Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration
Abstract
:1. Introduction
2. MSCs as Sources of Exosomes
3. Quality Control of EVs for Development of Therapeutic EVs
3.1. EV Quantity and Size
3.2. EV Identity
3.3. EV Purity
3.4. Potency Assays
4. Anti-Inflammation and Immunomodulation by MSC-Exosomes
4.1. Macrophage Polarization
4.2. T Cell Regulation
4.3. Inflammation in Skin
4.4. Immunomodulation in Other Inflammatory Diseases
5. Anti-Aging Effects of MSC-Exosomes
5.1. EVs in Senescence
5.2. Anti-Aging Effects
6. Cutaneous Wound Healing by MSC-Exosomes
6.1. Homeostasis Phase
6.2. Inflammatory Phase
6.3. Proliferative Phase
6.4. Remodeling Phase
6.5. Proteolytic Environment
6.6. Animal Models
6.7. ASC-Exosomes
7. MSC-Exosome-Induced Hair Growth
7.1. The Effects of DP-Exosomes on Hair Cells
7.2. The Effects of MSC-Exosomes on Hair Growth
8. Repair and Regeneration of Skin barrier by MSC-Exosomes
8.1. Skin Barrier
8.2. The Effects of ASC-Exosomes on Skin Barrier
9. Application of MSC-Exosomes for Regenerative Aesthetics
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yi, Y.W.; Lee, J.H.; Kim, S.Y.; Pack, C.G.; Ha, D.H.; Park, S.R.; Youn, J.; Cho, B.S. Advances in analysis of biodistribution of exosomes by molecular imaging. Int. J. Mol. Sci. 2020, 21, 665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar]
- Wang, Y.; Wang, Q.; Wei, X.; Shao, J.; Zhao, J.; Zhang, Z.; Chen, Z.; Bai, Y.; Wang, N.; Wang, Y.; et al. Global scientific trends on exosome research during 2007–2016: A bibliometric analysis. Oncotarget 2017, 8, 48460–48470. [Google Scholar] [PubMed] [Green Version]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmers, L.; Lim, S.K.; Arslan, F.; Armstrong, J.S.; Hoefer, I.E.; Doevendans, P.A.; Piek, J.J.; El Oakley, R.M.; Choo, A.; Lee, C.N.; et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2008, 1, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Zipkin, M. Exosome redux. Nat. Biotechnol. 2019, 37, 1395–1400. [Google Scholar] [CrossRef]
- Hildreth, C. Top 4 Most Richly Funded Exosome Startups. BioInformant. Available online: https://bioinformant.com/top-exosome-companies/ (accessed on 13 December 2019).
- Cross, R. Meet the Exosome, the Rising Star in Delivery. Chemical & Engineering News. 30 July 2018. Available online: https://cen.acs.org/business/start-ups/Meet-exosome-rising-star-drug/96/i31 (accessed on 13 December 2019).
- Plieth, J.; Armstrong, M. Exosomes Start to Deliver Deals. Vantage. 28 January 2019. Available online: https://www.evaluate.com/vantage/articles/news/snippets/exosomes-start-deliver-deals (accessed on 13 December 2019).
- Deatherage, B.L.; Cookson, B.T. Membrane vesicle release in bacterial, eukaryotes, and archeas: A conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012, 80, 1948–1957. [Google Scholar] [CrossRef] [Green Version]
- Cunnane, E.M.; Weinbaum, J.S.; O’Brien, F.J.; Dorp, D.A. Future perspective on the role of stem cells and extracellular vesicles in vascular tissue regeneration. Front. Cardiovasc. Med. 2018, 5, 86. [Google Scholar] [CrossRef] [Green Version]
- Koniusz, S.; Andrzejewsk, A.; Muraca, M.; Srivastava, A.K.; Janowski, M.; Lukomska, B. Extracellular vesicels in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Front. Cell. Neurosci. 2016, 10, 109. [Google Scholar] [CrossRef]
- Corso, G.; Mager, I.; Lee, Y.; Gorgens, A.; Bultema, J.; Giebel, B.; Wood, M.J.A.; Nordin, J.Z.; El Andaloussi, S. Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography. Sci. Rep. 2017, 7, 11561. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Sun, X.; Liu, L.; Jiang, H.; Shen, Y.; Xu, X.; Li, J.; Zhang, G.; Huang, J.; Lin, Z.; et al. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int. 2016, 2016, 7653489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspective. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phinney, D.G.; Pittenger, M.F. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; Oakley, R.M.E.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef]
- Cho, B.S.; Kim, J.O.; Ha, D.H.; Yi, Y.W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res. Ther. 2018, 9, 187. [Google Scholar] [CrossRef] [Green Version]
- Nooshabadi, V.T.; Mardpour, S.; Yousefi-Ahmadipour, A.; Allahverdi, A.; Izadpanah, M.; Daneshimehr, F.; Ai, J.; Banafshe, H.R.; Ebrahimi-Barough, S. The extracellular vesicles-derived from mesenchymal stromal cells: A new therapeutic option in regenerative medicine. J. Cell. Biochem. 2018, 119, 8048–8073. [Google Scholar] [CrossRef]
- Mendt, M.; Rezvani, K.; Shpall, E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019, 54, 789–792. [Google Scholar] [CrossRef]
- Phinney, D.G.; Prockop, D.J. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair-current views. Stem Cells 2007, 25, 2896–2902. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise review, mesenchymal stem cells, from roots to boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barberi, T.; Willis, L.M.; Socci, N.D.; Studer, L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005, 2, e161. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Hematti, P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp. Hematol. 2008, 36, 350–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabapathy, V.; Kumar, S. hiPSC-derived iMSCs, NextGen MSCs as an advanced therapeutically active cell resource for regenerative medicine. J. Cell. Mol. Med. 2016, 20, 1571–1588. [Google Scholar] [CrossRef] [Green Version]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Elahi, F.M.; Farwell, D.G.; Nolta, J.A.; Anderson, J.D. Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells 2019. [Google Scholar] [CrossRef] [Green Version]
- Katsuda, T.; Tsuchiya, R.; Kosaka, N.; Yoshioka, Y.; Takagaki, K.; Oki, K.; Takeshita, F.; Sakai, Y.; Kuroda, M.; Ochiya, T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep. 2013, 3, 1197. [Google Scholar] [CrossRef] [Green Version]
- Del Fattore, A.; Luciano, R.; Saracino, R.; Battafarano, G.; Rizzo, C.; Pascucci, L.; Alessandri, G.; Pessina, A.; Perrotta, A.; Fierabracci, A.; et al. Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert. Opin. Biol. Ther. 2015, 15, 495–504. [Google Scholar] [CrossRef]
- Lopez-Verrilli, M.A.; Caviedes, A.; Cabrera, A.; Sandoval, S.; Wyneken, U.; Khoury, M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 2016, 21, 129–139. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Villatoro, A.J.; Alcoholado, C.; Martín-Astorga, M.C.; Fernández, V.; Cifuentes, M.; Becerra, J. Comparative analysis and characterization of soluble factors and exosomes from cultured adipose tissue and bone marrow mesenchymal stem cells in canine species. Vet. Immunol. Immunopathol. 2019, 208, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Tracy, S.A.; Ahmed, A.; Tigges, J.C.; Ericsson, M.; Pal, A.K.; Zurakowski, D.; Fauza, D.O. A comparison of clinically relevant sources of mesenchymal stem cell-derived exosomes, bone marrow and amniotic fluid. J. Pediatr. Surg. 2019, 54, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Vakhshiteh, F.; Atyabi, F.; Ostad, S.N. Mesenchymal stem cell exosomes: A two-edged sword in cancer therapy. Int. J. Nanomed. 2019, 14, 2847–2859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahara, K.; Li, M.; Inamoto, T.; Nakagawa, T.; Ibuki, N.; Yoshikawa, Y.; Tsujino, T.; Uchimoto, T.; Saito, K.; Takai, T.; et al. microRNA-145 mediates the inhibitory effect of adipose tissue-derived stromal cells on prostate cancer. Stem Cells Dev. 2016, 25, 1290–1298. [Google Scholar] [CrossRef]
- Willis, G.R.; Mitsialis, S.A.; Kourembanas, S. “Good things come in small packages”: Application of exosome-based therapeutics in neonatal lung injury. Pediat. Res. 2018, 83, 298–307. [Google Scholar] [CrossRef]
- Pachler, K.; Ketteri, N.; Desgeorges, A.; Dunai, Z.A.; Laner-Plamberger, S.; Streif, D.; Strunk, D.; Rohde, E.; Gimona, M. An in vitro potency assay for monitoring the immunomodulatory potential of stromal cell-derived extracellular vesicles. Int. J. Mol. Sci. 2017, 18, 1413. [Google Scholar] [CrossRef]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef]
- Whitford, W.; Guterstam, P. Exosome manufacturing status. Future Med. Chem. 2019, 11, 1225–1236. [Google Scholar] [CrossRef] [Green Version]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, e99263. [Google Scholar] [CrossRef]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions, a position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Witwer, K.W.; Soekmadji, C.; Hill, A.F.; Wauben, M.H.; Buzas, E.I.; Di Vizio, D.; Falcon-Perez, J.M.; Gardiner, C.; Hochberg, F.; Kurochkin, I.V.; et al. Updating the minimal requirements for extracellular vesicle studies, building bridges to reproducibility. J. Extracell. Vesicles 2017, 6, 1396823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018), a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cell and Gene Therapy Products Division, Biopharmaceutical and Herbal Medicine Evaluation Department, National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety. Guideline on Quality, Non-Clinical and Clinical Assessment of Extracellular Vesicles Therapy Products. 2018. Available online: www.nifds.go.kr/brd/m_15/down.do?brd_id=167&seq=12625&data_tp=A&file_seq=1 (accessed on 13 December 2019).
- Pachler, K.; Lener, T.; Streif, D.; Dunai, Z.A.; Desgeorges, A.; Feichtner, M.; Öller, M.; Schallmoser, K.; Rohde, E.; Gimona, M. A good manufacturing practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy 2017, 19, 458–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andriolo, G.; Provasi, E.; Lo Cicero, V.; Brambilla, A.; Soncin, S.; Torre, T.; Milano, G.; Biemmi, V.; Vassalli, G.; Turchetto, L.; et al. Exosomes from human cardiac progenitor cells for therapeutic applications, development of a GMP-grade manufacturing method. Front. Physiol. 2018, 9, 1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koritzinsky, E.H.; Street, J.M.; Star, R.A.; Yuen, P.S. Quantification of Exosome. J. Cell. Physiol. 2018, 232, 1587–1590. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Alvarez, V.A. Advances in magnetic noble metal/iron-based oxide hybrid nanoparticles as biomedical devices. Bioengineering 2019, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, M.; Amorim, M.G.; Gadelha, C.; Milic, I.; Welsh, J.A.; Freitas, V.M.; Nawaz, M.; Akbar, N.; Couch, Y.; Makin, L.; et al. Technical challenges of working with extracellular vesicles. Nanoscale 2018, 10, 881–906. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research—Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef] [Green Version]
- Bachurski, D.; Schuldner, M.; Nguyen, P.H.; Malz, A.; Reiners, K.S.; Grenzi, P.C.; Babatz, F.; Schauss, A.C.; Hansen, H.P.; Hallek, M.; et al. Extracellular vesicle measurements with nanoparticle tracking analysis—An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. J. Extracell. Vesicles 2019, 8, 1596016. [Google Scholar] [CrossRef]
- van der Pol, E.; Coumans, F.A.; Grootemaat, A.E.; Gardiner, C.; Sargent, I.L.; Harrison, P.; Sturk, A.; van Leeuwen, T.G.; Nieuwland, R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J. Thromb. Haemost. 2014, 12, 1182–1192. [Google Scholar] [CrossRef]
- Vestad, B.; Llorente, A.; Neurauter, A.; Phuyal, S.; Kierulf, B.; Kierulf, P.; Skotland, T.; Sandvig, K.; Haug, K.B.F.; Ovstebo, R. Size and concentration analyses of extracellular vesicles by nanoparticle tracking analysis, a variation study. J. Extracell. Vesicles 2017, 6, 1344087. [Google Scholar] [CrossRef] [PubMed]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The methods of choice for extracellular vesicles (EVs) characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Nejadnik, M.R.; Baunsgaard, D.; Henriksen, A.; Rischel, C.; Jiskoot, W. A comprehensive evaluation of nanoparticle tracking analysis (NanoSight) for characterization of proteinaceous submicron particles. J. Pharm. Sci. 2016, 105, 3366–3375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnell-Morris, P.; Tannetta, D.; Siupa, A.; Hole, P.; Dragovic, R. Analysis of extracellular vesicles using fluorescence nanoparticle tracking analysis. Methods Mol. Biol. 2017, 1660, 153–173. [Google Scholar] [PubMed]
- Ma, L.; Zhu, S.; Tian, Y.; Zhang, W.; Wang, S.; Chen, C.; Wu, L.; Yan, X. Label-free analysis of single viruses with a resolution comparable to that of electron microscopy and the throughput of flow cytometry. Angew. Chem. Int. Ed. Engl. 2016, 55, 10239–10243. [Google Scholar] [CrossRef] [PubMed]
- Danielson, K.M.; Estanislau, J.; Tigges, J.; Toxavidis, V.; Camacho, V.; Felton, E.J.; Khoory, J.; Kreimer, S.; Ivanov, A.R.; Mantel, P.Y.; et al. Diurnal variations of circulating extracellular vesicles measured by nano flow cytometry. PLoS ONE 2016, 11, e0144678. [Google Scholar] [CrossRef]
- Nizamudeen, Z.; Markus, R.; Lodge, R.; Parmenter, C.; Platt, M.; Chakrabarti, L.; Sottile, V. Rapid and accurate analysis of stem cell-derived extracellular vesicles with super resolution microscopy and live imaging. Biochim. Biophys. Acta. Mol. Cell. Res. 2018, 1865, 1891–1900. [Google Scholar] [CrossRef]
- Kabe, Y.; Suematsu, M.; Sakamoto, S.; Hirai, M.; Koike, I.; Hishiki, T.; Matsuda, A.; Hasegawa, Y.; Tsujita, K.; Ono, M.; et al. Development of a highly sensitive device for counting the number of disease-specific exosomes in human sera. Clin. Chem. 2018, 64, 1463–1473. [Google Scholar] [CrossRef] [Green Version]
- Gorgens, A.; Bremer, M.; Ferrer-Tur, R.; Murke, F.; Tertel, T.; Horn, P.A.; Thalmann, S.; Welsh, J.A.; Probst, C.; Guerin, C.; et al. Optimisation of imaging flow cytometry for the analysis of single extracellular vesicles by using florescence-tagged vesicles as biological reference material. J. Extracell. Vesicles 2019, 8, 1597567. [Google Scholar]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Nat. Acad. Sci. USA 2016, 113, E977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; El Andaloussi, S.; Wood, M.J. Exosomes and microvesicles, extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, T.A.; Paul, J.W.; Chan, E.C.; Smith, R.; Tolosa, J.M. Misleading westerns, common quantification mistakes in western blot densitometry and proposed corrective measures. Biomed. Res. Int. 2019, 2019, 5214821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Center for Biologics Evaluation and Research, Food and Drug Administration, US Department of Health and Human Services. Guidance for Industry, Potency Tests for Cellular and Gene Therapy Products. Available online: www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (accessed on 21 December 2019).
