Concerted EP2 and EP4 Receptor Signaling Stimulates Autocrine Prostaglandin E2 Activation in Human Podocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. PGE2 Treatment and Inhibition of EP Receptors
2.3. Determination of Intracellular cAMP Levels
2.4. Reverse Transcription and Quantitative Real-Time PCR
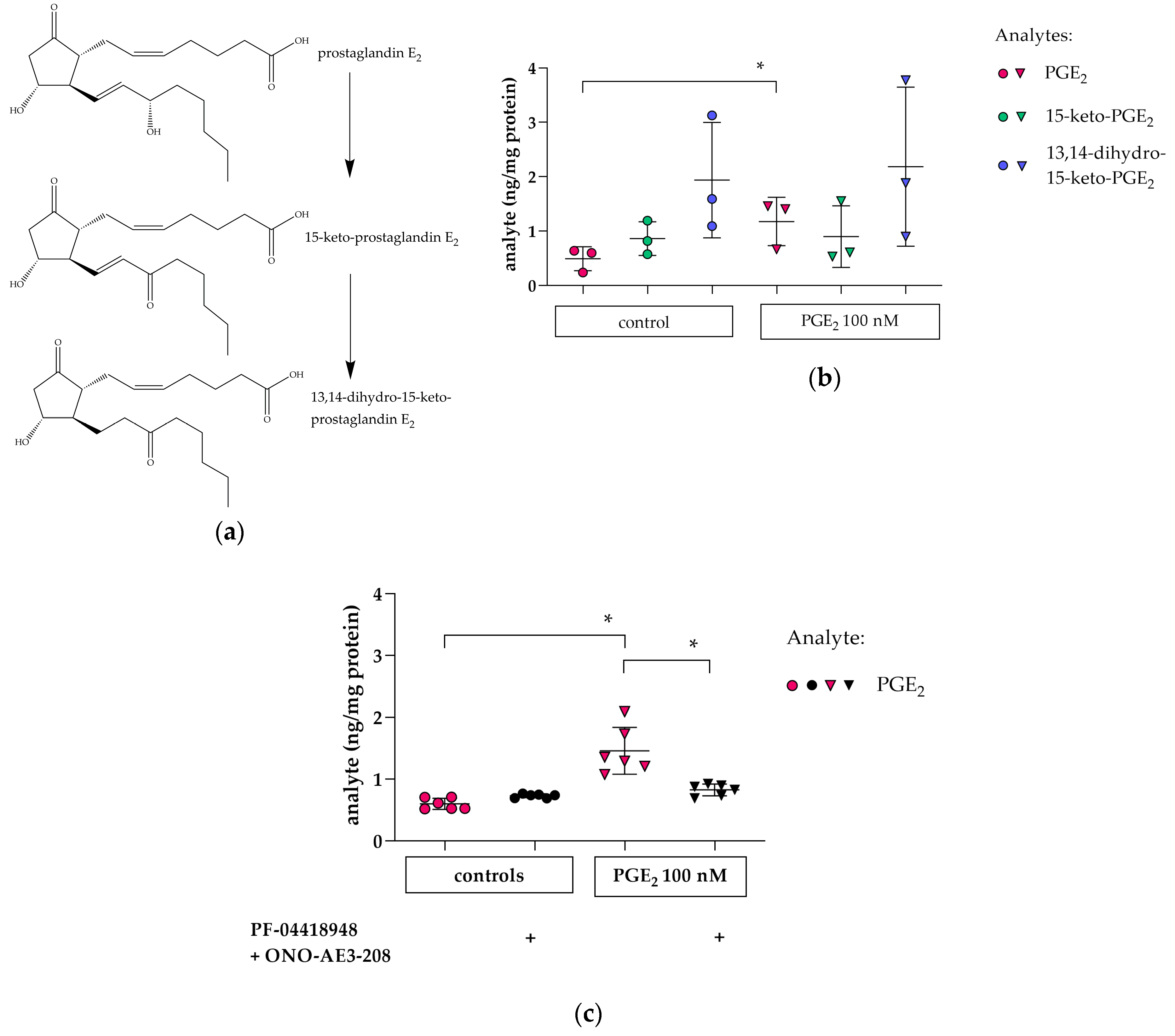
2.5. LC/ESI-MS/MS for Analysis of Prostaglandins
2.5.1. Sample Preparation
2.5.2. LC/ESI-MS/MS
2.6. FFSS
2.7. Animals
2.8. Statistics
3. Results
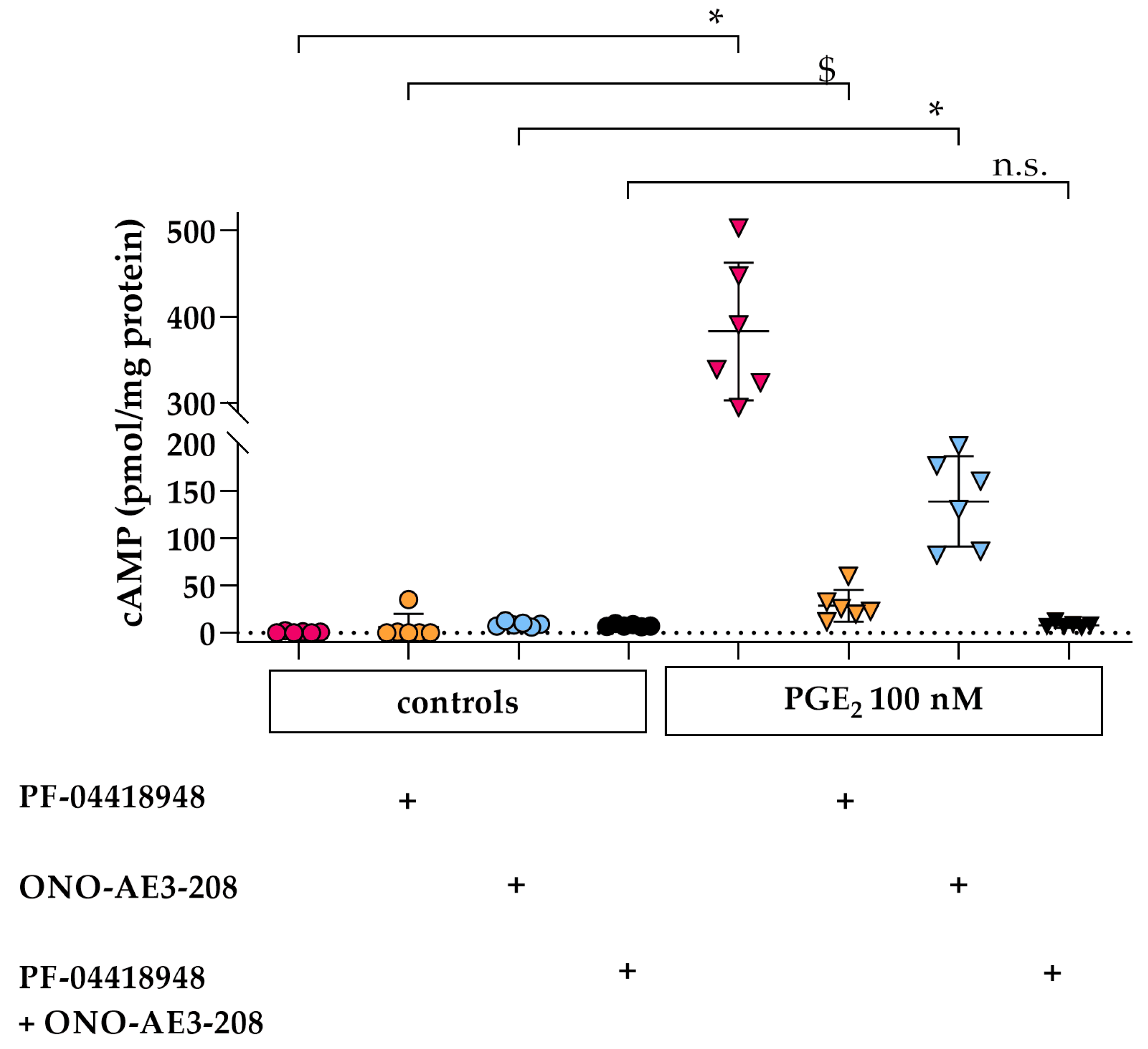
3.1. PGE2 Leads to EP2- and EP4- Dependent Increased cAMP Levels in Differentiated hPC
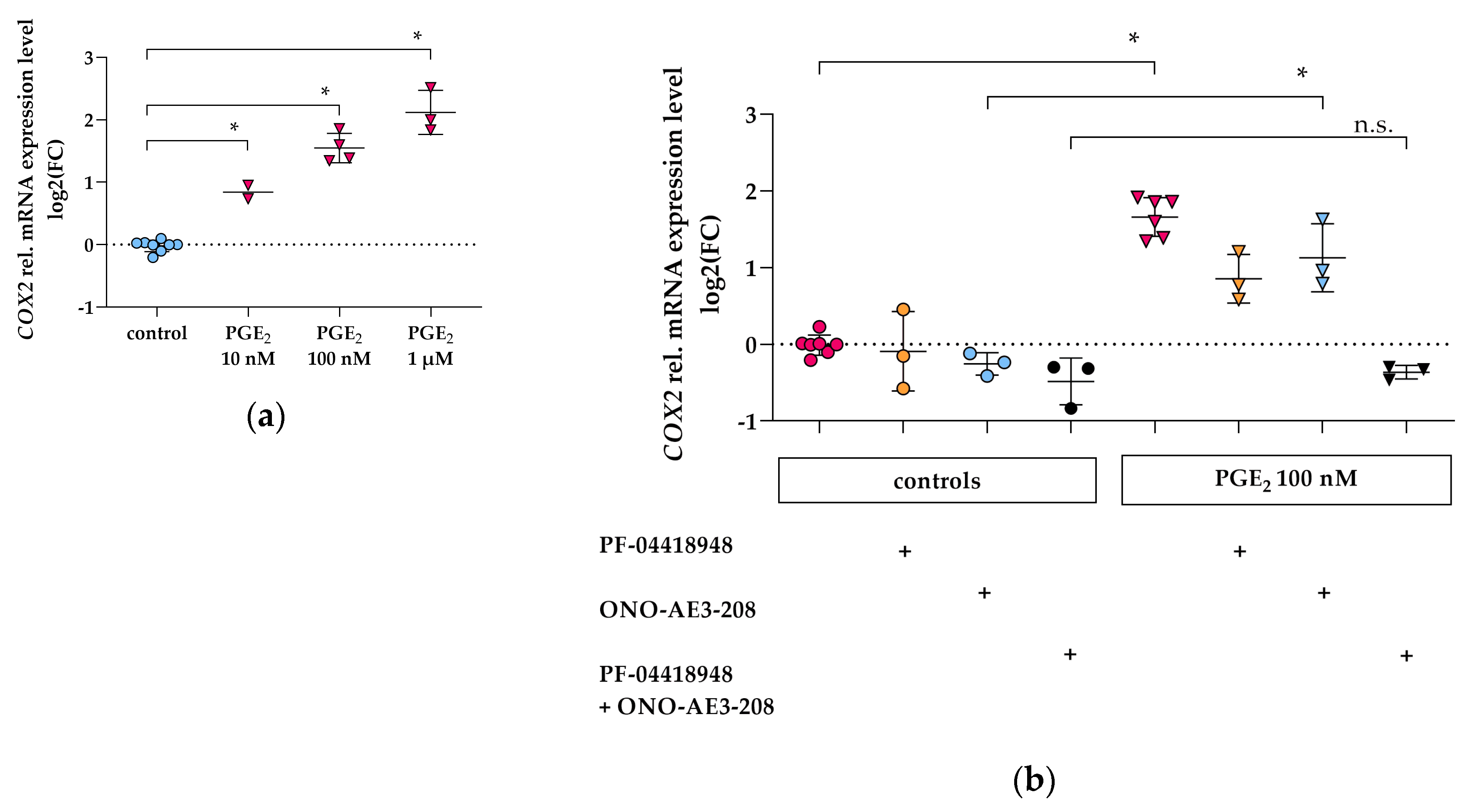
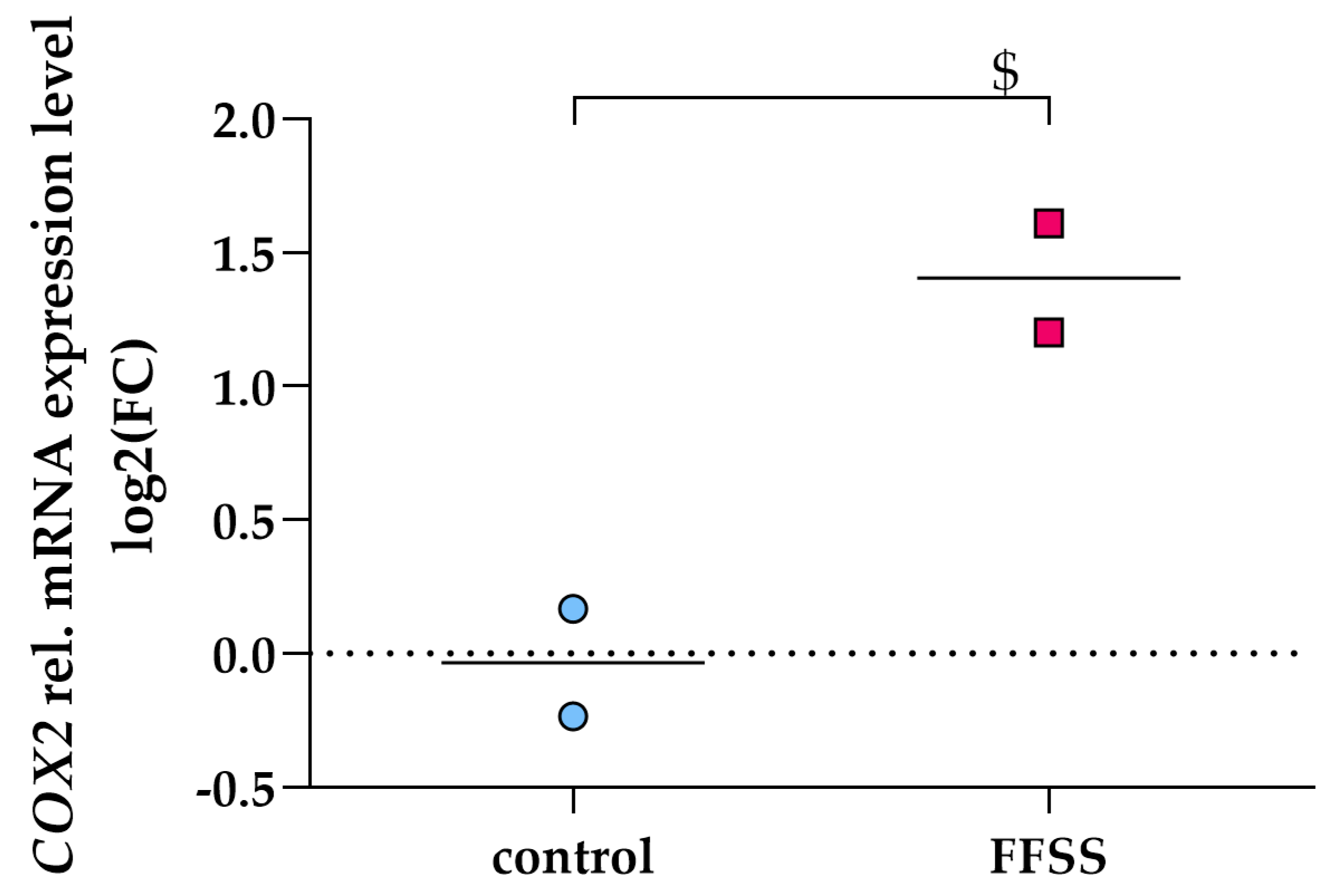
3.2. PGE2 Induces COX2 Gene Expression via EP2 and EP4 Signaling in Differentiated hPC
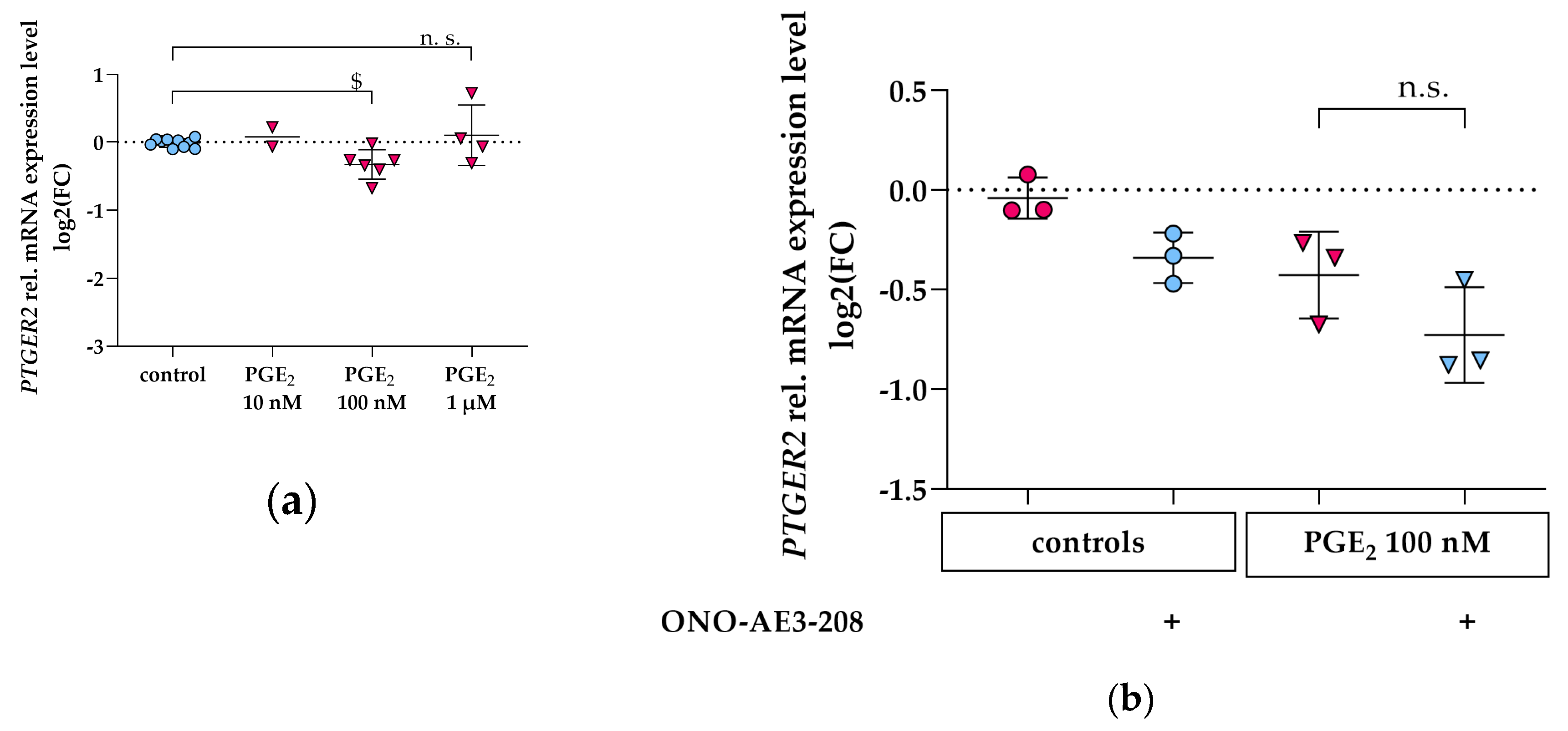
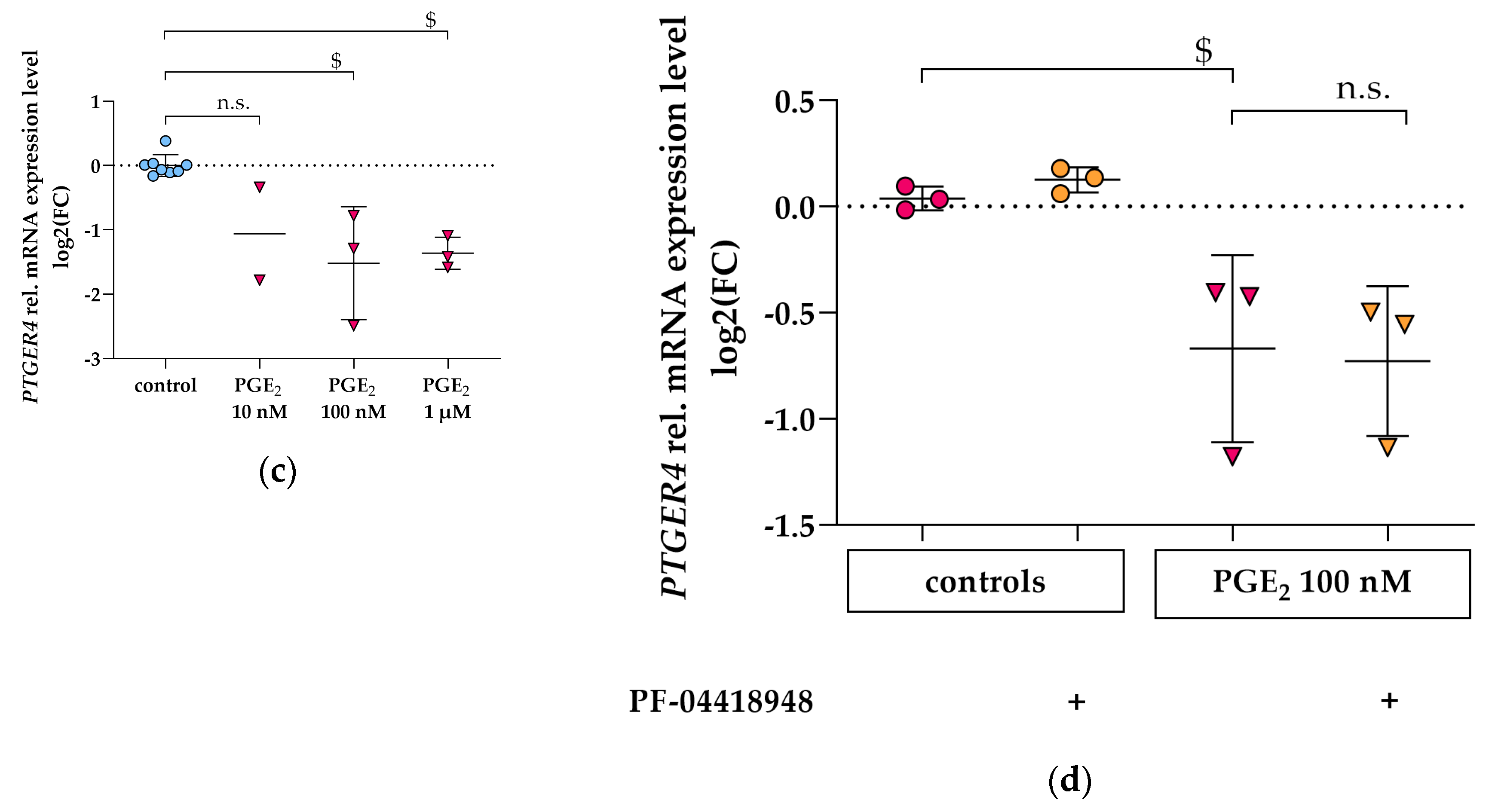
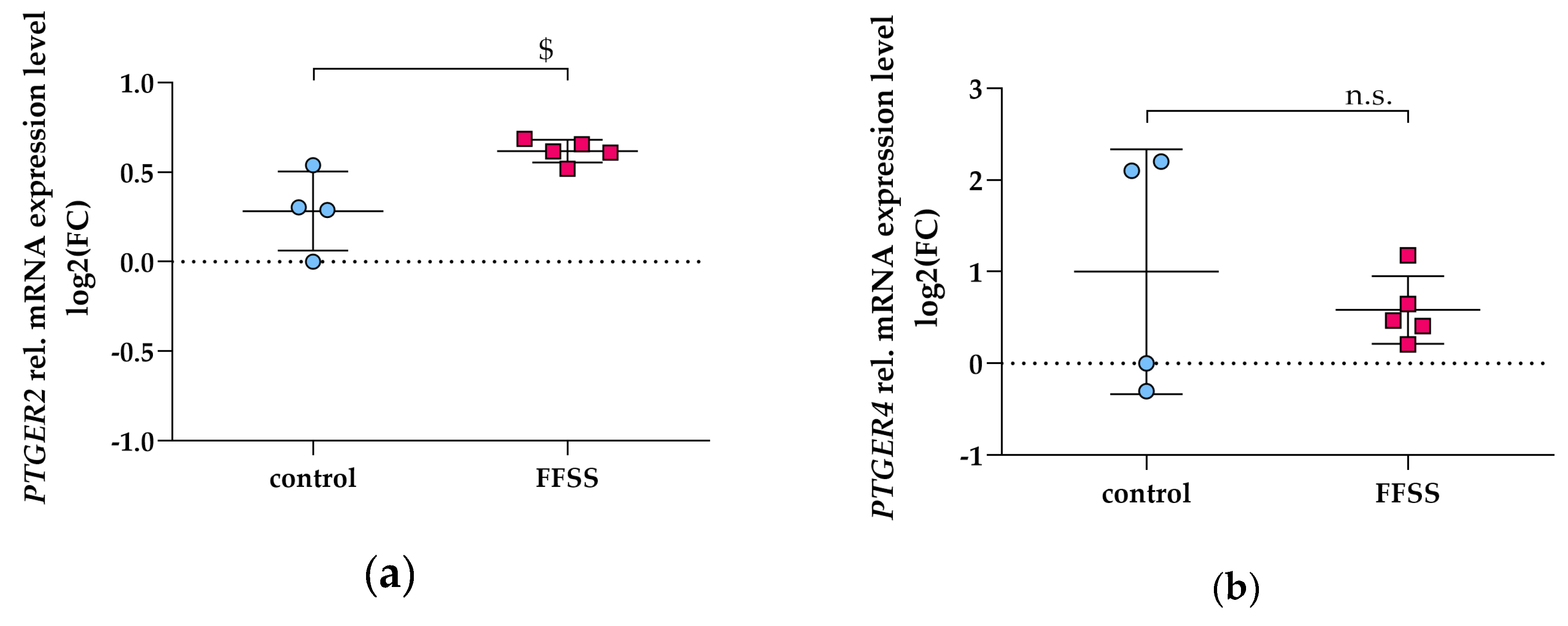
3.3. PGE2 Reduces PTGER2 and PTGER4 Gene Expression Which Is Not Modified by EP2 or EP4 Antagonists in Differentiated hPC
3.4. Cellular PGE2 and Metabolite Profile in hPC after PGE2 Stimulation: Effects of EP2 and EP4 Blockade
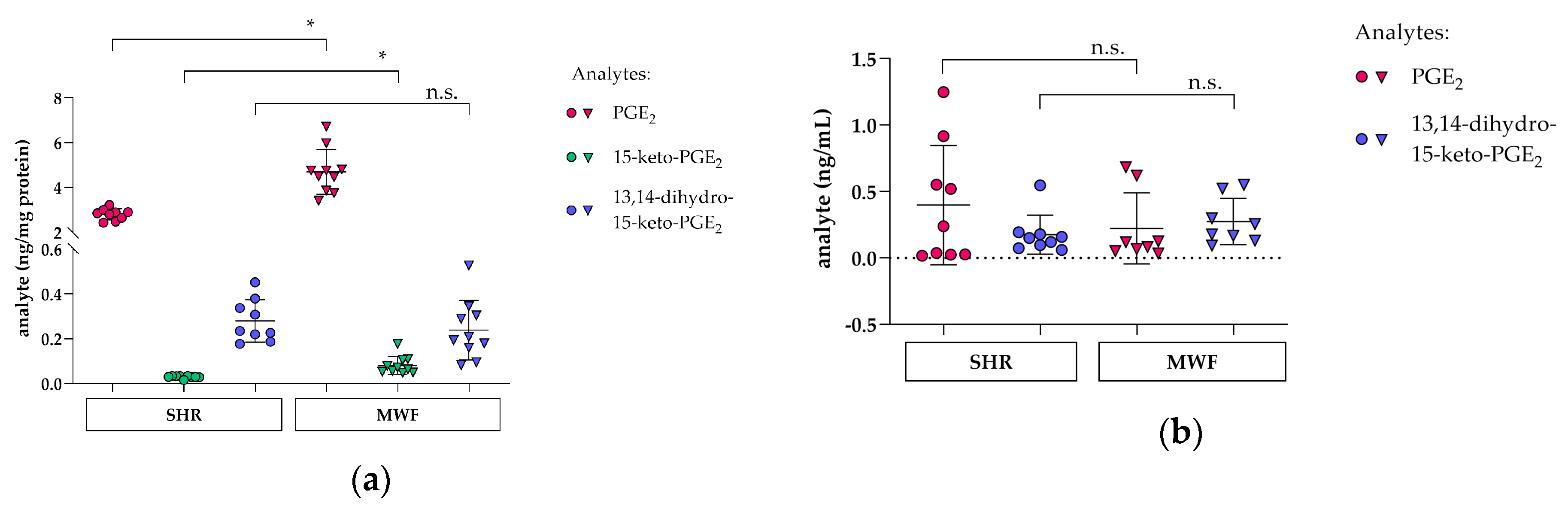
3.5. Glomerular PGE2 and Metabolite Profile in Glomeruli and Plasma in the CKD MWF Model
3.6. FFSS Increases COX2 and PTGER2 Gene Expression in hPC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pavenstadt, H.; Kriz, W.; Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003, 83, 253–307. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, T.; Hariharan, S.; Alon, U.S.; McCarthy, E.T.; Sharma, R.; El-Meanawy, A.; Savin, V.J.; Sharma, M. Hyperfiltration-mediated Injury in the Remaining Kidney of a Transplant Donor. Transplantation 2018, 102, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, T.; Celsi, G.E.; Sharma, M.; Dai, H.; McCarthy, E.T.; Ruiz, M.; Cudmore, P.A.; Alon, U.S.; Sharma, R.; Savin, V.A. Fluid flow shear stress over podocytes is increased in the solitary kidney. Nephrol Dial. Transpl. 2014, 29, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M. Nephron adaptation to renal injury or ablation. Am. J. Physiol. 1985, 249, F324–F337. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, R.; McCarthy, E.T.; Savin, V.J.; Srivastava, T. Hyperfiltration-associated biomechanical forces in glomerular injury and response: Potential role for eicosanoids. Prostaglandins Lipid Mediat. 2017, 132, 59–68. [Google Scholar] [CrossRef]
