Do Mast Cells Have a Role in Tendon Healing and Inflammation?
Abstract
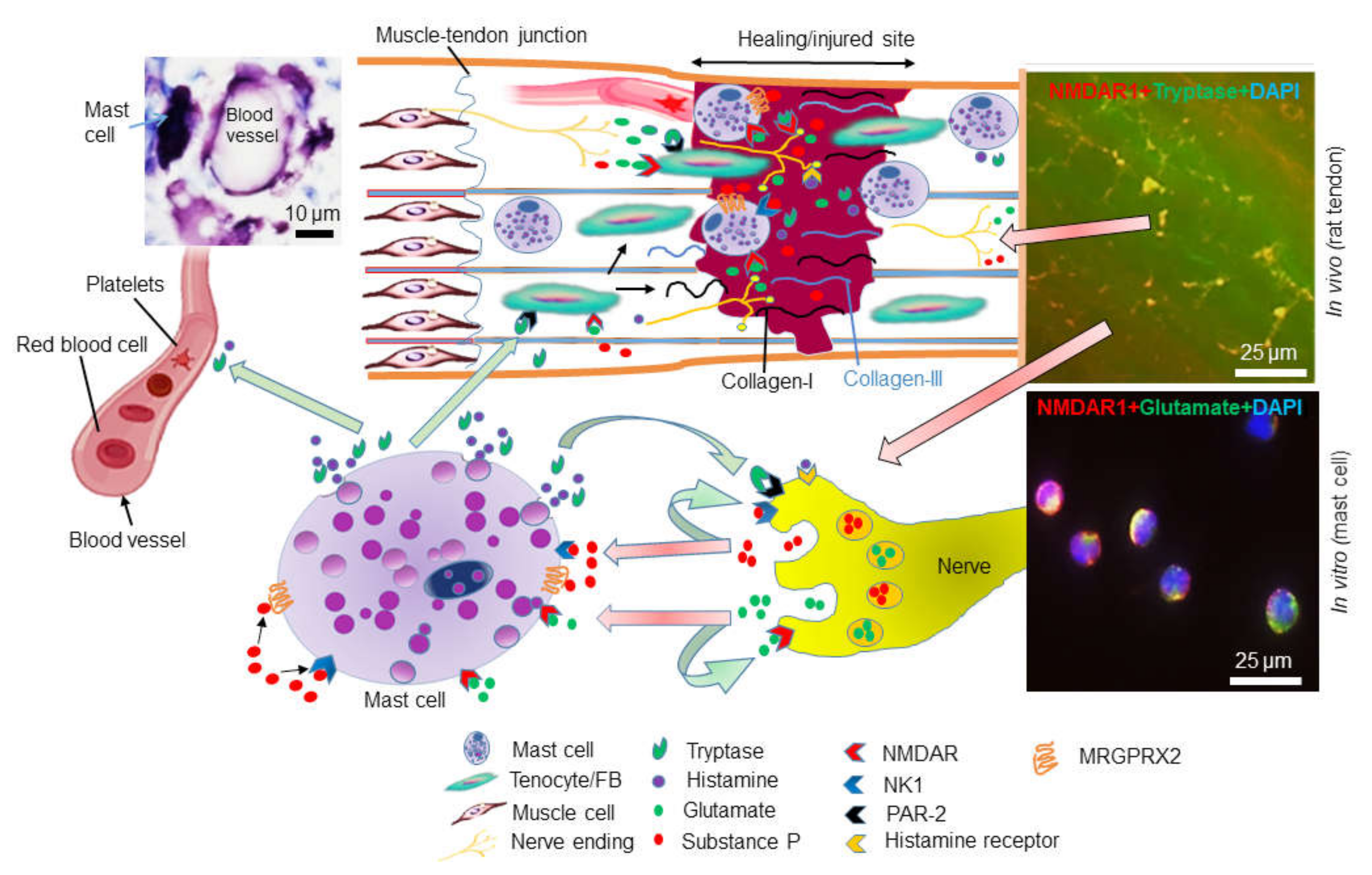
:1. Introduction
2. Tendon
3. Tendon Healing and Inflammation
4. Mast Cells
5. Mast Cells in Tendinopathy
6. Glutamate Receptors in Tendinopathy and Mast Cells
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Galli, S.J.; Starkl, P.; Marichal, T.; Tsai, M. Mast cells and IgE in defense against venoms: Possible “good side” of allergy? J. Allergol. Int. 2016, 65, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S. Mast-cell responses to pathogens. Nat. Rev. Immunol. 2004, 4, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Lian, O.; Bahr, R.; Hart, D.A.; Duronio, V.; Khan, K.M. Increased mast cell numbers in human patellar tendinosis: Correlation with symptom duration and vascular hyperplasia. Br. J. Sports Med. 2008, 42, 753–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pingel, J.; Wienecke, J.; Kongsgaard, M.; Behzad, H.; Abraham, T.; Langberg, H.; Scott, A. Increased mast cell numbers in a calcaneal tendon overuse model. Scand J. Med. Sci. Sports 2013, 23, e353–e360. [Google Scholar] [CrossRef]
- Alim, M.A.; Ackermann, P.W.; Eliasson, P.; Blomgran, P.; Kristiansson, P.; Pejler, G.; Peterson, M. Increased mast cell degranulation and co-localization of mast cells with the NMDA receptor-1 during healing after Achilles tendon rupture. Cell Tissue Res. 2017, 370, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Urist, M.; McLean, F. Accumulation of mast cells in endosteum of bones of calciumdeficient rats. Arch. Pathol. 1957, 63, 239–251. [Google Scholar]
- Ragipoglu, D.; Dudeck, A.; Haffner-Luntzer, M.; Voss, M.; Kroner, J.; Ignatius, A.; Fischer, V. The Role of Mast Cells in Bone Metabolism and Bone Disorders. Front. Immunol. 2020, 11, 163. [Google Scholar] [CrossRef] [Green Version]
- Lazarus, B.; Messina, A.; Barker, J.E.; Hurley, J.V.; Romeo, R.; Morrison, W.A.; Knight, K.R. The role of mast cells in ischaemia–reperfusion injury in murine skeletal muscle. The J. Pathol. 2000, 191, 443–448. [Google Scholar] [CrossRef]
- Nahirney, P.C.; Dow, P.R.; Ovalle, W.K. Quantitative morphology of mast cells in skeletal muscle of normal and genetically dystrophic mice. Anat. Rec. 1997, 247, 341–349. [Google Scholar] [CrossRef]
- Alim, M.A.; Grujic, M.; Ackermann, P.W.; Eliasson, P.; Kristiansson, P.; Peterson, M.; Pejler, G. Glutamate Triggers the Expression of Functional Ionotropic and Metabotropic Glutamate Receptors in Mast Cells. Cell Mol. Immunol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Maffulli, N. Tendinopathy and tendon injury: The future. Disabil. Rehabil. 2008, 30, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Nourissat, G.; Berenbaum, F.; Duprez, D. Tendon injury: From biology to tendon repair. Nat. Rev. Rheumatol. 2015, 11, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Nerlich, M.; Docheva, D. Tendon injuries: Basic science and new repair proposals. EFORT Open Rev. 2017, 2, 332–342. [Google Scholar] [CrossRef]
- Mienaltowski, M.J.; Birk, D.E. Structure, physiology, and biochemistry of collagens. Adv. Exp. Med. Biol. 2014, 802, 5–29. [Google Scholar]
- Vestergaard, P.; Jorgensen, J.O.L.; Olesen, J.L.; Bosnjak, E.; Holm, L.; Frystyk, J.; Langberg, H.; Kjaer, M.; Hansen, M. Local administration of growth hormone stimulates tendon collagen synthesis in elderly men. J. Appl. Physiol. 2012, 113, 1432–1438. [Google Scholar] [CrossRef] [Green Version]
- Alfredson, H. The chronic painful Achilles and patellar tendon: Research on basic biology and treatment. Scand J. Med. Sci. Sports 2005, 15, 252–259. [Google Scholar] [CrossRef]
- Khan, K.M.; Cook, J.L.; Bonar, F.; Harcourt, P.; Astrom, M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999, 27, 393–408. [Google Scholar] [CrossRef]
- Maffulli, N.; Khan, K.M.; Puddu, G. Overuse tendon conditions: Time to change a confusing terminology. Arthroscopy 1998, 14, 840–843. [Google Scholar] [CrossRef]