- Wills, G.R.; Kourembanas, S.; Mitsiallis, S.A. Toward exosome-based therapeutics, isolation, heterogeneity, and fit-for-purpose potency. Front. Cardiovasc. Med. 2017, 4, 63. [Google Scholar] [CrossRef] [Green Version]
- Pacienza, N.; Lee, R.H.; Bae, E.H.; Kim, D.K.; Liu, Q.; Prockop, D.J.; Yannarelli, G. In vitro macrophage assay predicts the in vivo anti-inflammatory potential of exosomes from human mesenchymal stromal cells. Mol. Ther. Methods Clin. Dev. 2018, 13, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Blazquez, R.; Sanchez-Margallo, F.M.; de la Rosa, O.; Dalemans, W.; Alvarez, V.; Tarazona, R.; Casado, J.G. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front. Immunol. 2014, 5, 556. [Google Scholar] [CrossRef] [Green Version]
- Conforti, A.; Scarsella, M.; Starc, N.; Giorda, E.; Biagini, S.; Proia, A.; Carsetti, R.; Locatelli, F.; Bernardo, M.E. Microvesicles derived from mesenchymal stromal cells are not as effective as their cellular counterpart in the ability to modulate immune responses in vitro. Stem Cells Dev. 2014, 23, 2591–2599. [Google Scholar] [CrossRef] [Green Version]
- Gouveia de Andrade, A.V.; Bertolino, G.; Riewaldt, J.; Bieback, K.; Karbanova, J.; Odendahl, M.; Bornhauser, M.; Schmitz, M.; Corbeil, D.; Tonn, T. Extracellular vesicles secreted by bone marrow- and adipose tissue-derived mesenchymal stromal cells fail to suppress lymphocyte proliferation. Stem Cells Dev. 2015, 24, 1374–1376. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inflammation. Wikipedia. Available online: en.wikipedia.org/wiki/Inflammation (accessed on 23 December 2019).
- Inflammatory Diseases. Nature. Available online: www.nature.com/subjects/inflammatory-diseases (accessed on 23 December 2019).
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of inflammation, what controls its onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inflammation, the Root Cause of all Disease? Available online: www.alkaway.com.au/blog/inflammation-the-root-cause-of-all-disease/ (accessed on 22 December 2019).
- Hunter, P. The inflammation theory of disease. The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO Rep. 2012, 13, 968–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.L.; Sung, P.H.; Chen, K.H.; Shao, P.L.; Yang, C.C.; Cheng, B.C.; Lin, K.C.; Chen, C.H.; Chai, H.T.; Chang, H.W.; et al. Adipose-derived mesenchymal stem cell-derived exosomes alleviate overwhelming systemic inflammatory reaction and organ damage and improve outcome in rat sepsis syndrome. Am. J. Transl. Res. 2018, 10, 1053–1070. [Google Scholar] [PubMed]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef] [Green Version]
- Börger, V.; Bremer, M.; Ferrer-Tur, R.; Gockeln, L.; Stambouli, O.; Becic, A.; Giebel, B. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int. J. Mol. Sci. 2017, 18, 1450. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef] [Green Version]
- Willis, G.R.; Fernandez-Gonzalez, A.; Anastas, J.; Vitali, S.H.; Liu, X.; Ericsson, M.; Kwong, A.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am. J. Respir. Crit. Care Med. 2018, 197, 104–116. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, L.; Yang, J.; Yu, Y.; Chai, J.; Wang, L.; Ma, L.; Yin, H. Exosome derived from human umbilical cord mesenchymal stem cell dedicates MiR-181c attenuating burn-induced excessive inflammation. EbioMedicine 2016, 8, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Dalirfardouei, R.; Jamialahmadi, K.; Jafarian, A.H.; Mahdipour, E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J. Tissue. Eng. Regen. Med. 2018, 13, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xue, H.; Li, T.; Chu, X.; Xin, D.; Xiong, Y.; Qiu, W.; Gao, X.; Qian, M.; Xu, J.; et al. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE-/- mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem. Biophys. Res. Commun. 2019, 510, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Liang, Z.; Wang, F.; Zhou, C.; Zheng, X.; Hu, T.; He, X.; Wu, X.; Lan, P. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight 2019, 4, 131273. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; Zhou, X.; Ge, Z.; Song, Y.; Wang, H.; Liu, X.; Zhang, D. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int. J. Biochem. Cell. Biol. 2019, 114, 105564. [Google Scholar] [CrossRef]
- Heo, J.S.; Choi, Y.; Kim, H.O. Adipose-derived mesenchymal stem cells promote M2 macrophage phenotype through exosomes. Stem Cells Int. 2019, 2019, 7921760. [Google Scholar] [CrossRef]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Huang, Y.; Han, J.; Yu, L.; Li, Y.; Lu, Z.; Li, H.; Liu, Z.; Shi, C.; Duan, F.; et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 2016, 64, 831–840. [Google Scholar] [CrossRef]
- Du, Y.M.; Zhuansun, Y.X.; Chen, R.; Lin, L.; Lin, Y.; Li, J.G. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp. Cell Res. 2018, 363, 114–120. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, L.; Liang, Y.; Guo, Z.; Wang, L.; Ma, C.; Wang, H. Exosomes originating from MSCs stimulated with TGF-β and IFN-γ promote Treg differentiation. J. Cell. Physiol. 2018, 233, 6832–6840. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, Y.; Lai, R.C.; Tan, S.S.; Choo, A.B.; Lim, S.K. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014, 23, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Nojehdehi, S.; Soudi, S.; Hesampour, A.; Rasouli, S.; Soleimani, M.; Hashemi, S.M. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J. Cell. Biochem. 2018, 119, 9433–9443. [Google Scholar] [CrossRef] [PubMed]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lässer, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.V.; Hutchins, E.; Hamamoto, A.; et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Uemoto, S.; Tabata, Y. Immunosuppressive effect of mesenchymal stem cell-derived exosomes on a concanavalin A-induced liver injury model. Inflamm. Regen. 2016, 36, 26. [Google Scholar] [CrossRef] [Green Version]
- Fattore, A.D.; Luciano, R.; Pascucci, L.; Goffredo, B.M.; Giorda, E.; Scapaticci, M.; Fierabracci, A.; Muraca, M. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant. 2015, 24, 2615–2627. [Google Scholar] [CrossRef] [Green Version]
- Trapani, M.D.; Bassi, G.; Midolo, M.; Gatti, A.; Kamga, P.T.; Cassaro, A.; Carusone, R.; Adamo, A.; Krampera, M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Roura, S.; Gálvez-Montón, C.; Pujal, J.M.; Aran, G.; Sanjurjo, L.; Franquesa, M.; Sarrias, M.R.; Bayes-Genis, A.; Borràs, F.E. Nanosized UCMSC-derived extracellular vesicles but not conditioned medium exclusively inhibit the inflammatory response of stimulated T cells, implications for nanomedicine. Theranostics 2017, 7, 270–284. [Google Scholar] [CrossRef]
- Khare, D.; Or, R.; Resnick, I.; Barkatz, C.; Almogi-Hazan, O.; Avni, B. Mesenchymal stromal cell-derived exosomes affect mRNA expression and function of B-lymphocytes. Front. Immunol. 2018, 9, 3053. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Li, Z.; Cores, J.; Huang, K.; Su, T.; Dinh, P.U.; Cheng, K. Needle-Free Injection of Exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ACS Nano 2019, 13, 11273–11282. [Google Scholar] [CrossRef]
- Bai, Y.; Han, Y.D.; Yan, X.L.; Ren, J.; Zeng, Q.; Li, X.D.; Pei, X.T.; Han, Y. Adipose mesenchymal stem cell-derived exosomes stimulated by hydrogen peroxide enhanced skin flap recovery in ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 2018, 500, 310–317. [Google Scholar] [CrossRef]
- Shin, K.O.; Ha, D.H.; Kim, J.O.; Crumrine, D.A.; Meyer, J.M.; Kim, H.K.; Lee, J.; Kwon, H.H.; Park, G.H.; Lee, J.H.; et al. Exosomes from human adipose tissue-derived mesenchymal stem cells promote epidermal barrier repair by inducing de novo synthesis of ceramides in atopic dermatitis. Cells 2020, 9, 680. [Google Scholar] [CrossRef] [Green Version]
- Alzahrani, F.A. Melatonin improves therapeutic potential of mesenchymal stem cells-derived exosomes against renal ischemia-reperfusion injury in rats. Am. J. Transl. Res. 2019, 11, 2887–2907. [Google Scholar]
- Shen, B.; Liu, J.; Zhang, F.; Wang, Y.; Qin, Y.; Zhou, Z.; Qiu, J.; Fan, Y. CCR2 Positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int. 2016, 2016, 1240301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Jia, H.; Zhang, B.; Wang, J.; Ji, C.; Zhu, X.; Yan, Y.; Yin, L.; Yu, J.; Qian, H.; et al. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res. Ther. 2017, 8, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, L.; Shao, H.; Wang, H.; Zhang, Z.; Su, C.; Dong, L.; Yu, B.; Chen, X.; Li, X.; Zhang, X. Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci. Rep. 2017, 7, 4323. [Google Scholar] [CrossRef] [Green Version]
- Bier, A.; Berenstein, P.; Kronfeld, N.; Morgoulis, D.; Ziv-Av, A.; Goldstein, H.; Kazimirsky, G.; Cazacu, S.; Meir, R.; Popovtzer, R.; et al. Placenta-derived mesenchymal stromal cells and their exosomes exert therapeutic effects in Duchenne muscular dystrophy. Biomaterials 2018, 174, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, S.; Thueson, S.; Ponnalagu, D.; Alam, M.A.; Gheorghe, C.P.; Aghai, Z.; Singh, H.; Bhandari, V. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Res. Ther. 2018, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.H.; Guo, H.D.; Li, H.; Zhai, Y.; Gong, Z.B.; Wu, J.; Liu, J.S.; Dong, Y.R.; Hou, S.X.; Liu, J.R. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun. Ageing 2019, 16, 10. [Google Scholar] [CrossRef] [Green Version]
- Doeppner, T.R.; Herz, J.; Görgens, A.; Schlechter, J.; Ludwig, A.K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.; Li, C.; Szalad, A.; Wang, L.; Pan, W.; Zhang, R.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia 2020, 63, 431–443. [Google Scholar] [CrossRef]
- Qi, H.; Liu, D.P.; Xiao, D.W.; Tian, D.C.; Su, Y.W.; Jin, S.F. Exosomes derived from mesenchymal stem cells inhibit mitochondrial dysfunction-induced apoptosis of chondrocytes via p38, ERK, and Akt pathways. In Vitro Cell. Dev. Biol. Anim. 2019, 55, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef]
- Jin, Z.; Ren, J.; Qi, S. Human bone mesenchymal stem cells-derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int. Immunopharmacol. 2019, 78, 105946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zeng, Z.; Fang, B.; Tao, M.; Gu, C.; Zheng, L.; Wang, Y.; Shi, Y.; Fang, C.; Mei, S.; et al. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic. Biol. Med. 2019, 143, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, P.; Bieler, L.; Scharler, C.; Pachler, K.; Kreutzer, C.; Zaunmair, P.; Jakubecova, D.; Mrowetz, H.; Benedetti, B.; Rivera, F.J.; et al. Extracellular vesicles can deliver anti-inflammatory and anti-scarring activities of mesenchymal stromal cells after spinal cord injury. Front. Neurol. 2019, 10, 1225. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Pei, S.; Han, L.; Guo, B.; Li, Y.; Duan, R.; Yao, Y.; Xue, B.; Chen, X.; Jia, Y. Mesenchymal stem cell-derived exosomes reduce A1 astrocytes via downregulation of phosphorylated NFκB p65 subunit in spinal cord injury. Cell. Physiol. Biochem. 2018, 50, 1535–1559. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Gong, F.; Rong, Y.; Luo, Y.; Tang, P.; Zhou, Z.; Zhou, Z.; Xu, T.; Jiang, T.; et al. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J. Neurotrauma 2019, 36, 469–484. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, Y.; Lan, B.; Wang, J.; Zhang, Z.; Zhang, L.; Xiao, P.; Meng, Q.; Geng, Y.J.; Yu, X.Y.; et al. MiRNA-Sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. Biomed. Res. Int. 2017, 2017, 4150705. [Google Scholar] [CrossRef]