- Friedrich, C.; Endlich, N.; Kriz, W.; Endlich, K. Podocytes are sensitive to fluid shear stress in vitro. Am. J. Physiol. Ren. Physiol. 2006, 291, F856–865. [Google Scholar] [CrossRef]
- Endlich, N.; Endlich, K. The challenge and response of podocytes to glomerular hypertension. Semin. Nephrol 2012, 32, 327–341. [Google Scholar] [CrossRef]
- Brenner, B.M.; Lawler, E.V.; Mackenzie, H.S. The hyperfiltration theory: A paradigm shift in nephrology. Kidney Int. 1996, 49, 1774–1777. [Google Scholar] [CrossRef]
- Futrakul, N.; Sridama, V.; Futrakul, P. Microalbuminuria–A biomarker of renal microvascular disease. Ren Fail. 2009, 31, 140–143. [Google Scholar] [CrossRef]
- Srivastava, T.; Alon, U.S.; Cudmore, P.A.; Tarakji, B.; Kats, A.; Garola, R.E.; Duncan, R.S.; McCarthy, E.T.; Sharma, R.; Johnson, M.L.; et al. Cyclooxygenase-2, prostaglandin E2, and prostanoid receptor EP2 in fluid flow shear stress-mediated injury in the solitary kidney. Am. J. Physiol. Ren. Physiol. 2014, 307, F1323–1333. [Google Scholar] [CrossRef]
- Smith, W.L. The eicosanoids and their biochemical mechanisms of action. Biochem. J. 1989, 259, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, B.; Granstrom, E.; Green, K.; Hamberg, M.; Hammarstrom, S. Prostaglandins. Annu. Rev. Biochem. 1975, 44, 669–695. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, T.; McCarthy, E.T.; Sharma, R.; Kats, A.; Carlton, C.G.; Alon, U.S.; Cudmore, P.A.; El-Meanawy, A.; Sharma, M. Fluid flow shear stress upregulates prostanoid receptor EP2 but not EP4 in murine podocytes. Prostaglandins Lipid Mediat. 2013, 104–105, 49–57. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science (N. Y.) 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Narumiya, S.; Ichikawa, A. Distribution and function of prostanoid receptors: studies from knockout mice. Prog Lipid Res. 2000, 39, 289–314. [Google Scholar] [CrossRef]