- Tran, P.H.T.; Malmgaard-Clausen, N.M.; Puggaard, R.S.; Svensson, R.B.; Nybing, J.D.; Hansen, P.; Schjerling, P.; Zinglersen, A.H.; Couppe, C.; Boesen, M.; et al. Early development of tendinopathy in humans: Sequence of pathological changes in structure and tissue turnover signaling. FASEB J. 2020, 34, 776–788. [Google Scholar] [CrossRef] [Green Version]
- Tuite, D.J.; Renstrom, P.A.; O’Brien, M. The aging tendon. Scand. J. Med. Sci. Sports 1997, 7, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Svensson, R.B.; Heinemeier, K.M.; Couppe, C.; Kjaer, M.; Magnusson, S.P. Effect of aging and exercise on the tendon. J. Appl. Physiol. (1985) 2016, 121, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Ranger, T.A.; Wong, A.M.; Cook, J.L.; Gaida, J.E. Is there an association between tendinopathy and diabetes mellitus? A systematic review with meta-analysis. Br. J. Sports Med. 2016, 50, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Murrell, G.A.; McInnes, I.B. Inflammatory mechanisms in tendinopathy—Towards translation. Nat. Rev. Rheumatol. 2017, 13, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Murrell, G.A.; McInnes, I.B. Alarmins in tendinopathy: Unravelling new mechanisms in a common disease. Rheumatology (Oxford) 2013, 52, 769–779. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, P.W.; Franklin, S.L.; Dean, B.J.F.; Carr, A.J.; Salo, P.T.; Hart, D.A. Neuronal pathways in tendon healing and tendinopathy—Update. Front. Biosci. 2014, 19, 1251–1278. [Google Scholar] [CrossRef] [Green Version]
- Bjur, D.; Alfredson, H.; Forsgren, S. The innervation pattern of the human Achilles tendon: Studies of the normal and tendinosis tendon with markers for general and sensory innervation. Cell Tissue Res. 2005, 320, 201–206. [Google Scholar] [CrossRef]
- Ackermann, P.W.; Li, J.; Lundeberg, T.; Kreicbergs, A. Neuronal plasticity in relation to nociception and healing of rat achilles tendon. J. Orthop. Res. 2003, 21, 432–441. [Google Scholar] [CrossRef]
- Schizas, N.; Lian, O.; Frihagen, F.; Engebretsen, L.; Bahr, R.; Ackermann, P.W. Coexistence of up-regulated NMDA receptor 1 and glutamate on nerves, vessels and transformed tenocytes in tendinopathy. Scand J. Med. Sci. Sports 2010, 20, 208–215. [Google Scholar] [CrossRef]
- Alfredson, H.; Lorentzon, R. Chronic tendon pain: No signs of chemical inflammation but high concentrations of the neurotransmitter glutamate. Implications for treatment? Curr. Drug Targets 2002, 3, 43–54. [Google Scholar] [CrossRef]
- Ljung, B.O.; Alfredson, H.; Forsgren, S. Neurokinin 1-receptors and sensory neuropeptides in tendon insertions at the medial and lateral epicondyles of the humerus. Studies on tennis elbow and medial epicondylalgia. J. Orthop. Res. 2004, 22, 321–327. [Google Scholar] [CrossRef]
- Spang, C.; Harandi, V.M.; Alfredson, H.; Forsgren, S. Marked innervation but also signs of nerve degeneration in between the Achilles and plantaris tendons and presence of innervation within the plantaris tendon in midportion Achilles tendinopathy. J. Musculoskelet. Neuronal Interact. 2015, 15, 197–206. [Google Scholar] [PubMed]
- Abate, M.; Silbernagel, K.G.; Siljeholm, C.; Di Iorio, A.; De Amicis, D.; Salini, V.; Werner, S.; Paganelli, R. Pathogenesis of tendinopathies: Inflammation or degeneration? Arthritis Res. Ther. 2009, 11, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. Wound healing: An overview. Plast. Reconstr. Surg. 2006, 117, 1e-S–32e-S. [Google Scholar] [CrossRef]
- Langberg, H.; Skovgaard, D.; Karamouzis, M.; Bulow, J.; Kjaer, M. Metabolism and inflammatory mediators in the peritendinous space measured by microdialysis during intermittent isometric exercise in humans. J. Physiol. 1999, 515, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M.; Langberg, H.; Skovgaard, D.; Olesen, J.; Bulow, J.; Krogsgaard, M.; Boushel, R. In vivo studies of peritendinous tissue in exercise. Scand. J. Med. Sci. Sports 2000, 10, 326–331. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.B.; Cafferty, W.B.J.; Marchand, F. Immune and glial cell factors as pain mediators and modulators. Exp. Neurol. 2005, 192, 444–462. [Google Scholar] [CrossRef]
