N.S.; Langenau, D.M.; Traver, D.; Look, A.T.; Zon, L.I. The use of zebrafish to understand immunity. Immunity 2004, 20, 367–379. [Google Scholar] [CrossRef]
- Chatzopoulou, A.; Heijmans, J.P.M.; Burgerhout, E.; Oskam, N.; Spaink, H.P.; Meijer, A.H.; Schaaf, M.J.M. Glucocorticoid-Induced Attenuation of the Inflammatory Response in Zebrafish. Endocrinology 2016, 157, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Yang, C.C.; Chen, I.H.; Liu, Y.M.L.; Chang, S.J.; Chuang, Y.J. Treatment of Glucocorticoids Inhibited Early Immune Responses and Impaired Cardiac Repair in Adult Zebrafish. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, X.-T.; Zhu, K.-Y.; Jin, Y.; Deng, M.; Le, H.-Y.; Fu, Y.-F.; Chen, Y.; Zhu, J.; Look, A.T.; et al. In vivo interstitial migration of primitive macrophages mediated by JNK-matrix metalloproteinase 13 signaling in response to acute injury. J. Immunol. 2008, 181, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.J.; Wicker, S.M.; Chien, A.-T.; Tromp, A.; Lawrence, L.M.; Sun, X.; Krissansen, G.W.; Crosier, K.E.; Crosier, P.S. Repositioning drugs for inflammatory disease—Fishing for new anti-inflammatory agents. Dis. Models Mech. 2014, 7, 1069–1081. [Google Scholar] [CrossRef]
- Xie, Y.; Tolmeijer, S.; Oskam, J.M.; Tonkens, T.; Meijer, A.H.; Schaaf, M.J.M. Glucocorticoids inhibit macrophage differentiation towards a proinflammatory phenotype upon wounding without affecting their migration. Dis. Models Mech. 2019, 12, dmm037887. [Google Scholar] [CrossRef]
- Schoonheim, P.J.; Chatzopoulou, A.; Schaaf, M.J.M. The zebrafish as an in vivo model system for glucocorticoid resistance. Steroids 2010, 75, 918–925. [Google Scholar] [CrossRef]
- Renshaw, S.A.; Loynes, C.A.; Trushell, D.M.I.; Elworthy, S.; Ingham, P.W.; Whyte, M.K.B. A transgenic zebrafish model of neutrophilic inflammation. Blood 2006, 108, 3976–3978. [Google Scholar] [CrossRef]
- Bernut, A.; Herrmann, J.-L.; Kissa, K.; Dubremetz, J.-F.; Gaillard, J.-L.; Lutfalla, G.; Kremer, L. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl. Acad. Sci. USA 2014, 111, E943–E952. [Google Scholar] [CrossRef]
- Ziv, L.; Muto, A.; Schoonheim, P.J.; Meijsing, S.H.; Strasser, D.; Ingraham, H.A.; Schaaf, M.J.M.; Yamamoto, K.R.; Baier, H. An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Mol. Psychiatry 2013, 18, 681–691. [Google Scholar] [CrossRef]
- The Organisation for Economic Co-operation and Development (OECD). Test No. 236: Fish Embryo Acute 3. Toxicity (FET) Test. Available online: http://dx.doi.org/10.1787/4.9789264203709-en (accessed on 26 July 2013).
- Van Pomeren, M.; Peijnenburg, W.J.G.M.; Brun, N.R.; Vijver, M.G. A novel experimental and modelling strategy for nanoparticle toxicity testing enabling the use of small quantities. Int. J. Environ. Res. Public Health 2017, 14, 1348. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish embryos as an alternative to animal experiments—A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. (Elmsford N.Y.) 2012, 33, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cho, J.Y. Anti-inflammatory effects of ginsenosides from Panax ginseng and their structural analogs. Afr. J. Biotechnol. 2009, 8, 3682–3690. [Google Scholar]
- Hu, C.; Lau, A.J.; Wang, R.; Chang, T.K.H. Comparative analysis of ginsenosides in human glucocorticoid receptor binding, transactivation, and transrepression. Eur. J. Pharmacol. 2017, 815, 501–511. [Google Scholar] [CrossRef]
- Karra, A.G.; Konstantinou, M.; Tzortziou, M.; Tsialtas, I.; Kalousi, F.D.; Garagounis, C.; Hayes, J.M.; Psarra, A.-M.G. Potential Dissociative Glucocorticoid Receptor Activity for Protopanaxadiol and Protopanaxatriol. Int. J. Mol. Sci. 2018, 20, 94. [Google Scholar] [CrossRef]
- Sundahl, N.; Bridelance, J.; Libert, C.; De Bosscher, K.; Beck, I.M. Selective glucocorticoid receptor modulation: New directions with non-steroidal scaffolds. Pharmacol. Ther. 2015, 152, 28–41. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Shi, Y.-Q.; Zhang, W.-Q.; Wen, Z.-L. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J. Biol. Chem. 2012, 287, 25353–25360. [Google Scholar] [CrossRef]
- Yoo, S.K.; Freisinger, C.M.; LeBert, D.C.; Huttenlocher, A. Early redox, Src family kinase, and calcium signaling integrate wound responses and tissue regeneration in zebrafish. J. Cell Biol. 2012, 199, 225–234. [Google Scholar] [CrossRef]
- Jiang, W.E.N.G.; Sanders, A.J.; Ruge, F.; Harding, K.G. Influence of interleukin-8 (IL-8) and IL-8 receptors on the migration of human keratinocytes, the role of PLC-γ and potential clinical implications. Exp. Ther. Med. 2012, 3, 231–236. [Google Scholar] [CrossRef]
- Hattori, N.; Mochizuki, S.; Kishi, K.; Nakajima, T.; Takaishi, H.; D’Armiento, J.; Okada, Y. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am. J. Pathol. 2009, 175, 533–546. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Halima, M.; Xie, Y.; Schaaf, M.J.M.; Meijer, A.H.; Wang, M. Ginsenoside Rg1 Acts as a Selective Glucocorticoid Receptor Agonist with Anti-Inflammatory Action without Affecting Tissue Regeneration in Zebrafish Larvae. Cells 2020, 9, 1107. https://doi.org/10.3390/cells9051107
He M, Halima M, Xie Y, Schaaf MJM, Meijer AH, Wang M. Ginsenoside Rg1 Acts as a Selective Glucocorticoid Receptor Agonist with Anti-Inflammatory Action without Affecting Tissue Regeneration in Zebrafish Larvae. Cells. 2020; 9(5):1107. https://doi.org/10.3390/cells9051107
Chicago/Turabian StyleHe, Min, Mahmoud Halima, Yufei Xie, Marcel J. M. Schaaf, Annemarie H. Meijer, and Mei Wang. 2020. "Ginsenoside Rg1 Acts as a Selective Glucocorticoid Receptor Agonist with Anti-Inflammatory Action without Affecting Tissue Regeneration in Zebrafish Larvae" Cells 9, no. 5: 1107. https://doi.org/10.3390/cells9051107
APA StyleHe, M., Halima, M., Xie, Y., Schaaf, M. J. M., Meijer, A. H., & Wang, M. (2020). Ginsenoside Rg1 Acts as a Selective Glucocorticoid Receptor Agonist with Anti-Inflammatory Action without Affecting Tissue Regeneration in Zebrafish Larvae. Cells, 9(5), 1107. https://doi.org/10.3390/cells9051107