- Negishi, M.; Sugimoto, Y.; Ichikawa, A. Molecular mechanisms of diverse actions of prostanoid receptors. Biochim. Biophys Acta 1995, 1259, 109–119. [Google Scholar] [CrossRef]
- Cheng, B.; Kato, Y.; Zhao, S.; Luo, J.; Sprague, E.; Bonewald, L.F.; Jiang, J.X. PGE(2) is essential for gap junction-mediated intercellular communication between osteocyte-like MLO-Y4 cells in response to mechanical strain. Endocrinology 2001, 142, 3464–3473. [Google Scholar] [CrossRef]
- Faour, W.H.; Gomi, K.; Kennedy, C.R. PGE(2) induces COX-2 expression in podocytes via the EP(4) receptor through a PKA-independent mechanism. Cell. Signal. 2008, 20, 2156–2164. [Google Scholar] [CrossRef]
- Pino, M.S.; Nawrocki, S.T.; Cognetti, F.; Abruzzese, J.L.; Xiong, H.Q.; McConkey, D.J. Prostaglandin E2 drives cyclooxygenase-2 expression via cyclic AMP response element activation in human pancreatic cancer cells. Cancer Biol. Ther. 2005, 4, 1263–1269. [Google Scholar] [CrossRef][Green Version]
- Ansari, K.M.; Sung, Y.M.; He, G.; Fischer, S.M. Prostaglandin receptor EP2 is responsible for cyclooxygenase-2 induction by prostaglandin E2 in mouse skin. Carcinogenesis 2007, 28, 2063–2068. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Muñoz, M.D.; Osma-García, I.C.; Fresno, M.; Iñiguez, M.A. Involvement of PGE2and the cAMP signalling pathway in the up-regulation of COX-2 and mPGES-1 expression in LPS-activated macrophages. Biochem. J. 2012, 443, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, T.; McCarthy, E.T.; Sharma, R.; Cudmore, P.A.; Sharma, M.; Johnson, M.L.; Bonewald, L.F. Prostaglandin E(2) is crucial in the response of podocytes to fluid flow shear stress. J. Cell Commun. Signal. 2010, 4, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Faupel-Badger, J.M.; Fuhrman, B.J.; Xu, X.; Falk, R.T.; Keefer, L.K.; Veenstra, T.D.; Hoover, R.N.; Ziegler, R.G. Comparison of liquid chromatography-tandem mass spectrometry, RIA, and ELISA methods for measurement of urinary estrogens. Cancer Epidemiol. Biomark. Prev. 2010, 19, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.S.; Budac, D.; Khayrullina, T.; Staal, R.; Chandrasena, G. Quantitative analysis of lipids: a higher-throughput LC-MS/MS-based method and its comparison to ELISA. Future Sci. OA 2017, 3, Fso157. [Google Scholar] [CrossRef] [PubMed]