- Bjorklund, E.; Forsgren, S.; Alfredson, H.; Fowler, C.J. Increased expression of cannabinoid CB(1) receptors in Achilles tendinosis. PLoS ONE 2011, 6, e24731. [Google Scholar] [CrossRef] [Green Version]
- Domeij-Arverud, E.; Labruto, F.; Latifi, A.; Nilsson, G.; Edman, G.; Ackermann, P.W. Intermittent pneumatic compression reduces the risk of deep vein thrombosis during post-operative lower limb immobilization: A prospective randomised trial of acute ruptures of the achilles tendon. Bone Jt. J. 2015, 97b, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Carter, D.R.; Beaupre, G.S.; Giori, N.J.; Helms, J.A. Mechanobiology of skeletal regeneration. Clin. Orthop. Relat. Res. 1998, S41–S55. [Google Scholar] [CrossRef] [Green Version]
- Nunamaker, D.M. Experimental models of fracture repair. Clin. Orthop. Relat. Res. 1998, S56–S65. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; von Hehn, C.A.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012, 15, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, P.W.; Ahmed, M.; Kreicbergs, A. Early nerve regeneration after achilles tendon rupture—A prerequisite for healing? A study in the rat. J. Orthop. Res. 2002, 20, 849–856. [Google Scholar] [CrossRef]
- Chen, C.C.; Grimbaldeston, M.A.; Tsai, M.; Weissman, I.L.; Galli, S.J. Identification of mast cell progenitors in adult mice. Proc. Natl. Acad. Sci. USA 2005, 102, 11408–11413. [Google Scholar] [CrossRef] [Green Version]
- Dahlin, J.S.; Hallgren, J. Mast cell progenitors: Origin, development and migration to tissues. Mol. Immunol. 2015, 63, 9–17. [Google Scholar] [CrossRef]
- Kitamura, Y.; Shimada, M.; Hatanaka, K.; Miyano, Y. Development of Mast-Cells from Grafted Bone-Marrow Cells in Irradiated Mice. Nature 1977, 268, 442–443. [Google Scholar] [CrossRef]
- Gurish, M.F.; Austen, K.F. Developmental origin and functional specialization of mast cell subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Pejler, G.; Ronnberg, E.; Waern, I.; Wernersson, S. Mast cell proteases: Multifaceted regulators of inflammatory disease. Blood 2010, 115, 4981–4990. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.J.; Nakae, S.; Tsai, M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005, 6, 135–142. [Google Scholar] [CrossRef]
- Erdei, A.; Andrasfalvy, M.; Peterfy, H.; Toth, G.; Pecht, I. Regulation of mast cell activation by complement-derived peptides. Immunol. Lett. 2004, 92, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Guhl, S.; Artuc, M.; Zuberbier, T. Allergic FcepsilonRI- and pseudo-allergic MRGPRX2-triggered mast cell activation routes are independent and inversely regulated by SCF. Allergy 2018, 73, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Reber, L.L.; Sibilano, R.; Mukai, K.; Galli, S.J. Potential effector and immunoregulatory functions of mast cells in mucosal immunity. Mucosal Immunol. 2015, 8, 444–463. [Google Scholar] [CrossRef] [Green Version]
- Johnzon, C.F.; Ronnberg, E.; Pejler, G. The Role of Mast Cells in Bacterial Infection. Am. J. Pathol. 2016, 186, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Abraham, S.N.; St John, A.L. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef] [Green Version]
- Metz, M.; Piliponsky, A.M.; Chen, C.-C.; Lammel, V.; Åbrink, M.; Pejler, G.; Tsai, M.; Galli, S.J. Mast Cells Can Enhance Resistance to Snake and Honeybee Venoms. Science 2006, 313, 526–530. [Google Scholar] [CrossRef] [Green Version]
- Akahoshi, M.; Song, C.H.; Piliponsky, A.M.; Metz, M.; Guzzetta, A.; Abrink, M.; Schlenner, S.M.; Feyerabend, T.B.; Rodewald, H.R.; Pejler, G.; et al. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J. Clin. Investig. 2011, 121, 4180–4191. [Google Scholar] [CrossRef] [Green Version]