- Teng, X.; Chen, L.; Chen, W.; Yang, J.; Yang, Z.; Shen, Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell. Physiol. Biochem. 2015, 37, 2415–2424. [Google Scholar] [CrossRef]
- Li, Q.C.; Liang, Y.; Su, Z.B. Prophylactic treatment with MSC-derived exosomes attenuates traumatic acute lung injury in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L1107–L1117. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Shao, Y.; Ye, K.; Qu, Y.; Memet, O.; He, D.; Shen, J. Mesenchymal stem cell-derived exosomes attenuate phosgene-induced acute lung injury in rats. Inhal. Toxicol. 2019, 31, 52–60. [Google Scholar] [CrossRef]
- Liu, J.; Chen, T.; Lei, P.; Tang, X.; Huang, P. Exosomes released by bone marrow mesenchymal stem cells attenuate lung injury induced by intestinal ischemia reperfusion via the TLR4/NF-κB pathway. Int. J. Med. Sci. 2019, 16, 1238–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansouri, N.; Willis, G.R.; Fernandez-Gonzalez, A.; Reis, M.; Nassiri, S.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight 2019, 4, 128060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nong, K.; Wang, W.; Niu, X.; Hu, B.; Ma, C.; Bai, Y.; Wu, B.; Wang, Y.; Ai, K. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy 2016, 18, 1548–1559. [Google Scholar] [CrossRef]
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013, 22, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lou, G.; Li, A.; Zhang, T.; Qi, J.; Ye, D.; Zheng, M.; Chen, Z. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EbioMedicine 2018, 36, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.J.; Wang, Y.H.; Li, Z.G.; Wang, Y.; Li, B.Y.; Kang, H.Y.; Wu, X.Y. Immunosuppressive effect of exosomes from mesenchymal stromal cells in defined medium on experimental colitis. Int. J. Stem Cells 2019, 12, 440–448. [Google Scholar] [CrossRef]
- Rager, T.M.; Olson, J.K.; Zhou, Y.; Wang, Y.; Besner, G.E. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J. Pediatr. Surg. 2016, 51, 942–947. [Google Scholar] [CrossRef] [Green Version]
- Spinosa, M.; Lu, G.; Su, G.; Bontha, S.V.; Gehrau, R.; Salmon, M.D.; Smith, J.R.; Weiss, M.L.; Mas, V.R.; Upchurch, G.R., Jr.; et al. Human mesenchymal stromal cell-derived extracellular vesicles attenuate aortic aneurysm formation and macrophage activation via microRNA-147. FASEB J. 2018, 32, 6038–6050. [Google Scholar] [CrossRef]
- Thomi, G.; Surbek, D.; Haesler, V.; Joerger-Messerli, M.; Schoeberlein, A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res. Ther. 2019, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Thomi, G.; Joerger-Messerli, M.; Haesler, V.; Muri, L.; Surbek, D.; Schoeberlein, A. Intranasally administered exosomes from umbilical cord stem cells have preventive neuroprotective effects and contribute to functional recovery after perinatal brain injury. Cells 2019, 8, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chopp, M.; Meng, Y.; Katakowski, M.; Xin, H.; Mahmood, A.; Xiong, Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015, 122, 856–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, N.A.; Moss, L.D.; Lee, J.Y.; Tajiri, N.; Acosta, S.; Hudson, C.; Parag, S.; Cooper, D.R.; Borlongan, C.V.; Bickford, P.C. Long noncoding RNA MALAT1 in exosomes derives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J. Neuroinflammation 2018, 15, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ophelders, D.R.; Wolfs, T.G.; Jellema, R.K.; Zwanenburg, A.; Andriessen, P.; Delhaas, T.; Ludwig, A.K.; Radtke, S.; Peters, V.; Janssen, L.; et al. Mesenchymal stromal cell-derived extracellular vesicles protect the fetal brain after hypoxia-ischemia. Stem Cells Transl. Med. 2016, 5, 754–763. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.C.; Wu, Y.P.; Li, X.D.; Chen, S.H.; Ye, X.J.; Xue, X.Y.; Xu, N. TNF-α-induced exosomal miR-146a mediates mesenchymal stem cell-dependent suppression of urethral stricture. J. Cell. Physiol. 2019, 234, 23243–23255. [Google Scholar] [CrossRef]
- Long, Q.; Upadhya, D.; Hattiangady, B.; Kim, D.K.; An, S.Y.; Shuai, B.; Prockop, D.J.; Shetty, A.K. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. USA 2017, 114, E3536–E3545. [Google Scholar] [CrossRef] [Green Version]
- Xian, P.; Hei, Y.; Wang, R.; Wang, T.; Yang, J.; Li, J.; Di, Z.; Liu, Z.; Baskys, A.; Liu, W.; et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics 2019, 9, 5956–5975. [Google Scholar] [CrossRef]
- Mathew, B.; Ravindran, S.; Liu, X.; Torres, L.; Chennakesavalu, M.; Huang, C.C.; Feng, L.; Zelka, R.; Lopez, J.; Sharma, M.; et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials 2019, 197, 146–160. [Google Scholar] [CrossRef]
- Yu, B.; Shao, H.; Su, C.; Jiang, Y.; Chen, X.; Bai, L.; Zhang, Y.; Li, Q.; Zhang, X.; Li, X. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep. 2016, 6, 34562. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gu, H.; Qin, D.; Yang, L.; Huang, W.; Essandoh, K.; Wang, Y.; Caldwell, C.C.; Peng, T.; Zingarelli, B.; et al. Exosomal miR-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Sci. Rep. 2015, 5, 13721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Gu, Z.; Zhao, X.; Yang, N.; Wang, F.; Deng, A.; Zhao, S.; Luo, L.; Wei, H.; Guan, L.; et al. Extracellular vesicles released from human umbilical cord-derived mesenchymal stromal cells prevent life-threatening acute graft-versus-host disease in a mouse model of allogeneic hematopoietic stem cell transplantation. Stem Cells Dev. 2016, 25, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-derived exosomes, a novel tool treat therapy-refractory graft-versus-host disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Zhuang, G.; Meng, C.; Guo, X.; Cheruku, P.S.; Shi, L.; Xu, H.; Li, H.; Wang, G.; Evans, A.R.; Safe, S.; et al. A novel regulator of macrophage activation, miR-223 in obesity-associated adipose tissue inflammation. Circulation 2012, 125, 2892–2903. [Google Scholar] [CrossRef] [Green Version]
- Teng, G.G.; Wang, W.H.; Dai, Y.; Wang, S.J.; Chu, Y.X.; Li, J. Let-7b is involved in the inflammation and immune responses associated with Helicobacter pylori infection by targeting Toll-like receptor 4. PLoS ONE 2013, 8, e56709. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, E.R.; Kawamoto, E.M.; Taub, D.D.; Lal, A.; Abdelmohsen, K.; Zhang, Y.; Wood, W.H., 3rd; Lehrmann, E.; Camandola, S.; Becker, K.G.; et al. Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia 2013, 61, 1018–1028. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.J.; Wu, X.Y.; Hong, Z.; Wei, W.S. MicroRNA-181c negatively regulates the inflammatory response in oxygen-glucose-deprived microglia by targeting Toll-like receptor 4. J. Neurochem. 2015, 132, 713–723. [Google Scholar] [CrossRef]
- Singla, D.K.; Johnson, T.A.; Dargani, Z.T. Exosome treatment enhances anti-inflammatory M2 macrophages and reduces inflammation-induced pyroptosis in doxorubicin-induced cardiomyopathy. Cells 2019, 8, 1224. [Google Scholar] [CrossRef] [Green Version]
- Castellani, M.L.; Felaco, P.; Galzio, R.J.; Tripodi, D.; Toniato, E.; De Lutiis, M.A.; Fulcheri, M.; Caraffa, A.; Antinolfi, P.; Tetè, S.; et al. IL-31 a Th2 cytokine involved in immunity and inflammation. Int. J. Immunopathol. Pharmacol. 2010, 23, 709–713. [Google Scholar] [CrossRef]
- Sehra, S.; Yao, Y.; Howell, M.D.; Nguyen, E.T.; Kansas, G.S.; Leung, D.Y.; Travers, J.B.; Kaplan, M.H. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J. Immunol. 2010, 184, 3186–3190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, J.D.; Ungar, B.; Guttman-Yassky, E. Drug evaluation review: Dupilumab in atopic dermatitis. Immunotherapy 2015, 7, 1043–1058. [Google Scholar] [CrossRef] [PubMed]
- Dodig, S.; Cepelak, I.; Pavic, I. Hallmarks of senescence and aging. Biochem. Med. 2019, 29, 030501. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Mchugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2017, 217, 65–77. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Baar, M.P.; Brandt, R.M.C.; Putavet, D.A.; Klein, J.D.D.; Derks, K.W.J.; Bourgeois, B.R.M.; Stryeck, S.; Rijksen, Y.; van Willigenburg, H.; Feijtel, D.A.; et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 2017, 169, 132–147. [Google Scholar] [CrossRef] [Green Version]
- Farr, J.N.; Xu, M.; Weivoda, M.M.; Monroe, D.G.; Fraser, D.G.; Onken, J.L.; Negley, B.A.; Sfeir, J.G.; Ogrodnik, M.B.; Hachfeld, C.M.; et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 2017, 23, 1072–1079. [Google Scholar] [CrossRef]
- Jeon, O.H.; Kim, C.; Laberge, R.M.; Demaria, M.; Tahod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–778. [Google Scholar] [CrossRef]
- Borghesan, M.; Fafian-Labora, J.; Eleftheriadou, O.; Carpintero-Fernandez, C.; Paez-Ribes, M.; Vizcay-Barrena, G.; Swisa, A.; Kolodkin-Gal, D.; Ximenez-Embun, P.; Lowe, R.; et al. Small extracellular vesicles are key regulators of non-cell autonomous intercellular communication in senescence via the interferon protein IFITM3. Cell Rep. 2019, 27, 3956–3971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbanelli, L.; Buratta, S.; Sagini, K.; Tancini, B.; Emiliani, C. Extracellular vesicles as new players in cellular senescence. Int. J. Mol. Sci. 2016, 17, 1408. [Google Scholar] [CrossRef] [PubMed]
- Terlecki-Zaniewicz, L.; Lammermann, I.; Latreille, J.; Bobbili, M.R.; Pils, V.; Schosserer, M.; Weinmullner, R.; Dellago, H.; Skalicky, S.; Pum, D.; et al. Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging 2018, 10, 1103–1132. [Google Scholar] [CrossRef]
- D’Anca, M.; Fenoglio, C.; Serpente, M.; Arosio, B.; Cesari, M.; Scarpini, E.A.; Galimberti, D. Exosomes determinants of physiological aging and age-related neurodegenerative diseases. Front. Aging Neurosci. 2019, 11, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; Luca, M.D.; Ottaviani, E.; Benedictis, G.D. Inflamm-aging: An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2006, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Giunta, S. Is inflammaging an auto[innate]immunity subclinical syndrome? Immunity Ageing 2006, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Maggio, M.; Guralnik, J.M.; Longo, D.L.; Ferrucci, L. Interleukin-6 in Aging and Chronic Disease: A Magnificent Pathway. J. Gerontol. Ser. A 2006, 61, 575–584. [Google Scholar] [CrossRef]
- Wang, X.; Bao, W.; Liu, J.; Ouyang, Y.-Y.; Wang, D.; Rong, S.; Xiao, X.; Shan, Z.-L.; Zhang, Y.; Yao, P.; et al. Inflammatory Markers and Risk of Type 2 Diabetes: A systematic review and meta-analysis. Diabetes Care 2012, 36, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Prattichizzo, F.; Nigris, V.D.; Sala, L.L.; Procopio, A.D.; Olivieri, F.; Ceriello, A. “Inflammaging” as a Druggable Target: A Senescence-Associated Secretory Phenotype—Centered View of Type 2 Diabetes. Oxidative Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Paine, M.S.; Brooks, A.M.; McCubrey, J.A.; Renegar, R.H.; Wang, R.; Terrian, D.M. Senescence-associated exosome release from human prostate cancer cells. Cancer. Res. 2008, 68, 7864–7871. [Google Scholar] [CrossRef] [Green Version]