- Mesaros, C.; Lee, S.H.; Blair, I.A. Analysis of epoxyeicosatrienoic acids by chiral liquid chromatography/electron capture atmospheric pressure chemical ionization mass spectrometry using [13C]-analog internal standards. Rapid Commun. Mass Spectrom. 2010, 24, 3237–3247. [Google Scholar] [CrossRef]
- Nithipatikom, K.; Laabs, N.D.; Isbell, M.A.; Campbell, W.B. Liquid chromatographic-mass spectrometric determination of cyclooxygenase metabolites of arachidonic acid in cultured cells. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 785, 135–145. [Google Scholar] [CrossRef]
- Kempen, E.C.; Yang, P.; Felix, E.; Madden, T.; Newman, R.A. Simultaneous quantification of arachidonic acid metabolites in cultured tumor cells using high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Anal. Biochem. 2001, 297, 183–190. [Google Scholar] [CrossRef]
- Rund, K.M.; Ostermann, A.I.; Kutzner, L.; Galano, J.M.; Oger, C.; Vigor, C.; Wecklein, S.; Seiwert, N.; Durand, T.; Schebb, N.H. Development of an LC-ESI(-)-MS/MS method for the simultaneous quantification of 35 isoprostanes and isofurans derived from the major n3- and n6-PUFAs. Anal. Chim. Acta 2018, 1037, 63–74. [Google Scholar] [CrossRef]
- Deems, R.; Buczynski, M.W.; Bowers-Gentry, R.; Harkewicz, R.; Dennis, E.A. Detection and quantitation of eicosanoids via high performance liquid chromatography-electrospray ionization-mass spectrometry. Methods Enzym. 2007, 432, 59–82. [Google Scholar] [CrossRef]
- Bollinger, J.G.; Thompson, W.; Lai, Y.; Oslund, R.C.; Hallstrand, T.S.; Sadilek, M.; Turecek, F.; Gelb, M.H. Improved sensitivity mass spectrometric detection of eicosanoids by charge reversal derivatization. Anal. Chem. 2010, 82, 6790–6796. [Google Scholar] [CrossRef] [PubMed]