- Steinhoff, M.; Buddenkotte, J.; Lerner, E.A. Role of mast cells and basophils in pruritus. Immunol. Rev. 2018, 282, 248–264. [Google Scholar] [CrossRef]
- Marichal, T.; Tsai, M.; Galli, S.J. Mast cells: Potential positive and negative roles in tumor biology. Cancer Immunol. Res. 2013, 1, 269–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Church, M.K.; Kolkhir, P.; Metz, M.; Maurer, M. The role and relevance of mast cells in urticaria. Immunol. Rev. 2018, 282, 232–247. [Google Scholar] [CrossRef]
- Metz, M.; Grimbaldeston, M.A.; Nakae, S.; Piliponsky, A.M.; Tsai, M.; Galli, S.J. Mast cells in the promotion and limitation of chronic inflammation. Immunol. Rev. 2007, 217, 304–328. [Google Scholar] [CrossRef] [PubMed]
- Bradding, P.; Pejler, G. The controversial role of mast cells in fibrosis. Immunol. Rev. 2018, 282, 198–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karasuyama, H.; Miyake, K.; Yoshikawa, S.; Yamanishi, Y. Multifaceted roles of basophils in health and disease. J. Allergy Clin. Immunol. 2018, 142, 370–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, P.; Singh, B.; Jaggi, A.S.; Singh, N. Mast cells: An expanding pathophysiological role from allergy to other disorders. Naunyn-Schmiedebergs Arch. Pharmacol. 2012, 385, 657–670. [Google Scholar] [CrossRef]
- Shi, M.A.; Shi, G.P. Different roles of mast cells in obesity and diabetes: Lessons from experimental animals and humans. Front. Immunol. 2012, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Gupta, K.; Harvima, I.T. Mast cell-neural interactions contribute to pain and itch. Immunol. Rev. 2018, 282, 168–187. [Google Scholar] [CrossRef]
- Mittal, A.; Sagi, V.; Gupta, M.; Gupta, K. Mast Cell Neural Interactions in Health and Disease. Front. Cell Neurosci. 2019, 13, 110. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, C.; Ishida, Y.; Kitoh, A.; Otsuka, A.; Kabashima, K. Interaction of peripheral nerves and mast cells, eosinophils, and basophils in the development of pruritus. Exp. Dermatol. 2019, 28, 1405–1411. [Google Scholar] [CrossRef] [Green Version]
- Chatterjea, D.; Martinov, T. Mast cells: Versatile gatekeepers of pain. Mol. Immunol. 2015, 63, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stalman, A.; Bring, D.; Ackermann, P.W. Chemokine expression of CCL2, CCL3, CCL5 and CXCL10 during early inflammatory tendon healing precedes nerve regeneration: An immunohistochemical study in the rat. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2682–2689. [Google Scholar] [CrossRef]
- Grutzkau, A.; Kruger-Krasagakes, S.; Baumeister, H.; Schwarz, C.; Kogel, H.; Welker, P.; Lippert, U.; Henz, B.M.; Moller, A. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: Implications for the biological significance of VEGF206. Mol. Biol. Cell 1998, 9, 875–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leon, A.; Buriani, A.; Dal Toso, R.; Fabris, M.; Romanello, S.; Aloe, L.; Levi-Montalcini, R. Mast cells synthesize, store, and release nerve growth factor. Proc. Natl. Acad. Sci. USA 1994, 91, 3739–3743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindstedt, K.A.; Wang, Y.; Shiota, N.; Saarinen, J.; Hyytiainen, M.; Kokkonen, J.O.; Keski-Oja, J.; Kovanen, P.T. Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: A novel function for chymase. FASEB J. 2001, 15, 1377–1388. [Google Scholar] [CrossRef] [Green Version]
- Maltby, S.; Khazaie, K.; McNagny, K.M. Mast cells in tumor growth: Angiogenesis, tissue remodelling and immune-modulation. Biochim. Biophys. Acta 2009, 1796, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Coussens, L.M.; Raymond, W.W.; Bergers, G.; Laig-Webster, M.; Behrendtsen, O.; Werb, Z.; Caughey, G.H.; Hanahan, D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999, 13, 1382–1397. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Y.; Yang, G.; Zhang, Q.; Huang, X.; Yu, L.; Dong, X. Mast cell chymase promotes hypertrophic scar fibroblast proliferation and collagen synthesis by activating TGF-beta1/Smads signaling pathway. Exp. Ther. Med. 2017, 14, 4438–4442. [Google Scholar]