- Takasugi, M.; Okada, R.; Takahashi, A.; Chen, D.V.; Watanabe, S.; Hara, E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat. Commun. 2017, 8, 15728. [Google Scholar] [CrossRef] [PubMed]
- Eitan, E.; Green, J.; Bodogai, M.; Mode, N.A.; Baek, R.; Jorgensen, M.M.; Freeman, D.W.; Witwer, K.W.; Zonderman, A.B.; Biragyn, A.; et al. Age-related changes in plasma extracellular vesicle characteristics and internalization by leukocytes. Sci. Rep. 2017, 7, 1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertoldi, K.; Cechinel, L.R.; Schallenberger, B.; Corssac, G.B.; Davies, S.; Guerreiro, I.C.K.; Bello-Klein, A.; Araujo, A.S.R.; Sigueira, I.R. Circulating extracellular vesicles in the aging process, impact of aerobic exercise. Mol. Cell. Biochem. 2018, 440, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Alibhai, F.J.; Lim, F.; Yeganeh, A.; Distefano, P.V.; Binesh-Marvasti, T.; Belfiore, A.; Wlodarek, L.; Gustafson, D.; Millar, S.; Li, S.H.; et al. Cellular senescence contributes to age-dependent changes in circulating extracellular vesicle cargo and function. Aging Cell 2020, 19. [Google Scholar] [CrossRef] [Green Version]
- Mitsuhashi, M.; Taub, D.D.; Kapogiannis, D.; Eitan, E.; Zukley, L.; Mattson, M.P.; Ferrucci, L.; Schwartz, J.B.; Goetzl, E.J. Aging enhances release of exosomal cytokine mRNAs by Aβ1-42-stimulated macrophages. FASEB J. 2013, 27, 5141–5150. [Google Scholar] [CrossRef] [Green Version]
- Pusic, A.D.; Kraig, R.P. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia 2014, 62, 284–299. [Google Scholar] [CrossRef] [Green Version]
- Weilner, S.; Keider, V.; Winter, M.; Harreither, E.; Salzer, B.; Weiss, F.; Schraml, E.; Messner, P.; Pietschmann, P.; Hildner, F.; et al. Vesicular galectin-3 levels decrease with donor age and contribute to the reduced osteo-inductive potential of human plasma derived extracellular vesicles. Aging 2016, 8, 16–33. [Google Scholar] [CrossRef] [Green Version]
- Buratta, S.; Urbanelli, L.; Sagini, K.; Giovagnoli, S.; Caponi, S.; Fioretto, D.; Mitro, N.; Caruso, D.; Emiliani, C. Extracellular vesicles released by fibroblasts undergoing H-Ras induced senescence show changes in lipid profile. PloS ONE 2017, 12, e0188840. [Google Scholar] [CrossRef]
- Takasugi, M. Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell 2018, 17, e12734. [Google Scholar] [CrossRef]
- Khayrullin, A.; Krishnan, P.; Martinez-Nater, L.; Mendhe, B.; Fulzele, S.; Liu, Y.; Mattison, J.A.; Hamrick, M.W. Very long-chain C24,1 ceramide is increased in serum extracellular vesicles with aging and can induce senescence in bone-derived mesenchymal stem cells. Cells 2019, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Mobarak, H.; Heidarpour, M.; Lolicato, F.; Nouri, M.; Rahbarghazi, R.; Mahdipour, M. Physiological impact of extracellular vesicles on female reproductive system, highlights to possible restorative effects on female-age-related fertility. Biofactors 2019, 45, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Dukes, A.; Drewry, M.; Helwa, I.; Johnson, M.H.; Isales, C.M.; Hill, W.D.; Liu, Y.; Shi, X.; Fulzele, S.; et al. MicroRNA-183-5p increases with age in bone-derived extracellular vesicles, suppresses bone marrow stromal (stem) cell proliferation, and induces stem cell senescence. Tissue Eng. Part A 2017, 23, 1231–1340. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Zhou, Q.; Fu, T.; Zhao, R.; Yang, J.; Kong, X.; Zhang, Z.; Sun, C.; Bao, Y.; Ge, X.; et al. Circulating exosomes derived-miR-146a from systemic lupus erythematosus patients regulates senescence of mesenchymal stem cells. Biomed. Res. Int. 2019, 2019, 6071308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, O.H.; Wilson, D.R.; Clement, C.C.; Rathod, S.; Cherry, C.; Powell, B.; Lee, Z.; Khalil, A.M.; Green, J.J.; Campisi, J.; et al. Senescence cell-associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight 2019, 4, e125019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalyfa, A.; Marin, J.M.; Qiao, Z.; Rubio, D.S.; Kheirandish-Gozal, L.; Gozal, D. Plasma exosomes in OSA patients promote endothelial senescence, effect of long-term adherent continuous positive airway pressure. Sleep 2019, zsz217. [Google Scholar] [CrossRef]
- Menon, R. Initiation of human parturition, signaling from senescent fetal tissues via extracellular vesicle mediated paracrine mechanism. Obstet. Gynecol. Sci. 2019, 62, 199–211. [Google Scholar] [CrossRef]
- Wong, P.F.; Tong, K.L.; Jamal, J.; Khor, E.S.; Lai, S.L.; Mustafa, M.R. Senescent HUVECs-secreted exosomes trigger endothelial barrier dysfunction in young endothelial cells. Exclij 2019, 18, 764–776. [Google Scholar]
- Cao, Q.; Guo, Z.; Yan, Y.; Wu, J.; Song, C. Exosomal long noncoding RNAs in aging and age-related diseases. Iumbm Life 2019, 71, 1846–1856. [Google Scholar] [CrossRef]
- Chen, L.; Yang, W.; Guo, Y.; Chen, W.; Zheng, P.; Zeng, J.; Tong, W. Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. PLoS ONE 2017, 12, e0185406. [Google Scholar] [CrossRef]
- Ruan, Y.; Lin, N.; Ma, Q.; Chen, R.; Zhang, Z.; Wen, W.; Chen, H.; Sun, J. Circulating LncRNAs Analysis in Patients with Type 2 Diabetes Reveals Novel Genes Influencing Glucose Metabolism and Islet β-Cell Function. Cell. Physiol. Biochem. 2018, 46, 335–350. [Google Scholar] [CrossRef]
- Borrelli, C.; Ricci, B.; Vulpis, E.; Fionda, C.; Ricciardi, M.R.; Petrucci, M.T.; Masuelli, L.; Peri, A.; Cippitelli, M.; Zingoni, A.; et al. Drug-induced senescent multiple myeloma cells elicit NK cell proliferation by direct or exosome-mediated IL15 trans-presentation. Cancer Immunol. Res. 2018, 6, 860–869. [Google Scholar] [CrossRef] [Green Version]
- Prattichizzo, F.; Giuliani, A.; Sabbatinelli, J.; Mensa, E.; De Nigris, V.; La Sala, L.; de Candia, P.; Olivieri, F.; Ceriello, A. Extracellular vesicles circulating in young organisms promote healthy longevity. J. Extracell. Vesicles 2019, 8, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, M.; Satoh, A.; Lin, J.B.; Mills, K.F.; Sasaki, Y.; Rensing, N.; Wong, M.; Apte, R.S.; Imai, S.I. Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metab. 2019, 30, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Kim, J.H.; Choi, E.S.; Cho, J.H.; Kim, E. Effects of young exosomes injected in aged mice. Int. J. Nanomed. 2018, 13, 5335–5345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wang, L.; Ruan, L.; Oh, J.; Dong, X.; Zhuge, Q.; Su, D.M. Extracellular vesicles extracted from young donor serum attenuated inflammaging via partially rejuvenating aged T-cell immunotolerance. FASEB J. 2018, 21, fj201800059R. [Google Scholar]
- Zhang, Y.; Kim, M.S.; Jia, B.; Yan, J.; Zuniga-Hertz, J.P.; Han, C.; Cai, D. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 2017, 548, 52–57. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 29. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Zhang, L.; Liang, C.; Liu, B.; Pan, X.; Wang, Y.; Zhang, Y.; Zhang, Y.; Xie, W.; Yan, B.; et al. Stem cell-derived exosomes prevent aging-induced cardiac dysfunction through a novel exosome/lncRNA MALAT1/NK-κB/TNF-α signaling pathway. Oxid. Med. Cell. Longev. 2019, 2019, 9739258. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Zhou, J.; Liu, B.; Liang, C.; Pan, X.; Zhang, Y.; Zhang, Y.; Wang, Y.; Shao, L.; Zhu, B.; et al. Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 322–332. [Google Scholar] [CrossRef]
- Liu, S.; Mahairaki, V.; Bai, H.; Ding, Z.; Li, J.; Witwer, K.W.; Cheng, L. Highly purified human extracellular vesicles produced by stem cells alleviate aging cellular phenotypes of senescent human cells. Stem Cells 2019, 37, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Tofino-Vian, M.; Guillen, M.I.; del Caz, M.D.P.; Castejon, M.A.; Alcaraz, M.J. Extracellular vesicles from adipose-derived mesenchymal stem cells downregulate senescence features in osteoarthritic osteoblasts. Oxid. Med. Cell. Longev. 2017, 2017, 7197598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, R.; Liu, M.; Wang, Y.; Li, J.; Wang, W.; Wu, J.; Sun, C.; Li, B.; Wang, Z.; Lan, W.; et al. BM-MSC-derived exosomes alleviate radiation-induced bone loss by restoring the function of recipient BM-MSCs and activating Wnt/β-catenin signaling. Stem Cell Res. Ther. 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, Y.U.; Son, Y.; Kim, C.H.; Kim, K.S.; Hyn, S.H.; Woo, H.G.; Jee, B.A.; Choi, J.H.; Sung, H.K.; Choi, H.C.; et al. Embryonic stem cell-derived mmu-miR-291a-3p inhibits cellular senescence in human dermal fibroblasts through the TGF-β receptor 2 pathway. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Sun, Y.; Zhang, J.; Zhu, Q.; Yang, Y.; Niu, X.; Deng, Z.; Li, Q.; Wang, Y. Human embryonic stem cell-derived exosomes promote pressure ulcer healing in aged mice by rejuvenating senescent endothelial cells. Stem Cell Res. Ther. 2019, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Oh, M.; Lee, J.; Kim, Y.J.; Rhee, W.J.; Park, J.H. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int. J. Mol. Sci. 2018, 19, 1715. [Google Scholar] [CrossRef] [Green Version]
- Ding, Q.; Sun, R.; Wang, P.; Zhang, H.; Xiang, M.; Meng, D.; Sun, N.; Chen, A.F.; Chen, S. Protective effects of human induced pluripotent stem cell-derived exosomes on high glucose-induced injury in human endothelial cells. Exp. Ther. Med. 2018, 15, 4791–4797. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Lim, W.; Park, J.; Park, S.; You, S.; Song, G. Anti-inflammatory effects of mesenchymal stem cell-derived exosomal microRNA-146a-5p and microRNA-548e-5p on human trophoblast cells. Mol. Hum. Reprod. 2019, 25, 755–771. [Google Scholar] [CrossRef]
- van Balkom, B.W.M.; de Jong, O.G.; Smits, M.; Brummelman, J.; den Ouden, K.; de Bree, P.M.; van Eijndhoven, M.A.J.; Pegtel, D.M.; Stoorvogel, W.; Wurdinger, T.; et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 2013, 121, 3997–4006. [Google Scholar] [CrossRef] [Green Version]
- Vomund, S.; Schafer, A.; Parnham, M.J.; Brune, B.; von Knethen, A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [Green Version]
- Banfai, K.; Garai, K.; Ernszt, D.; Pongracz, J.E.; Kvell, K. Transgenic exosomes for thymus regeneration. Front. Immunol. 2019, 10, 862. [Google Scholar] [CrossRef]
- Wound. Wikipedia. Available online: en.wikipedia.org/wiki/Wound (accessed on 21 December 2019).
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Margolis, D.J.; Pecoraro, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch. Dermatol. 1994, 130, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarbrink, K.; Ni, G.; Sonnergren, H.; Schmidtchen, A.; Pang, C.; Bajapai, R.; Car, J. Prevalence and incidence of chronic wounds and related complications, a protocol for a systematic review. Syst. Rev. 2016, 5, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruben, B. Wound Healing, Reasons Wounds will not Hill. WoundSource. Available online: www.woundsource.com/blog/wound-healing-reasons-wounds-will-not-heal (accessed on 21 December 2019).