- Le Faouder, P.; Baillif, V.; Spreadbury, I.; Motta, J.P.; Rousset, P.; Chene, G.; Guigne, C.; Terce, F.; Vanner, S.; Vergnolle, N.; et al. LC-MS/MS method for rapid and concomitant quantification of pro-inflammatory and pro-resolving polyunsaturated fatty acid metabolites. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 932, 123–133. [Google Scholar] [CrossRef]
- Saleem, M.A.; O’Hare, M.J.; Reiser, J.; Coward, R.J.; Inward, C.D.; Farren, T.; Xing, C.Y.; Ni, L.; Mathieson, P.W.; Mundel, P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002, 13, 630–638. [Google Scholar] [PubMed]
- Ni, L.; Saleem, M.; Mathieson, P.W. Podocyte culture: Tricks of the trade. Nephrology (Carlton Vic.) 2012, 12, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Aoki, T.; Tsuruyama, T.; Narumiya, S. Definition of Prostaglandin E2-EP2 Signals in the Colon Tumor Microenvironment That Amplify Inflammation and Tumor Growth. Cancer Res. 2015, 75, 2822–2832. [Google Scholar] [CrossRef] [PubMed]
- af Forselles, K.J.; Root, J.; Clarke, T.; Davey, D.; Aughton, K.; Dack, K.; Pullen, N. In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP(2) receptor antagonist. Br. J. Pharmacol. 2011, 164, 1847–1856. [Google Scholar] [CrossRef]
- Thieme, K.; Majumder, S.; Brijmohan, A.S.; Batchu, S.N.; Bowskill, B.B.; Alghamdi, T.A.; Advani, S.L.; Kabir, M.G.; Liu, Y.; Advani, A. EP4 inhibition attenuates the development of diabetic and non-diabetic experimental kidney disease. Sci. Rep. 2017, 7, 3442. [Google Scholar] [CrossRef]
- Kabashima, K.; Saji, T.; Murata, T.; Nagamachi, M.; Matsuoka, T.; Segi, E.; Tsuboi, K.; Sugimoto, Y.; Kobayashi, T.; Miyachi, Y.; et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J. Clin. Investig. 2002, 109, 883–893. [Google Scholar] [CrossRef]
- Bek, M.; Nusing, R.; Kowark, P.; Henger, A.; Mundel, P.; Pavenstadt, H. Characterization of prostanoid receptors in podocytes. J. Am. Soc. Nephrol. 1999, 10, 2084–2093. [Google Scholar]
- Srivastava, T.; Dai, H.; Heruth, D.P.; Alon, U.S.; Garola, R.E.; Zhou, J.; Duncan, R.S.; El-Meanawy, A.; McCarthy, E.T.; Sharma, R.; et al. Mechanotransduction signaling in podocytes from fluid flow shear stress. Am. J. Physiol. Ren. Physiol. 2018, 314, F22–F34. [Google Scholar] [CrossRef]