- Lang, Y.D.; Chang, S.F.; Wang, L.F.; Chen, C.M. Chymase mediates paraquat-induced collagen production in human lung fibroblasts. Toxicol. Lett. 2010, 193, 19–25. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhao, L.Y.; Zheng, Q.S.; Su, J.L.; Guan, H.; Shang, F.J.; Niu, X.L.; He, Y.P.; Lu, X.L. Chymase induces profibrotic response via transforming growth factor-beta 1/Smad activation in rat cardiac fibroblasts. Mol. Cell Biochem. 2008, 310, 159–166. [Google Scholar] [CrossRef]
- Hartmann, T.; Ruoss, S.J.; Raymond, W.W.; Seuwen, K.; Caughey, G.H. Human tryptase as a potent, cell-specific mitogen: Role of signaling pathways in synergistic responses. Am. J. Physiol. 1992, 262, L528–L534. [Google Scholar] [CrossRef] [PubMed]
- Ruoss, S.J.; Hartmann, T.; Caughey, G.H. Mast cell tryptase is a mitogen for cultured fibroblasts. J. Clin. Investig. 1991, 88, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Tchougounova, E.; Lundequist, A.; Fajardo, I.; Winberg, J.O.; Abrink, M.; Pejler, G. A key role for mast cell chymase in the activation of promatrix metalloprotease-9 and promatrix metalloprotease-2. J. Biol. Chem. 2005, 280, 9291–9296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saarinen, J.; Kalkkinen, N.; Welgus, H.G.; Kovanen, P.T. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J. Biol. Chem. 1994, 269, 18134–18140. [Google Scholar]
- Gruber, B.L.; Marchese, M.J.; Suzuki, K.; Schwartz, L.B.; Okada, Y.; Nagase, H.; Ramamurthy, N.S. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J. Clin. Investig. 1989, 84, 1657–1662. [Google Scholar] [CrossRef] [Green Version]
- Magarinos, N.J.; Bryant, K.J.; Fosang, A.J.; Adachi, R.; Stevens, R.L.; McNeil, H.P. Mast cell-restricted, tetramer-forming tryptases induce aggrecanolysis in articular cartilage by activating matrix metalloproteinase-3 and -13 zymogens. J. Immunol. 2013, 191, 1404–1412. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, P.W.; Finn, A.; Ahmed, M. Sensory neuropeptidergic pattern in tendon, ligament and joint capsule. A study in the rat. Neuroreport 1999, 10, 2055–2060. [Google Scholar] [CrossRef]
- Lian, O.; Dahl, J.; Ackermann, P.W.; Frihagen, F.; Engebretsen, L.; Bahr, R. Pronociceptive and antinociceptive neuromediators in patellar tendinopathy. Am. J. Sports Med. 2006, 34, 1801–1808. [Google Scholar] [CrossRef]
- Raddant, A.C.; Russo, A.F. Calcitonin gene-related peptide in migraine: Intersection of peripheral inflammation and central modulation. Expert Rev. Mol. Med. 2011, 13, e36. [Google Scholar] [CrossRef] [Green Version]
- Assas, B.M.; Pennock, J.I.; Miyan, J.A. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front. Neurosci. 2014, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Li, W.W.; Guo, T.Z.; Liang, D.Y.; Sun, Y.; Kingery, W.S.; Clark, J.D. Substance P signaling controls mast cell activation, degranulation, and nociceptive sensitization in a rat fracture model of complex regional pain syndrome. Anesthesiology 2012, 116, 882–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.P.; Limjunyawong, N.; Gour, N.; Pundir, P.; Dong, X. A Mast-Cell-Specific Receptor Mediates Neurogenic Inflammation and Pain. Neuron 2019, 101, 412–420.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadi, S.; Alysandratos, K.D.; Angelidou, A.; Miniati, A.; Sismanopoulos, N.; Vasiadi, M.; Zhang, B.; Kalogeromitros, D.; Theoharides, T.C. Substance P (SP) induces expression of functional corticotropin-releasing hormone receptor-1 (CRHR-1) in human mast cells. J. Investig. Dermatol. 2012, 132, 324–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eftekhari, S.; Warfvinge, K.; Blixt, F.W.; Edvinsson, L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J. Pain. 2013, 14, 1289–1303. [Google Scholar] [CrossRef] [Green Version]