- Igbal, A.; Jan, A.; Wajid, M.A.; Tarig, S. Management of chronic non-healing wounds by hirudotherapy. Worldj. Plat. Surg. 2017, 6, 9–17. [Google Scholar]
- Karppinen., S.M.; Heljasvaara, R.; Gullberg, D.; Tasanen, K.; Pihlajaniemi, T. Toward understanding scarless skin wound healing and pathological scarring. F1000Research 2019, 8. F1000 Faculty Rev-787. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K. Human wounds and its burden, an updated compendium of estimates. Avd. Wound Care 2019, 8, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.D.F.; Gomes, D.A. Stem cell extracellular vesicles in skin repair. Bioengineering 2018, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Kasuya, A.; Tokura, Y. Attempts to accelerate wound healing. J. Dermal. Sci. 2014, 76, 169–172. [Google Scholar] [CrossRef]
- Kawasumi, A.; Sagawa, N.; Hayashi, S.; Yokoyama, H.; Tamura, K. Wound healing in mammals and amphibians, toward limb regeneration in mammals. Curr. Top. Microbiol. Immunol. 2013, 367, 33–49. [Google Scholar]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin wound healing, an update on the current knowledge and concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Santoro, M.M.; Gaudino, G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp. Cell. Res. 2005, 304, 274–286. [Google Scholar] [CrossRef]
- Svolacchia, F.; De Francesco, F.; Trovato, L.; Graziano, A.; Ferraro, G.A. An innovative regenerative treatment of scars with dermal micrografts. J. Cosmet. Dermatol. 2016, 15, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Pelizzo, G.; Avanzini, M.A.; Icaro Cornaglia, A.; De Silvestri, A.; Mantelli, M.; Travaglino, P.; Scoce, S.; Romano, P.; Avolio, L.; Lacob, G.; et al. Extracellular vesicles derived from mesenchymal cells, perspective treatment for cutaneous wound healing in pediatrics. Regen. Med. 2018, 13, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.D.F.; Cunha, P.D.S.; Carregal, V.M.; da Silva, P.S.; de Miranda, M.C.; Kunrath-Limam, M.; de Melo, M.I.A.; Faraco, C.C.F.; Barbosa, J.L. Extracellular vesicles from adipose-derived mesenchymal stem/stromal cells accelerate migration and activate AKT pathway in human keratinocytes and fibroblasts independently of miR-205 activity. Stem. Cells. Int. 2017, 2017, 9841035. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mesenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodeling. Sci. Rep. 2017, 7, 13321. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiao, Y.; Pan, Y.; Zhang, L.; Gong, H.; Qi, Y.; Wang, M.; Gong, H.; Shao, M.; Wang, X.; et al. Fetal dermal mesenchymal stem cell-derived exosomes accelerate cutaneous wound healing by activating Notch signaling. Stem Cells Int. 2019, 2019, 2402916. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef]
- Sung, D.K.; Chang, Y.W.; Sung, S.I.; Park, W.S. Thrombin preconditioning of extracellular vesicles derived from mesenchymal stem cells accelerates cutaneous wound healing by boosting their biogenesis and enriching cargo content. J. Clin. Med. 2019, 8, 533. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W.; et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl. Med. 2015, 4, 513–522. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, J.; Niu, X.; Hu, G.; Guo, S.; Li, Q.; Xie, Z.; Zhang, C.; Wang, Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 2015, 13, 49. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef] [PubMed]
- El-Tookhy, M.S.; Shamaa, A.A.; Shehab, G.G.; Abdallah, A.N.; Azzam, O.M. Histological evaluation of experimentally induced critical size defect skin wounds using exosomal solution of mesenchymal stem cells derived microvesicles. Int. J. Stem Cells 2017, 10, 144–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silachev, D.N.; Goryunov, K.V.; Shpilyuk, M.A.; Beznoschenko, O.S.; Morozova, N.Y.; Kraevaya, E.E.; Popkov, V.A.; Pevzner, I.B.; Zorova, L.D.; Evtushenko, E.A.; et al. Effect of MSCs and MSC-derived extracellular vesicles on human blood coagulation. Cells 2019, 8, 258. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair, molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation, a critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [Green Version]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage phenotypes regulate scar formation and chronic wound healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef] [Green Version]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; van Badiavas, E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.K.; Kim, H.; Kim, T.N. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int. J. Mol. Sci. 2018, 19, 3119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell. Sci. 2016, 129, 2182–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nooshabadi, V.T.; Verdi, J.; Ebrahimi-Barough, S.; Mowla, J.; Ali Atlasi, M.; Mazoochi, T.; Valipour, E.; Shafiei, S.; Ai, J.; Banafshe, H.R. Endometrial mesenchymal stem cell-derived exosome promote endothelial cell angiogenesis in a dose dependent manner, a new perspective on regenerative medicine and cell-free therapy. Arch. Neurosci. 2019, 6, e94041. [Google Scholar]
- McCarty, S.M.; Percival, S.L. Proteases and delayed wound healing. Adv. Wound Care 2013, 2, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Sabino, F.; Auf dem Keller, U. Matrix metalloproteinases in impaired wound healing. Metalloproteinases Med. 2015, 2, 1–8. [Google Scholar]
- Westby, M.J.; Dumville, J.C.; Stubbs, N.; Norman, G.; Wong, J.K.; Cullum, N.; Riley, R.D. Protease activity as a prognostic factor for wound healing in venous leg ulcers. Cochrane Database Syst. Rev. 2018, 9, CD012841. [Google Scholar] [CrossRef] [Green Version]
- International Consensus. The Role of Proteases in Wound Healing Diagnostics; An Expert Working Group Review; Wounds International: London, UK, 2011. [Google Scholar]
- Lobmann, R.; Schultz, G.; Lehnert, H. Proteases and the diabetic foot syndrome, mechanisms and therapeutic implications. Diabetes Care 2005, 28, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Hernandez, M.A.; Kirkpatrick, V.E.; Liang, L.J.; Nouvong, A.L.; Gordon, I.I. Topical platelet-derived growth factor vs placebo therapy of diabetic foot ulcers offloaded with windowed casts, a randomized, controlled trial. Wounds 2015, 27, 83–91. [Google Scholar]
- Stacey, M. Combined topical growth factor and protease inhibitor in chronic wound healing, protocol for a randomized controlled proof-of-concept study. JMIR Res. Protoc. 2018, 7, e97. [Google Scholar] [CrossRef] [Green Version]
- Longattie, A.; Shindler, C.; Collinson, A.; Jenkinson, L.; Matthews, C.; Fitzpatrick, L.; Blundy, M.; Minter, R.; Vaughan, T.; Shwa, M.; et al. High affinity single-chain variable fragments are specific and versatile targeting motifs for extracellular vesicles. Nanoscale 2018, 10, 14230–14244. [Google Scholar] [CrossRef] [Green Version]
- Charoenviriyakul, C.; Takahashi, T.; Morishita, M.; Nishikawa, M.; Takakura, Y. Role of extracellular vesicle surface proteins in the pharmacokinetics of extracellular vesicles. Mol. Pharm. 2018, 15, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Boeringer, T.; Gould, L.J.; Koria, P. Protease-resistant growth factor formulations for the healing of chronic wounds. Adv. Wound Care 2019. [Google Scholar] [CrossRef]
- Wang, J.F.; Olson, M.E.; Reno, C.R.; Kulyk, W.; Wright, J.B.; Hart, D.A. Molecular and cell biology of skin wound healing in a pig model. Connect. Tissue Res. 2000, 41, 195–211. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The pig as a model for human wound healing. Wound Repair Regen. 2001, 9, 66–76. [Google Scholar] [CrossRef]
- Seaton, M.; Hocking, A.; Gibran, N.S. Porcine models of cutaneous wound healing. ILAR J. 2015, 56, 127–138. [Google Scholar] [CrossRef]
- Jung, Y.; Son, D.; Kwon, S.; Kim, J.; Han, K. Experimental pig model of clinically relevant wound healing delay by intrinsic factors. Int. Wound J. 2013, 10, 295–305. [Google Scholar] [CrossRef]
- Grada, A.; Mervis, J.; Falanga, V. Research techniques made simple, animal models of wound healing. J. Investig. Dermatol. 2018, 138, 2095–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eun, S.C. Stem cell and research in plastic surgery. J. Kor. Med. Sci. 2014, 29, S167–S169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Sun, Y.; Yang, X.Y.; Ji, S.Z.; Han, S.; Xia, Z.F. Mobilised bone marrow-derived cells accelerate wound healing. Int. Wound J. 2012, 10, 479. [Google Scholar] [CrossRef]
- Castella, M.A.; Mosna, F.; Micheletti, A.; Lisi, V.; Tamassia, N.; Cont, C.; Calzetti, F.; Pelletier, M.; Pizzolo, G.; Krampera, M. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells 2011, 29, 1001–1011. [Google Scholar] [CrossRef] [Green Version]
- Faulknor, R.A.; Olekson, M.A.; Ekwueme, E.C.; Krzyszczyk, P.; Freeman, J.W.; Berthiaume, F. Hypoxia impairs mesenchymal stromal cell-induced macrophage M1 to M2. Technology 2017, 5, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A new mesenchymal stem cell (MSC) paradigm, polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, N.; Kharlampieva, D.; Loguinova, M.; Butenko, I.; Pobeguts, O.; Efimenko, A.; Ageeva, L.; Sharonov, G.; Ischenko, D.; Alekseev, D.; et al. Characterization of secretomes provides evidence for adipose-derived mesenchymal stromal cells subtypes. Stem Cell Res. Ther. 2015, 6, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukho, P.; Hesselink, J.W.; Kops, N.; Kirpensteijn, J.; Verseijden, F.; Bastiaansen-Jenniskens, Y.M. Human mesenchymal stromal cell sheets induce macrophages predominantly to an anti-inflammatory phenotype. Stem Cells Dev. 2018, 27, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Alonso, L.; Fuchs, E. The hair cycle. J. Cell. Sci. 2006, 119, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef] [Green Version]
- Festa, E.; Fretz, J.; Berry, R.; Schmidt, B.; Rodeheffer, M.; Horowitz, M.; Horsley, V. Adipocyte Lineage Cells Contribute to the Skin Stem Cell Niche to Drive Hair Cycling. Cell 2011, 146, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Won, C.H.; Yoo, H.G.; Kwon, O.S.; Sung, M.Y.; Kang, Y.J.; Chung, J.H.; Park, B.S.; Sung, J.-H.; Kim, W.S.; Kim, K.H. Hair growth promoting effects of adipose tissue-derived stem cells. J. Dermatol. Sci. 2010, 57, 134–137. [Google Scholar] [CrossRef]
- Huang, C.-F.; Chang, Y.-J.; Hsueh, Y.-Y.; Huang, C.-W.; Wang, D.-H.; Huang, T.-C.; Wu, Y.-T.; Su, F.-C.; Hughes, M.; Chuong, C.-M.; et al. Assembling Composite Dermal Papilla Spheres with Adipose-derived Stem Cells to Enhance Hair Follicle Induction. Sci. Rep. 2016, 6, 26436. [Google Scholar]
- Fukuoka, H.; Narita, K.; Suga, H. Hair Regeneration Therapy: Application of Adipose-Derived Stem Cells. Curr. Stem Cell Res. Ther. 2017, 12, 531. [Google Scholar] [CrossRef]
- Kishimoto, J.; Burgeson, R.E.; Morgan, B.A. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000, 14, 1181–1185. [Google Scholar] [PubMed]
- Chen, D.; Jarrell, A.; Guo, C.; Lang, R.; Atit, R. Dermal β-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development 2012, 139, 1522–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hébert, J.M.; Rosenquist, T.; Götz, J.; Martin, G.R. FGF5 as a regulator of the hair growth cycle, Evidence from targeted and spontaneous mutations. Cell 1994, 78, 1017–1025. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Kim, K.-T.; Jo, S.J.; Cho, A.-R.; Jeon, S.-I.; Choi, H.-D.; Kim, K.H.; Park, G.S.; Pack, J.K.; Kwon, O.S.; et al. Induction of hair growth by insulin-like growth factor-1 in 1,763 MHz radiofrequency-irradiated hair follicle cells. PLoS ONE 2011, 6, e28474. [Google Scholar] [CrossRef] [Green Version]
- Trueb, R.; Rezende, H.; Dias, M.R.G. A comment on the science of hair aging. Int. J. Trichol. 2018, 10, 245. [Google Scholar] [CrossRef]
- Horev, L. Environmental and cosmetic factors in hair loss and destruction. Curr. Probl. Dermatol. 2007, 35, 103–117. [Google Scholar]
- Hagenaars, S.P.; Hill, W.D.; Harris, S.E.; Ritchie, S.J.; Davies, G.; Liewald, D.C.; Gale, C.R.; Porteous, D.J.; Deary, I.J.; Marioni, R.E. Genetic prediction of male pattern baldness. PLoS Genet. 2017, 13, e1006594. [Google Scholar] [CrossRef]
- Mysore, V. Finasteride and sexual side effects. Indian Dermatol. Online J. 2012, 3, 62. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders, a review. Drug Des. Devel. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef] [Green Version]
- Kerure, A.; Patwardhan, N. Complications in hair transplantation. J. Cutan. Aesthet. Surg. 2018, 11, 182. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, H.; Jing, J.; Yu, L.; Wu, X.; Lu, Z. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem. Biophys. Res. Commun. 2018, 500, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Seo, C.H.; Gangadaran, P.; Ahn, B.C.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Exosomes derived from human dermal papilla cells promote hair growth in cultured human hair follicles and augment the hair-inductive capacity of cultured dermal papilla spheres. Exp. Dermatol. 2019, 28, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Gao, Y.; Ding, Q.; Liu, J.; Li, Y.; Jin, M.; Xu, H.; Ma, S.; Wang, X.; Zeng, W.; et al. Exosomal Micro RNAs Derived from Dermal Papilla Cells Mediate Hair Follicle Stem Cell Proliferation and Differentiation. Int. J. Biol. Sci. 2019, 15, 1368–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Xu, X.; Zhang, B.; Xu, J.; Pan, Z.; Gong, A.; Zhang, X.; Li, R.; Sun, Y.; Yan, Y.; et al. 3,3′-Diindolylmethane stimulates exosomal Wnt11 autocrine signaling in human umbilical cord mesenchymal stem cells to enhance wound healing. Theranostics 2017, 7, 1674–1688. [Google Scholar] [CrossRef]
- Rajendran, R.L.; Gangadaran, P.; Bak, S.S.; Oh, J.M.; Kalimuthu, S.; Lee, H.W.; Baek, S.H.; Zhu, L.; Sung, Y.K.; Jeong, S.Y.; et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci. Rep. 2017, 7, 15560. [Google Scholar]
- Trüeb, R.M. Further clinical evidence for the effect of IGF-1 on hair growth and alopecia. Ski. Appendage Disord. 2017, 4, 90–95. [Google Scholar] [CrossRef]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [Green Version]
- Fore, J. A review of skin and the effects of aging on skin structure and function. Ostomy Wound Manag. 2006, 52, 24–35. [Google Scholar]
- Human Skin. Wikipedia. Available online: en.wikipedia.org/wiki/Human_skin (accessed on 19 December 2019).