- Dey, I.; Giembycz, M.A.; Chadee, K. Prostaglandin E(2) couples through EP(4) prostanoid receptors to induce IL-8 production in human colonic epithelial cell lines. Br. J. Pharmacol. 2009, 156, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Aoki, T.; Thumkeo, D.; Siriwach, R.; Yao, C.; Narumiya, S. T cell-intrinsic prostaglandin E2-EP2/EP4 signaling is critical in pathogenic TH17 cell-driven inflammation. J. Allergy Clin. Immunol. 2019, 143, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, P.; Wang, W.; Li, W.; Shu, Q.; Chen, Z.J.; Myatt, L.; Sun, K. Inhibition of lysyl oxidase by prostaglandin E2 via EP2/EP4 receptors in human amnion fibroblasts: Implications for parturition. Mol. Cell. Endocrinol. 2016, 424, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Muller, N.V.; van de Lest, N.A.; Eisenreich, A.; Schmidbauer, M.; Barysenka, A.; Purfurst, B.; Sporbert, A.; Lorenzen, T.; Meyer, A.M.; et al. Analysis of the genomic architecture of a complex trait locus in hypertensive rat models links Tmem63c to kidney damage. eLife 2019, 8. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; McGiff, J.C.; Quilley, J.; Sacerdoti, D.; Reddy, L.M.; Falck, J.R.; Zhang, F.; Lerea, K.M.; Wong, P.Y. Identification of 5,6-trans-epoxyeicosatrienoic acid in the phospholipids of red blood cells. J. Biol. Chem. 2004, 279, 36412–36418. [Google Scholar] [CrossRef]
- Schulz, A.; Kreutz, R. Mapping genetic determinants of kidney damage in rat models. Hypertens. Res. 2012, 35, 675–694. [Google Scholar] [CrossRef][Green Version]
- Vogt, L.; Laverman, G.D.; Navis, G. Time for a comeback of NSAIDs in proteinuric chronic kidney disease? Neth. J. Med. 2010, 68, 400–407. [Google Scholar]
- Liu, J.; Zhang, Y.D.; Chen, X.L.; Zhu, X.L.; Chen, X.; Wu, J.H.; Guo, N.F. The protective effect of the EP2 receptor on TGF-beta1 induced podocyte injury via the PI3K/Akt signaling pathway. PLoS ONE 2018, 13, e0197158. [Google Scholar] [CrossRef]
- Lemieux, L.I.; Rahal, S.S.; Kennedy, C.R. PGE2 reduces arachidonic acid release in murine podocytes: evidence for an autocrine feedback loop. Am. J. Physiol. Cell Physiol. 2003, 284, C302–309. [Google Scholar] [CrossRef]