- Murphy, P.G.; Hart, D.A. Plasminogen activators and plasminogen activator inhibitors in connective tissues and connective tissue cells: Influence of the neuropeptide substance P on expression. Biochim. Biophys. Acta 1993, 1182, 205–214. [Google Scholar] [CrossRef]
- Hart, D.A.; Archambault, J.M.; Kydd, A.; Reno, C.; Frank, C.B.; Herzog, W. Gender and neurogenic variables in tendon biology and repetitive motion disorders. Clin. Orthop. Relat. Res. 1998, 44–56. [Google Scholar] [CrossRef]
- Le, D.D.; Schmit, D.; Heck, S.; Omlor, A.J.; Sester, M.; Herr, C.; Schick, B.; Daubeuf, F.; Fahndrich, S.; Bals, R.; et al. Increase of Mast Cell-Nerve Association and Neuropeptide Receptor Expression on Mast Cells in Perennial Allergic Rhinitis. Neuroimmunomodulation 2016, 23, 261–270. [Google Scholar] [CrossRef]
- Bring, D.K.; Reno, C.; Renstrom, P.; Salo, P.; Hart, D.A.; Ackermann, P.W. Joint immobilization reduces the expression of sensory neuropeptide receptors and impairs healing after tendon rupture in a rat model. J. Orthop. Res. 2009, 27, 274–280. [Google Scholar] [CrossRef]
- Okayama, Y.; Ono, Y.; Nakazawa, T.; Church, M.K.; Mori, M. Human skin mast cells produce TNF-alpha by substance P. Int. Arch. Allergy Immunol. 1998, 117, 48–51. [Google Scholar] [CrossRef]
- Kulka, M.; Sheen, C.H.; Tancowny, B.P.; Grammer, L.C.; Schleimer, R.P. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008, 123, 398–410. [Google Scholar] [CrossRef]
- Piotrowski, W.; Foreman, J.C. Some effects of calcitonin gene-related peptide in human skin and on histamine release. Br. J. Dermatol. 1986, 114, 37–46. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Schwengberg, S.; Lorentz, A.; Manns, M.P.; Bektas, H.; Sann, H.; Levi-Schaffer, F.; Shanahan, F.; Schemann, M. Substance P and other neuropeptides do not induce mediator release in isolated human intestinal mast cells. Neurogastroenterol. Motil. 2004, 16, 185–193. [Google Scholar] [CrossRef]
- Kashiba, H.; Senba, E. Primary sensory neurons expressing histamine H1-receptor mRNA. Folia Pharmacol. Jpn. 2001, 118, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashiba, H.; Fukui, H.; Morikawa, Y.; Senba, E. Gene expression of histamine H1 receptor in guinea pig primary sensory neurons: A relationship between H1 receptor mRNA-expressing neurons and peptidergic neurons. Brain Res. Mol. Brain Res. 1999, 66, 24–34. [Google Scholar] [CrossRef]
- Morales, M.; Wang, S.D. Differential composition of 5-hydroxytryptamine3 receptors synthesized in the rat CNS and peripheral nervous system. J. Neurosci. 2002, 22, 6732–6741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amenta, F.; Ricci, A.; Tayebati, S.K.; Zaccheo, D. The peripheral dopaminergic system: Morphological analysis, functional and clinical applications. Ital. J. Anat. Embryol. 2002, 107, 145–167. [Google Scholar] [PubMed]
- Akers, I.A.; Parsons, M.; Hill, M.R.; Hollenberg, M.D.; Sanjar, S.; Laurent, G.J.; McAnulty, R. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. Am. J. Physiol. Cell. Mol. Physiol. 2000, 278, L193–L201. [Google Scholar] [CrossRef]
- Saito, T.; Bunnett, N.W. Protease-activated receptors—Regulation of neuronal function. Neuromol. Med. 2005, 7, 79–99. [Google Scholar] [CrossRef]
- Akula, S.; Paivandy, A.; Fu, Z.; Thorpe, M.; Pejler, G.; Hellman, L. Quantitative In-Depth Analysis of the Mouse Mast Cell Transcriptome Reveals Organ-Specific Mast Cell Heterogeneity. Cells 2020, 9, 211. [Google Scholar] [CrossRef] [Green Version]
- Motakis, E.; Guhl, S.; Ishizu, Y.; Itoh, M.; Kawaji, H.; de Hoon, M.; Lassmann, T.; Carninci, P.; Hayashizaki, Y.; Zuberbier, T.; et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 2014, 123, e58–e67. [Google Scholar] [CrossRef]
- Caughey, G.H.; Leidig, F.; Viro, N.F.; Nadel, J.A. Substance P and vasoactive intestinal peptide degradation by mast cell tryptase and chymase. J. Pharmacol. Exp. Ther. 1988, 244, 133–137. [Google Scholar] [PubMed]