- Eyerich, S.; Eyerich, K.; Traidl-Hoffmann, C.; Biedermann, T. Cutaneous barriers and skin immunity, differentiating a connected network. Trends Immunol. 2018, 39, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Nibbering, B.; Ubags, N.D.J. Microbial interaction in the atopic march. Clin. Exp. Immunol. 2020, 199, 12–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappas, A. Epidermal surface lipids. Dermatoendocrinolology 2009, 1, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fluhr, J.W.; Kao, J.; Jain, M.; Ahn, S.K.; Feingold, K.R.; Elias, P.M. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J. Investig. Dermatol. 2001, 117, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K.O. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef] [Green Version]
- Das, C.; Olmsted, P.D. The physics of stratum corneum lipid membranes. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150126. [Google Scholar] [CrossRef] [Green Version]
- Stratum Corneum. Wikipedia. Available online: en.wikipedia.org/wiki/Stratum_corneum (accessed on 19 December 2019).
- Brandner, J.M.; Zorn-Kruppa, M.; Yoshida, T.; Moll, I.; Beck, L.A.; De Benedetto, A. Epidermal tight junctions in health and disease. Tissue Barriers 2015, 3, e974451. [Google Scholar] [CrossRef] [Green Version]
- Kubo, A.; Nagao, K.; Yokouchi, M.; Sasaki, H.; Amagai, M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 2009, 206, 2937–2946. [Google Scholar] [CrossRef] [Green Version]
- Addor, F.A. Skin barrier in rosacea. An. Bras. Dermatol. 2016, 91, 59–63. [Google Scholar] [CrossRef]
- Rocha, M.A.; Bagatin, E. Skin barrier and microbiome in acne. Arch. Dermatol. Res. 2018, 310, 181–185. [Google Scholar] [CrossRef]
- van der Schaft, J.; Thijs, J.L.; de Bruin-Weller, M.S.; Balak, D.M.W. Dupilumab after the 2017 approval for the treatment of atopic dermatitis, what’s new and what’s next? Curr. Opin. Allergy Clin. Immunol. 2019, 19, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.; Sekhon, S.; Yan, D.; Afifi, L.; Nakamura, M.; Bhutani, T. Monoclonal antibodies inhibiting IL-12, -23, and -17 for the treatment of psoriasis. Hum. Vaccin. Immunother. 2017, 13, 2247–2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivero, A.L.; Whitfeld, M. An update on the treatment of rosacea. Aust. Prescr. 2018, 41, 20–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathi, S.K. Acne vulgaris treatment, the current scenario. Indian J. Dermatol. 2011, 56, 7–13. [Google Scholar] [CrossRef]
- Elias, P.M.; Wakefield, J.S.; Man, M.Q. Moisturizers versus current and next-generation barrier repair therapy for the management of atopic dermatitis. Ski. Pharmacol. Physiol. 2019, 32, 1–7. [Google Scholar] [CrossRef]
- Man, M.Q.; Hatano, Y.; Lee, S.H.; Man, M.; Chang, S.; Feingold, K.R.; Leung, D.Y.; Holleran, W.; Uchida, Y.; Elias, P.M. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis, structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J. Investig. Dermatol. 2008, 128, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.A.; Gilchrest, B.A. Psychosocial aspects of aging skin. Dermatol. Clin. 2005, 23, 643–648. [Google Scholar] [CrossRef]
- Amirthalingam, M.; Seetharam, R.N. Stem cell derived cosmetic products, an overview. Manipal J. Med. Sci. 2016, 1, 46–52. [Google Scholar]
- Lactic Acid. The Environmental Working Group. Available online: www.ewg.org/skindeep/ingredients/703350-LACTIC_ACID (accessed on 20 December 2019).
- Ammonia. The Environmental Working Group. Available online: www.ewg.org/skindeep/ingredients/700353-AMMONIA (accessed on 20 December 2019).
- Reiner, A.; Witwer, K.W.; van Balkom, B.W.M.; de Beer, J.; Brondie, C.; Corteling, R.L.; Gabrielsson, S.; Gimona, M.; Ibrahim, A.G.; de Kleijn, D.; et al. Concise review, developing best-practice models for the therapeutic use of extracellular vesicles. Stem Cells Transl. Med. 2017, 6, 1730–1739. [Google Scholar] [CrossRef] [Green Version]
- Ha, D.H.; Kim, S.D.; Cho, B.S.; Lee, J.; Lee, J.H.; Park, S.R.; Youn, J.; Lee, S.H.; Kim, J.E.; Lim, J.; et al. Toxicological evaluation of exosomes derived from human adipose tissue-derived mesenchymal stem/stromal cells. Regul. Toxicol. Pharmacol. under review.
- Maguire, G. The safe and efficacious use of secretome from fibroblasts and adipose-derived (but not bone marrow-derived) mesenchymal stem cells for skin therapeutics. J. Clin. Aesthet. Dermatol. 2019, 12, E57–E69. [Google Scholar] [PubMed]
- Reza, A.M.M.T.; Choi, Y.J.; Yasuda, H.; Kim, J.O. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducting anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci. Rep. 2016, 6, 38498. [Google Scholar] [CrossRef] [PubMed]
- Mesoblast, FDA Agree on Pathway to BLA for Heart Failure Cell Therapy. Genetic Engineering & Biotechnology News. 27 August 2019. Available online: www.genengnews.com/news/mesoblast-fda-agree-on-pathway-to-bla-for-heart-failure-cell-therapy/ (accessed on 13 December 2019).
- Chen, T.S.; Arslan, F.; Yin, Y.; Tan, S.S.; Lai, R.C.; Choo, A.B.; Padmandabhan, J.; Lee, C.N.; de Kleijn, D.P.; Lim, S.K. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J. Tansl. Med. 2011, 9, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, R.C.; Yeo, R.W.; Padmanabhan, J.; Choo, A.; de Kleijn, D.P.; Lim, S.K. Isolation and characterization of exosome from human embryonic stem cell-derived c-Myc-immortalized mesenchymal stem cells. Methods Mol. Biol. 2016, 1416, 477–494. [Google Scholar] [PubMed]
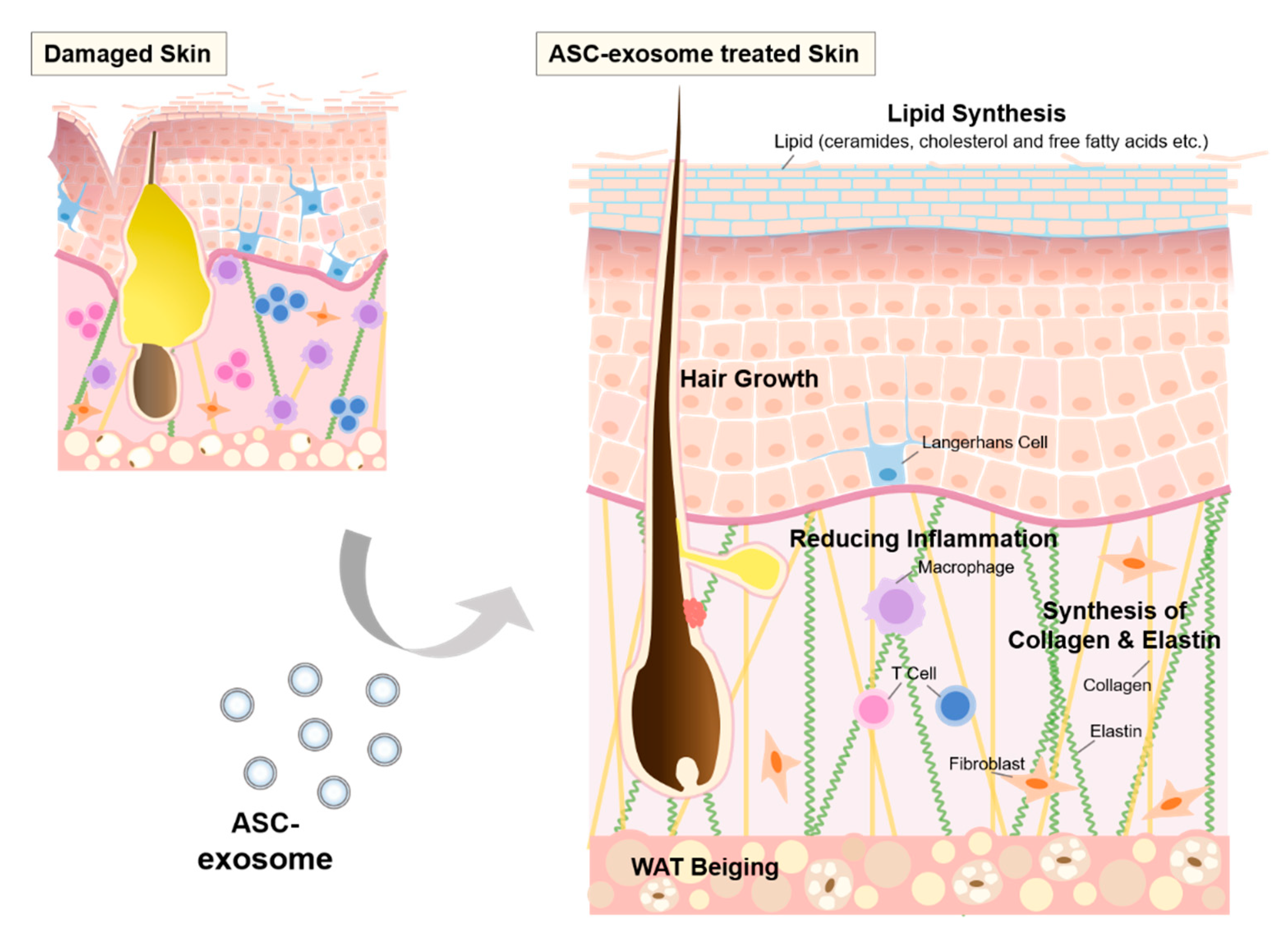
| Diseases/Focuses | Nomenclature | Exosome Isolation | MSC Origin | Outcome | Reference |
|---|---|---|---|---|---|
| Alzheimer’s disease | Exosomes | Ultracentrifugation | Human adipose tissue | Adipose stem cell (ASC)-exosomes had superior effects compared to bone marrow (BM)-MSC-exosomes Decreased Aβ peptide in the N2a cells | [30] |
| Human bone marrow | |||||
| Glioblastoma | Extracellular Vesicles (EVs) | Ultrafiltration | Human bone marrow | Decreased U87MG cell proliferation Induced apoptosis in the U87MG cells | [31] |
| Human Wharton’s jelly | |||||
| Human adipose tissue | Increased U87MG cell proliferation No apoptotic effect | ||||
| Neurodegenerative disease | Exosomes | Ultracentrifugation | Human menstrual fluid | Promoted neurite outgrowth in cortical and sensory neurons | [32] |
| Human bone marrow | |||||
| Human chorion | No effect | ||||
| Human umbilical cord | |||||
| Osteoarthritis (OA) | Exosomes | Ultrafiltration | Human iPSCs | Attenuated OA in a murine model Stimulated chondrocyte migration and proliferation Induced pluripotent stem cell-derived MSC (iMSC)-exosomes exert superior therapeutic effects compared to synovial membrane (SM)-MSC-exosomes | [33] |
| Human synovial membrane | |||||
| Exosome release | Exosomes | Ultracentrifugation | Canine bone marrow | BM-MSCs released higher amount of exosome compared to ASCs | [34] |
| Canine adipose tissue | |||||
| Exosomes | Total Exosome Isolation Kit (Invitrogen) | Human amniotic fluid | Amniotic fluid (AF)-MSCs released higher amount of exosome compared to BM-MSCs | [35] | |
| Human bone marrow |
| QC Criteria | Examples in Guidelines | Examples in GMP Settings | |||
|---|---|---|---|---|---|
| ISEV Recommendation [43,44,45] | MFDS Guideline (2018) [46] | Pachler et al. [47] | Andriolo et al. [48] | Mendt et al. [42] | |
| Exosome Quantity | Particle number by NTA, high-resolution FCM RPS, cryo-EM, AFM, etc. | Particle number by NTA or compatible methods 1 | (ZetaVeiw NTA) | (NanoSight NTA) | (NanoSight NTA) |
| Total protein amount | 2 | - | (BCA assay) | (microBCA assay) | |
| Total lipid amount | - | - | - | - | |
| Total RNA amount | - | - | - | - | |
| Quantification of specific molecules | - | - | TSG101 ELISA | - | |
| Exosome Size | NTA | NTA 1 | |||
| - | DLS 1 | - | - | - | |
| RPS | RPS 1 | - | - | - | |
| High-resolution FCM | - | - | - | - | |
| AF4 | - | - | - | - | |
| - | - | - | - | ||
| FCS | FCS 1 | - | - | - | |
| Identity | Proteins | Proteins | (WB: CD9, CD81, TSG101) | (FCM: CD9, CD63, CD81 ELISA: TSG101) | (FCM: CD47, CD63, CD81, CD9, CD29, CD90) |
| Phospholipids | Lipids | - | - | - | |
| Nucleic acids | RNAs | - | - | - | |
| Purity | Ratio of protein:particle | - | - | - | - |
| Ratio of lipids:particle | - | - | - | - | |
| Ratio of lipids:protein | - | - | - | - | |
| Proteins that are expected not to be enriched in exosomes | Proteins that are expected not to be enriched in exosomes | (WB: GM130) | - | - | |
| Process impurities depending on the source of exosomes | Process impurities (serum albumin, antibiotics, etc.) | - | - | - | |
| Potency Assays | Dose-response assessment | Biological assay, which can represent MoA | - | Anti-apoptotic activity; Pro-angiogenic activity | Apoptosis assay |
| Others | Not mentioned | Mycoplasma test | - | - | - |
| Sterility test | - | Microbiological Control for Cellular Products | - | ||
| Endotoxin test | - | Quantitative LAL test | - | ||
| Adventitious virus test | - | - | - | ||
| Category | Exosome Source | Nomenclature | Exosome Isolation | Related Exosomal Cargo | Secreted Factors or Expressed Genes Affected | Immunomodulatory Effects | Reference |
|---|---|---|---|---|---|---|---|
| Macrophage polarization | Human jaw bone marrow (JM-MSCs) Human BM-MSCs | Exosomes | Ultracentrifugation ExoQuick (System Biosciences) | miR-223 | TNF-α ↓ IL-10 ↑ | Accelerated wound healing in mice Induced M2 macrophage polarization (CD206+ macrophage ↑) | [85] |