- Regan, J.W. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003, 74, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Fujino, H.; West, K.A.; Regan, J.W. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J. Biol. Chem. 2002, 277, 2614–2619. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.H.; Lin, Y.M.; Shen, C.Y.; Shibu, M.A.; Li, S.Y.; Chang, S.H.; Lin, C.C.; Chen, R.J.; Viswanadha, V.P.; Shih, H.N.; et al. Prostaglandin E2-Induced COX-2 Expressions via EP2 and EP4 Signaling Pathways in Human LoVo Colon Cancer Cells. Int. J. Mol. Sci. 2017, 18, 1132. [Google Scholar] [CrossRef] [PubMed]
- Fujino, H.; Xu, W.; Regan, J.W. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J. Biol. Chem. 2003, 278, 12151–12156. [Google Scholar] [CrossRef]
- Kitase, Y.; Barragan, L.; Qing, H.; Kondoh, S.; Jiang, J.X.; Johnson, M.L.; Bonewald, L.F. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the beta-catenin and PKA pathways. J. Bone Min. Res. 2010, 25, 2657–2668. [Google Scholar] [CrossRef]
- Cherian, P.P.; Cheng, B.; Gu, S.; Sprague, E.; Bonewald, L.F.; Jiang, J.X. Effects of mechanical strain on the function of Gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J. Biol. Chem. 2003, 278, 43146–43156. [Google Scholar] [CrossRef]
- Fujino, H.; Salvi, S.; Regan, J.W. Differential regulation of phosphorylation of the cAMP response element-binding protein after activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Mol. Pharmacol. 2005, 68, 251–259. [Google Scholar] [CrossRef]
- Lu, J.W.; Wang, W.S.; Zhou, Q.; Gan, X.W.; Myatt, L.; Sun, K. Activation of prostaglandin EP4 receptor attenuates the induction of cyclooxygenase-2 expression by EP2 receptor activation in human amnion fibroblasts: implications for parturition. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 8148–8160. [Google Scholar] [CrossRef]
- Majumder, M.; Xin, X.; Liu, L.; Tutunea-Fatan, E.; Rodriguez-Torres, M.; Vincent, K.; Postovit, L.M.; Hess, D.; Lala, P.K. COX-2 Induces Breast Cancer Stem Cells via EP4/PI3K/AKT/NOTCH/WNT Axis. Stem Cells 2016, 34, 2290–2305. [Google Scholar] [CrossRef]