- Tam, E.K.; Caughey, G.H. Degradation of airway neuropeptides by human lung tryptase. Am. J. Respir Cell Mol. Biol. 1990, 3, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Reber, L.L.; Frossard, N. Targeting mast cells in inflammatory diseases. Pharmacol. Ther. 2014, 142, 416–435. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Hueber, A.J.; Reilly, J.H.; Xu, Y.; Fazzi, U.G.; Murrell, G.A.; McInnes, I.B. Inflammation is present in early human tendinopathy. Am. J. Sports Med. 2010, 38, 2085–2091. [Google Scholar] [CrossRef]
- Matthews, T.J.; Hand, G.C.; Rees, J.L.; Athanasou, N.A.; Carr, A.J. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J. Bone Jt. Surg. Br. 2006, 88, 489–495. [Google Scholar] [CrossRef]
- Berglund, M.E.; Hildebrand, K.A.; Zhang, M.; Hart, D.A.; Wiig, M.E. Neuropeptide, mast cell, and myofibroblast expression after rabbit deep flexor tendon repair. J. Hand Surg. Am. 2010, 35, 1842–1849. [Google Scholar] [CrossRef]
- Nedergaard, M.; Takano, T.; Hansen, A.J. Beyond the role of glutamate as a neurotransmitter. Nat. Rev. Neurosci. 2002, 3, 748–755. [Google Scholar] [CrossRef]
- Swanson, C.J.; Bures, M.; Johnson, M.P.; Linden, A.M.; Monn, J.A.; Schoepp, D.D. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat. Rev. Drug Discov. 2005, 4, 131–144. [Google Scholar] [CrossRef]
- Celli, R.; Santolini, I.; Van Luijtelaar, G.; Ngomba, R.T.; Bruno, V.; Nicoletti, F. Targeting metabotropic glutamate receptors in the treatment of epilepsy: Rationale and current status. Expert Opin. Ther. Targets 2019, 23, 341–351. [Google Scholar] [CrossRef]
- Fazio, F.; Ulivieri, M.; Volpi, C.; Gargaro, M.; Fallarino, F. Targeting metabotropic glutamate receptors for the treatment of neuroinflammation. Curr. Opin. Pharmacol. 2018, 38, 16–23. [Google Scholar] [CrossRef]
- Kalsi, G.; Whiting, P.; Bourdelles, B.L.; Callen, D.; Barnard, E.A.; Gurling, H. Localization of the human NMDAR2D receptor subunit gene (GRIN2D) to 19q13.1-qter, the NMDAR2A subunit gene to 16p13.2 (GRIN2A), and the NMDAR2C subunit gene (GRIN2C) to 17q24-q25 using somatic cell hybrid and radiation hybrid mapping panels. Genomics 1998, 47, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, C.; Venkatesan, C.; Go, C.G.; Mong, J.A.; Dawson, T.M. Cellular and subcellular localization of NMDA-R1 subunit immunoreactivity in the visual cortex of adult and neonatal rats. J. Neurosci. 1994, 14, 5202–5222. [Google Scholar] [CrossRef] [PubMed]
- Janssens, N.; Lesage, A.S. Glutamate receptor subunit expression in primary neuronal and secondary glial cultures. J. Neurochem. 2001, 77, 1457–1474. [Google Scholar] [CrossRef]
- Fan, D.; Grooms, S.Y.; Araneda, R.C.; Johnson, A.B.; Dobrenis, K.; Kessler, J.A.; Zukin, R.S. AMPA receptor protein expression and function in astrocytes cultured from hippocampus. J. Neurosci. Res. 1999, 57, 557–571. [Google Scholar] [CrossRef]
- Spreafico, R.; Frassoni, C.; Arcelli, P.; Battaglia, G.; Wenthold, R.J.; De Biasi, S. Distribution of AMPA selective glutamate receptors in the thalamus of adult rats and during postnatal development. A light and ultrastructural immunocytochemical study. Dev. Brain Res. 1994, 82, 231–244. [Google Scholar] [CrossRef]
- Werner, P.; Voigt, M.; Keinanen, K.; Wisden, W.; Seeburg, P.H. Cloning of a putative high-affinity kainate receptor expressed predominantly in hippocampal CA3 cells. Nature 1991, 351, 742–744. [Google Scholar] [CrossRef]
- Contractor, A.; Swanson, G.T.; Sailer, A.; O’Gorman, S.; Heinemann, S.F. Identification of the kainate receptor subunits underlying modulation of excitatory synaptic transmission in the CA3 region of the hippocampus. J. Neurosci. 2000, 20, 8269–8278. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.; Alfredson, H.; Forsgren, S. VGluT2 expression in painful Achilles and patellar tendinosis: Evidence of local glutamate release by tenocytes. J. Orthop. Res. 2008, 26, 685–692. [Google Scholar] [CrossRef]