| Human JM-MSCs Human BM-MSCs | Exosomes | Ultracentrifugation | - | Collagen, Il-6, Ccl2, Cd206, Ccl7, Ccl17, Tnfα, Retnia ↓ Arg1 ↑ | Reduced BPD through macrophage M22 polarization | [86] | |
| Human umbilical cord (UC)-MSCs | Exosomes | Ultracentrifugation | let-7b | TLR4, p-p65, iNOS ↓ p-STAT3, p-AKT, ARG1 ↑ | Alleviated inflammation and enhanced diabetic cutaneous wound healing in rats Induced M2 macrophage polarization Inhibited TLR4 signaling pathway | [87] | |
| Human UC-MSCs | Exosomes | PureExo (101Bio) | miR-181c | TNF-α, IL-1β, TLR4, p65, p-p65 ↓ IL-10 ↑ | Reduced burn-induced inflammation in rats Reduced neutrophil and macrophage infiltration (MPO+ cell, CD68+ cell ↓) Inhibited TLR4 signaling pathway | [88] | |
| Human menstrual blood derived MSCs (MenSCs) | Exosomes | Ultracentrifugation | - | iNOS ↓ ARG1, VEGF ↑ | Resolved inflammation and ameliorate cutaneous non-healing wounds in diabetic mice Induced M2 macrophage polarization | [89] | |
| Mouse BM-MSCs | Exosomes | HPLC | let-7 | HMGA2, IGF2BP1 ↓ | Attenuated atherosclerosis in mice Reduced area of atherosclerotic plaques Promoted M2 macrophage polarization | [90] | |
| Mouse BM-MSCs | Exosomes | Ultracentrifugation | miR-182 | IL-6, iNOS, IL-1 β, IL-6, TNF-α ↓ ARG1, IL-10, TGF-β ↑ | Reduced myocardial ischemic-reperfusion injury in mice Reduced infarct size and inflammation Promoted M2 macrophage polarization | [91] | |
| Human BM-MSCs | Exosomes | Ultracentrifugation | MT2A | IFN-γ, IL-1β, IL-6, TNF-α ↓ IL-10, Lyz1, Defa20, Defa29, Ang4 ↑ | Reduced IBD by polarizing M2 macrophage in mice | [92] | |
| Rat ASCs | Exosomes | Ultracentrifugation | - | S1P, SphK1, S1PR1 ↑ AGR1, Ym1, TGF-β1, IL-10 ↑ IL-1β, IL-6, TNF-α, IFN-γ, p65 ↓ | Reduced cardiac damage in rats Reduced fibrosis and apoptosis Promoted M2 macrophage polarization | [93] | |
| Human ASCs | Exosomes | Exosome Isolation Kit (System Biosciences) | - | CD163, ARG1, CD206, STAT6, MafB ↑ | Increased the expression of M2 macrophage markers | [94] | |
| Mouse ASCs | Exosomes | Ultrafiltration | STAT3 | ARG1, IL-10, tyrosine hydroxylase ↑ TNF-α, IL-12 ↓ | Induced M2 macrophage polarization in obese mice ASC-exosome-educated M2 macrophage promoted WAT beiging | [95] | |
| T cell regulation | Human BM-MSCs | Exosomes | ExoQuick (System Biosciences) | - | TNF-α, IL-1β ↓ TGF-β ↑ | Induced conversion of Th1 into Th2 Reduced differentiation of Th17 Increased the level of Tregs Induced apoptosis of PBMCs and CD3+ T cells | [96] |
| Human BM-MSCs | Exosomes | Ultracentrifugation | - | IL-10, TGF-β ↑ | Promoted proliferation and immune-suppression capacity of Tregs | [97] | |
| Human UC-MSCs | Exosomes | PEG6000 precipitation | - | IL-10, IDO ↑ | Induced an increase of Tregs in PBMCs Inhibited proliferation of PBMCs | [98] | |
| Human embryonic stem cell (ES)-MSCs | Exosomes | Tangential flow filtration + HPLC | EDA-FN | TNF-α, IL-1β, IL-6, IL-12p40 ↓ IL-10 ↑ | Induced Tregs through activation of APCs in the MyD88-dependent manner Enhanced allogeneic skin graft | [99] | |
| Mouse ASCs | Exosomes | Ultracentrifugation | - | IL-17, IFN-γ ↓ IL-4, IL-10, TGF-β ↑ | Ameliorated autoimmune type 1 diabetes mellitus by increasing Tregs in mice | [100] | |
| Human BM-MSCs | Exosomes | Ultracentrifugation | - | IL-6, IL-12p70, IL-22, IL-17AF ↓ IDO ↑ | Improved motor skill in the MS mouse experimental autoimmune encephalomyelitis model Increased Tregs and decreased infiltration and proliferation of pro-inflammatory T cells | [101] | |
| Mouse BM-MSCs | Exosomes | Ultracentrifugation | - | IL-1, IL-2, IL-4, IL-10, TNF-α, IFN-γ ↓ | Decreased aminotransferase (ALT), liver necrotic areas, and apoptosis in Con A-induced liver injury in mice Increased Tregs | [102] | |
| UC-MSCs | EVs | Size exclusion chromatography | - | - | Suppressed T cell proliferation | [105] | |
| B cell regulation | Human BM-MSCs | Exosomes | Ultracentrifugation | - | MZB1, CXCL8 ↑ IgM ↓ | Reduced proliferation of T and B cells | [106] |
| Photoaging | Human BM-MSCs | Exosomes | Ultrafiltration | - | TNF-α, IL-1β ↓ TGF-β, CTLA4 ↑ | Reduced photoaging of skin in mice Ameliorated inflammation | [107] |
| Skin flap | Human ASCs | Exosomes | Ultracentrifugation | - | - | Enhanced neovascularization and survival of the skin flap in rats Reduced inflammation and apoptosis | [108] |
| Atopic dermatitis (AD) | Human ASCs | Exosomes | Tangential flow filtration | - | IgE, IL-4, IL-5, IL-13, IL-17, IL-23, IL-31, IFN-γ, TNF-α, TSLP ↓ | Reduced pathological symptoms of AD in mice Reduced mast cell infiltration Reduced inflammatory dendritic epidermal cells (CD86+/CD206+ cells↓) | [20,109] |
| Renal injury | Rat BM-MSCs | Exosomes | Ultracentrifugation | - | MDA, HIF1α, NOX2, Caspase 3, BAX, PARP1, MPO, ICAM1, IL-1β, NF-κB ↓ SOD, CAT, GPX, HO-1, BCL2, IL-10, bFGF, HGF, SOX9, VEGF ↑ | Decreased histopathological score of kidney injury in rats Reduced the levels of blood urea nitrogen (BUN) and creatinine Reduced the level of oxidative stress Increased anti-oxidant status Reduced apoptosis and inflammation Improved regeneration and enhanced angiogenesis | [110] |
| Mouse BM-MSCs | Exosomes | Ultracentrifugation | CCR2 | TNF-α, IL-6, IL-1β ↓ | Reduced BUN and creatinine in the mouse IR model Reduced infiltration of macrophages | [111] | |
| Human UC-MSCs | Exosomes | Ultracentrifugation | - | PCNA, BCL-XL, BCL2, IL-1β, 4E-BP1 ↑ Bax, cytochrome C, Caspase-3, p65, TNF-α, IL-6, IL-1β, p-mTOR ↓- | Reduced cisplatin-induced AKI in rats Reduced BUN and creatinine | [112] | |
| Uveitis | Human UC-MSCs | Exosomes | Ultracentrifugation | - | - | Reduced experimental autoimmune uveitis in rats Reduced infiltration of Gr-1+, CD161+, CD68+ and CD4+ cells in retina | [113] |
| Duchenne muscular dystrophy (DMD) | Human Placenta MSCs | Exosomes | Ultracentrifugation | miR-29c | TGF-β, creatine kinase, collagen I, collagen IV, TNF-α, IL-6 ↓ Utrophin ↑ | Reduced DMD in mice Decreased the tissue fibrosis and inflammation | [114] |
| Bronchopulmonary dysplasia (BPD) | Human UC-MSCs | Exosomes | Ultracentrifugation | TSG-6 | Neutrophil ↓ | Improved pathology of lung, cardiac and brain in neonatal mice with BPD Reduced pulmonary inflammation and alveolar-capillary leak | [115] |
| Alzheimer’s disease | Mouse BM-MSCs | Exosomes | Ultracentrifugation | - | TNF-α, IL-1β, IL-6 ↓ IL-10, IL-4, IL-13 ↑ | Improved cognitive function in transgenic APP/PS1 mice Reduced plaque deposition and Aβ levels Reduced activation of astrocytes | [116] |
| Post-stroke neuroregeneration | Human BM-MSCs | EVs | PEG6000 precipitation | - | Dcx, NeuN, CD31 ↑ | Improved neurological impairment (motor coordination) and long-term neuroprotection (neuronal survival and cell proliferation) in stroke mice Reduced post-ischemic immunosuppression and lymphopenia Stimulated post-ischemic neurogenesis and angiogenesis | [117] |
| Diabetic peripheral neuropathy | Mouse BM-MSCs | Exosomes | Ultracentrifugation | miR-17 miR-23a miR-125b | TNF-α, IL-1β, iNOS, TLR4, IRAK1, p65 ↓ ARG1, IL-10, TGF-β ↑ | Decreased the threshold for thermal and mechanical stimuli in mice Increased nerve conduction velocity, the number of intraepidermal nerve fibers, myelin thickness, and axonal diameters | [118] |
| OA | Rabbit BM-MSCs | Exosomes | Ultracentrifugation | - | p-p38, p-ERK ↓ p-AKT ↑ | Increased chondrocytes viability under IL-1β-induced inflammatory status through activating AKT pathway | [119] |
| Human ES-MSCs | Exosomes | Tangential flow filtration | CD73 | α-SMA, MMP-13, IL-1β, iNOS ↓ PCNA, s-GAG ↑ | Promoted repair and regeneration of temporomandibular joint OA in rats through the AKT/ERK/AMPK-dependent manner | [120] | |
| Human BM-MSCs | Exosomes | ExoQuick (System Biosciences) | miR-26a-5p | PTGS, Bcl-2, IL-6, TNF-α, IL-8, IL-1β ↓ Bax, caspase-3 ↑ | Alleviated OA damage in rats treated with pentobarbital | [121] | |
| Human ES-MSCs | Exosomes | Tangential flow filtration | CD73 | TNF-α, IL-1β ↓ PCNA ↑ | Induced cartilage repair through the CD73-mediated activation of AKT and ERK pathway | [122] | |
| Intervertebral disc degeneration (IVDD) | Mouse BM-MSCs | Exosomes | Ultrafiltration | - | Caspase-9/3, iNOS, MMP-3/13, caspase-1, IL-1β, TXNIP, NLRP3 ↓ COL2A, SOX9 ↑ | Prevented progression of IVDD in rabbit Suppressed activation of NLRP3 inflammasome | [123] |
| Spinal cord injury | Human UC-MSCs | EVs | Ultracentrifugation | IL-1β, IL-6 ↓ | Demonstrated anti-inflammatory and anti-scarring activities in the spinal cord parenchyma in rats | [124] | |
| Rat BM-MSCs | Exosomes | Ultracentrifugation | - | C3, GFAP, TNF-α, IL-1α, IL-1β, p-p65, p-IκBα ↓ | Reduced spinal cord injury-induced A1 astrocytes in rats | [125] | |
| BM-MSCs | Exosomes | Ultrafiltration | - | NO, Bax, caspase-3, TNF-α, IL-1β, IL-6 ↓ Bcl2, VEGF, NF200 ↑ | Improved functional behavioral recovery in rats Attenuated neuronal cells apoptosis, suppressed glial scar formation Suppressed activation of microglia, A1 neurotoxic reactive astrocytes and neuroinflammation | [126] | |
| Myocardial infarction | Rat BM-MSCs | Exosomes | Total Exosome Isolation Kit (Invitrogen) | miR-29, miR-24 | - | Inhibited cardiac fibrosis, inflammation, and improved cardiac function in rat myocardial infarction model | [127] |
| Rat BM-MSCs | Exosomes | ExoQuick (System Biosciences) | - | NO, Bax, caspase-3/9 ↓ Bcl2 ↑ | Improved microenvironment of infarcted myocardium in rats through angiogenesis and anti-inflammation | [128] | |
| Acute lung injury (ALI) | Rat BM-MSCs | Exosomes | Exosome extractant (Ribobio Co., Ltd.) | miR-124-3p | P2X7, TNF-α, IL-6, IL-8 ↓ GSH, SOD ↑ | Increased survival rate of rats | [129] |