- Greve, K.; Labruto, F.; Edman, G.; Bring, D.; Nilsson, G.; Ackermann, P.W.; Domeij-Arverud, E. Metabolic activity in early tendon repair can be enhanced by intermittent pneumatic compression. Scand. J. Med. Sci. Sports 2012, 22, e55–e63. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Kazey, V.I.; Leinsoo, T.A.; Mashkina, A.P.; Tyulina, O.V.; Johnson, P.; Tuneva, J.O.; Chittur, S.; Carpenter, D.O. Rodent lymphocytes express functionally active glutamate receptors. Biochem. Biophys. Res. Commun. 2004, 324, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.L.; Diemel, L.T.; Cuzner, M.L.; Pocock, J.M. Activation of group II metabotropic glutamate receptors underlies microglial reactivity and neurotoxicity following stimulation with chromogranin A, a peptide up-regulated in Alzheimer’s disease. J. Neurochem. 2002, 82, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Miglio, G.; Varsaldi, F.; Lombardi, G. Human T lymphocytes express N-methyl-D-aspartate receptors functionally active in controlling T cell activation. Biochem. Biophys. Res. Commun. 2005, 338, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Pocock, J.M.; Kettenmann, H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007, 30, 527–535. [Google Scholar] [CrossRef]
- Hamasato, E.K.; Ligeiro-De-Oliveira, A.P.; Lino-Dos-Santos-Franco, A.; Ribeiro, A.; De Paula, V.F.; Peron, J.P.S.; Damazo, A.S.; Tavares-De-Lima, W.; Neto, J.P. Effects of MK-801 and amphetamine treatments on allergic lung inflammatory response in mice. Int. Immunopharmacol. 2013, 16, 436–443. [Google Scholar] [CrossRef]
- Wagner, E.F. Bone development and inflammatory disease is regulated by AP-1 (Fos/Jun). Ann. Rheum Dis. 2010, 69, i86–i88. [Google Scholar] [CrossRef]
- Eliasson, P.; Andersson, T.; Hammerman, M.; Aspenberg, P. Primary gene response to mechanical loading in healing rat Achilles tendons. J. Appl. Physiol. (1985) 2013, 114, 1519–1526. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, J.B.; Jin, M.; Chai, D.H.; Siparsky, P.; Fanning, P.; Grodzinsky, A.J. Shear- and compression-induced chondrocyte transcription requires MAPK activation in cartilage explants. J. Biol. Chem. 2008, 283, 6735–6743. [Google Scholar] [CrossRef] [Green Version]
- Ohnishi, Y.N.; Sakumi, K.; Yamazaki, K.; Ohnishi, Y.H.; Miura, T.; Tominaga, Y.; Nakabeppu, Y. Antagonistic regulation of cell-matrix adhesion by FosB and DeltaFosB/Delta2DeltaFosB encoded by alternatively spliced forms of fosB transcripts. Mol. Biol. Cell 2008, 19, 4717–4729. [Google Scholar] [CrossRef] [Green Version]
- Shirai, K.; Okada, Y.; Saika, S.; Senba, E.; Ohnishi, Y. Expression of transcription factor AP-1 in rat lens epithelial cells during wound repair. Exp. Eye Res. 2001, 73, 461–468. [Google Scholar] [CrossRef]
- Rangaswami, H.; Marathe, N.; Zhuang, S.; Chen, Y.; Yeh, J.C.; Frangos, J.A.; Boss, G.R.; Pilz, R.B. Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction. J. Biol. Chem. 2009, 284, 14796–14808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crupi, R.; Impellizzeri, D.; Cuzzocrea, S. Role of Metabotropic Glutamate Receptors in Neurological Disorders. Front. Mol. Neurosci. 2019, 12, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alim, M.A.; Peterson, M.; Pejler, G. Do Mast Cells Have a Role in Tendon Healing and Inflammation? Cells 2020, 9, 1134. https://doi.org/10.3390/cells9051134
Alim MA, Peterson M, Pejler G. Do Mast Cells Have a Role in Tendon Healing and Inflammation? Cells. 2020; 9(5):1134. https://doi.org/10.3390/cells9051134
Chicago/Turabian StyleAlim, Md Abdul, Magnus Peterson, and Gunnar Pejler. 2020. "Do Mast Cells Have a Role in Tendon Healing and Inflammation?" Cells 9, no. 5: 1134. https://doi.org/10.3390/cells9051134
APA StyleAlim, M. A., Peterson, M., & Pejler, G. (2020). Do Mast Cells Have a Role in Tendon Healing and Inflammation? Cells, 9(5), 1134. https://doi.org/10.3390/cells9051134