| Rat BM-MSCs | Exosomes | Ultracentrifugation | TNF-α, IL-1β, IL-6, MMP-9 ↓ IL-10, SP-C ↑ | Attenuated phosgene-induced ALI in rats | [130] | ||
| Rat BM-MSCs | Exosomes | Ultracentrifugation | - | Caspase-3, TNF-α, IL-1β, IL-6, TLR4, NF-κB ↓ | Attenuated ischemia repurfusion (IR)-induced lung injury in rats Decreased apoptosis and inflammation | [131] | |
| Induced pulmonary fibrosis (IPF) | Human BM-MSCs | Exosomes | Ultracentrifugation | - | CCL2, ARG1 ↓ | Reduce bleomycin-induced IPF in mice Reduced collagen deposition and apoptosis | [132] |
| Hepatic IR injury | Human iMSCs | Exosomes | Ultrafiltration | TNF-α, IL-6, HMGB1, caspase-3, Bax ↓ GSH, GSH-Px, SOD ↑ | Suppressed hepatocyte necrosis and sinusoidal congestion Reduced the AST and ALT | [133] | |
| Liver fibrosis | Human UC-MSCs | Exosomes | Ultrafiltration | - | AST ↑ Collagen I/III, TGF-β 1, p-Smad2 ↓ | Alleviated hepatic inflammation and collagen deposition in the CCl4-induced fibrotic liver of mice | [134] |
| Acute liver failure | Mouse ASCs | Exosomes | Total Exosome Isolation Kit (Invitrogen) | miR-17 | TNF-α, IFN-γ, IL-1β, IL-6, IL-18, TXNIP, NLRP3, ASC, caspase-1 ↓ | Ameliorated acute liver failure by reducing ALT and AST in mice Reduced activation of TXNIP/NLRP3 inflammasome in macrophages | [135] |
| Intestinal bowel disease (IBD) | Human UC-MSCs | Exosomes | Ultracentrifugation | - | TNF-α, IFN-γ, IL-1β, IL-6, IL-17 ↓ TGF-β 1, IL-10 ↑ | Ameliorated DSS-induced IBD in mice | [136] |
| Necrotizing enterocolitis (NEC) | Mouse BM-MSCs | Exosomes | PureExo (101Bio) | - | - | Reduced incidence and severity of NEC in premature newborn rats | [137] |
| Abdominal aortic aneurysm | Human UC-MSCs | EVs | Ultracentrifugation | miR-147 | IL-6, IL-17, IFN-γ, IL-23, RANTES, KC, MCP-1, MIP-1α, HMGB1 ↓ | Reduced inflammation and macrophage activation in a mouse abdominal aortic aneurysm model | [138] |
| Perinatal brain injury | Human Wharton’s jelly (WJ)-MSCs | Exosomes | Ultracentrifugation | - | TNF-α, IL-6, IL-1β, CXCL10, IκBα, p-ERK1/2, p-JNK, p-p38 ↓ | Reduced neuroinflammation in rats with perinatal brain injury | [139] |
| Human WJ-MSCs | Exosomes | Ultracentrifugation | - | Mbp, Map 2 ↑ | Reduced neuron-specific cell death in rats with perinatal brain injury | [140] | |
| Traumatic brain injury (TBI) | Rat BM-MSCs | Exosomes | ExoQuick (System Biosciences) | - | GFAP ↑ | Improved spatial learning in rats with TBI | [141] |
| Human ASCs | Exosomes | ExoQuick (System Biosciences) | MALAT1 | TNF-α, IL-1β, IFN-γ ↓ | Improved motor behavior in rats with TBI | [142] | |
| Hypoxic-ischemic brain injury | Human BM-MSCs | EVs | PEG6000 precipitation | - | - | Improved function of brain by reducing the total number and duration of seizures in sheep | [143] |
| Urethral stricture | Human UC-MSCs | Exosomes | Ultracentrifugation | miR-146a | α-SMA, collagen I/III, IL-6, IL-1β, IRAK1, TRAF6, NF-κB ↓ | Reduced urethral fibrosis and stricture in rats | [144] |
| Status epilepticus (SE) | Human BM-MSCs | Exosomes | Anion exchange chromatography | - | TNF-α, IL-1β, MCP-1, SCF, MIP-1a, GM-CSF ↓ IL-10, PDGF-B, IL-6, IL-2 ↑ | Reduced pilocarpine-induced SE in mice Reduced loss of glutamatergic and GABAergic neurons Reduced inflammation in hippocampus | [145] |
| Human UC-MScs | Exosomes | Ultracentrifugation | - | GFAP, TNF-α, IL-1β ↓ | Ameliorated SE-induced learning and memory impairment in mice | [146] | |
| Retinal IR injury | Human BM-MSCs | EVs | ExoQuick (System Biosciences) | - | TNF-α, IL-6, caspase-3 ↓ | Reduced neuro-inflammation and apoptosis | [147] |
| Laser-induced retinal injury | Mouse ASCs Human UC-MSCs | Exosomes | Ultracentrifugation | MCP-1 ↓ | Reduced damage, inhibited apoptosis, and suppressed inflammatory responses in mice | [148] | |
| Sepsis | Mouse BM-MSCs | Exosomes | Ultracentrifugation | miR-223 | TNF-α, IL-1β, IL-6 ↓ | Protected cardiomyocytes from cecal ligation and puncture-induced sepsis in mice through downregulation of SEMA3A and STAT3 | [149] |
| Graft versus Host Disease (GvHD) | Human UC-MSCs | EVs | Ultracentrifugation | - | IL-2, TNF-α, IFN-γ ↓ IL-10 ↑ | Prevented acute GvHD in a mouse model of allogeneic hematopoietic stem cell transplantation | [150] |
| Human BM-MSCs | Exosomes | PEG6000 precipitation | - | TNF-α, IL-1β, IFN-γ ↓ | Modulated the patient’s immune cells | [151] |
| Exosome Source | Nomenclature | Exosome Isolation | Potential MoA | Senescent Cells | In Vitro Effects | In Vivo Effects | Reference |
|---|---|---|---|---|---|---|---|
| Human ASCs | Exosomes | ExoQuick (System Biosciences) | NFR2 | HG-induced senescent EPCs | Cell viability, Tube formation ↑ SMP30, p-VEGFR2 ↑ NOX1, NOX4, IL-6, IL-1β, TNF-α ↓ | Wound healing in diabetic rat | [205] |
| Human UC-MSCs | Exosomes | Total exosome isolation kit (Invitrogen) | Reducing NF-κB/TNFα signaling by lncRNA MALAT1 | H2O2-treated H9C2 | SA-β-gal ↓ NF-κB activation, p21, TNFα ↓ Cell proliferation ↑ | Improvement cardiac function in D-gal-induced aged mouse | [206] |
| Human UC-MSCs | Exosome | Ultracentrifugation | TGF-β1 downregulation by miR-675 | H2O2-treated H9C2 | SA-β-gal, p21, TGF-β1 ↓ | Perfusion in ischemic hindlimb | [207] |
| Human BM-MSCs Human iPSCs | EVs | Size exclusion chromatography | Reduction of ROS by PRDXs enriched in exosomes | RS MSCs Progerin-induced senescent MSCs | Cell growth ↑ SA-β-gal, IL-1A, IL-6, γ-H2AX↓ ↓ p21, p53 mRNAs ↓ | ND | [208] |
| Human ASCs | Exosomes | Ultracentrifugation | Unknown | IL-1β-treated OA osteoblasts | SA-β-gal, γ-H2AX ↓ IL-6 and Prostaglandin E2 ↓ Oxidative stress, Mitochondrial membrane potential ↓ | ND | [209] |
| Rat BM-MSCs | Exosomes | Ultracentrifugation | Activation of Wnt/β-catenin signaling | Irradiated rat BM-MSCs | Oxidative stress ↓ γ-H2AX, Rb, p53, p21, p16 ↓ SOD1/2, Catalase ↑ | Attenuating radiation-induced bone loss in rat | [210] |
| Mouse ESCs | Exosome | ExoQuick (System Biosciences) or Ultracentrifugation | TGF-β Receptor 2 inhibition by mouse miR-291a-3p (human miR-371a-3p | RS HDFs AS HDFs | SA-β-gal ↓ Cell proliferation, migration ↑ | ND | [211] |
| Human ESCs | Exosome | Ultracentrifugation | KEAP1 downregulation by miR-200a | D-gal-induced HUVECs | SA-β-gal, p16, p21 ↓ ROS ↓ Cell proliferation, migration, tube formation ↑ | Pressure ulcer healing in D-gal-induced aged mouse | [212] |
| Human iPSCs | Exosomes | ExoQuick (System Biosciences) | Unknown | RS HDFs Photoaged HDFs | SA-β-gal, MMP-1/3 ↓ Collagen Type I ↑ | ND | [213] |
| Human iPSCs | Exosomes | Ultracentrifugation | Unknown | HG-injured HUVECs | SA-β-gal ↓Cell viability, Tube formation↑ | ND | [214] |
| Exosome Source | Nomenclature | Exosome Isolation | Related Exosomal Cargo | Factors Affected | Animal for In Vivo Study | Reference |
|---|---|---|---|---|---|---|
| Human JM-MSCs Human BM-MSCs | Exosomes | Ultracentrifugation ExoQuick (System Biosciences) | miR-223 | TNF-α ↓ IL-10 ↑ | Mouse | [85] |
| Human UC-MSCs | Exosomes | Ultracentrifugation | let-7b | TLR4, p-p65, iNOS ↓ p-STAT3, p-AKT, ARG1 ↑ | Rat | [87] |
| Human UC-MSCs | Exosomes | PureExo (101Bio) | miR-181c | TNF-α, IL-1β, TLR4, p65, p-p65 ↓ IL-10 ↑ | Rat | [88] |
| Human ASCs | Exosomes | ExoQuick (System Biosciences) | - | NOX1, NOX4, IL-6, IL-1β, TNF-α ↓ SMP30, p-VEGFR2 ↑ | Rat | [205] |
| Rabbit ASCs Rabbit BM-MSCs | EVs | Ultracentrifugation | - | - | Rabbit | [232] |
| Human ASCs | EVs | Ultracentrifugation | - | - | Rat | [233] |
| Human ASCs | Exosomes | ExoQuick (System Biosciences) | - | N-cadherin, cyclin 1, PCNA, collagen I/III, elastin ↑ | Mouse | [234] |
| Human ASCs | Exosomes | ExoQuick (System Biosciences) | - | Collagen I/II, TGF-β1/3, MMP1/3 α-SMA ↓ | Mouse | [235] |
| Human fetal dermal MSCs | Exosomes | ExoQuick (System Biosciences) | Jagged 1 | Collagen I/III, elastin, fibronectin mRNA ↑ | Mouse | [236] |
| Human UC-MSCs | Exosomes | Ultracentrifugation | Wnt4 | CK19, PCNA, collagen I ↑ | Rat | [237] |
| Human UC blood-MSCs | Exosomes | Ultracentrifugation | - | Ang, Ang1, HFG, VEGF ↑ | Rat | [238] |
| Human UC-MSCs | Exosomes | Ultracentrifugation | Wnt4 | β-catenin, N-cadherin, PCNA, Cyclin D3 ↑ | Rat | [239] |
| Human iPSC-MSCs | Exosomes | Ultracentrifugation | - | Collagen I/III, elastin, ↑ | Rat | [240] |
| Human UC-MSCs | Exosomes | Ultracentrifugation | - | α-SMA, collagen I ↓ | Mouse | [241] |
| Human gingival MSCs | Exosomes | Size exclusion chromatography | - | Collagen ↑ | Rat | [242] |
| Dog BM-MSCs | Exosomes | Ultracentrifugation | - | α-SMA ↓ | Dog | [243] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, D.H.; Kim, H.-k.; Lee, J.; Kwon, H.H.; Park, G.-H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. https://doi.org/10.3390/cells9051157
Ha DH, Kim H-k, Lee J, Kwon HH, Park G-H, Yang SH, Jung JY, Choi H, Lee JH, Sung S, et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells. 2020; 9(5):1157. https://doi.org/10.3390/cells9051157
Chicago/Turabian StyleHa, Dae Hyun, Hyun-keun Kim, Joon Lee, Hyuck Hoon Kwon, Gyeong-Hun Park, Steve Hoseong Yang, Jae Yoon Jung, Hosung Choi, Jun Ho Lee, Sumi Sung, and et al. 2020. "Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration" Cells 9, no. 5: 1157. https://doi.org/10.3390/cells9051157
APA StyleHa, D. H., Kim, H.-k., Lee, J., Kwon, H. H., Park, G.-H., Yang, S. H., Jung, J. Y., Choi, H., Lee, J. H., Sung, S., Yi, Y. W., & Cho, B. S. (2020). Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells, 9(5), 1157. https://doi.org/10.3390/cells9051157