- Vo, B.T.; Morton, D., Jr.; Komaragiri, S.; Millena, A.C.; Leath, C.; Khan, S.A. TGF-beta effects on prostate cancer cell migration and invasion are mediated by PGE2 through activation of PI3K/AKT/mTOR pathway. Endocrinology 2013, 154, 1768–1779. [Google Scholar] [CrossRef]
- Gao, F.; Zafar, M.I.; Juttner, S.; Hocker, M.; Wiedenmann, B. Expression and Molecular Regulation of the Cox2 Gene in Gastroenteropancreatic Neuroendocrine Tumors and Antiproliferation of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs). Med. Sci. Monit. 2018, 24, 8125–8140. [Google Scholar] [CrossRef] [PubMed]
- Mesa, J.; Alsina, C.; Oppermann, U.; Pares, X.; Farres, J.; Porte, S. Human prostaglandin reductase 1 (PGR1): Substrate specificity, inhibitor analysis and site-directed mutagenesis. Chem. Biol. Interact. 2015, 234, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Chang, Y.C.; Su, T.H.; Nong, J.Y.; Li, C.C.; Chuang, L.M. Prostaglandin reductase-3 negatively modulates adipogenesis through regulation of PPARgamma activity. J. Lipid Res. 2013, 54, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.H.; Ensor, C.M.; Tong, M.; Zhou, H.; Yan, F. Prostaglandin catabolizing enzymes. Prostaglandins Lipid Mediat. 2002, 68–69, 483–493. [Google Scholar] [CrossRef]
- Reid, G.; Wielinga, P.; Zelcer, N.; van der Heijden, I.; Kuil, A.; de Haas, M.; Wijnholds, J.; Borst, P. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc. Natl. Acad. Sci. USA 2003, 100, 9244–9249. [Google Scholar] [CrossRef]
- Chan, B.S.; Satriano, J.A.; Pucci, M.; Schuster, V.L. Mechanism of prostaglandin E2 transport across the plasma membrane of HeLa cells and Xenopus oocytes expressing the prostaglandin transporter ‘’PGT”. J. Biol. Chem. 1998, 273, 6689–6697. [Google Scholar] [CrossRef]
- Shirasaka, Y.; Shichiri, M.; Kasai, T.; Ohno, Y.; Nakanishi, T.; Hayashi, K.; Nishiura, A.; Tamai, I. A role of prostaglandin transporter in regulating PGE(2) release from human bronchial epithelial BEAS-2B cells in response to LPS. J. Endocrinol. 2013, 217, 265–274. [Google Scholar] [CrossRef]
- Kosaka, T.; Miyata, A.; Ihara, H.; Hara, S.; Sugimoto, T.; Takeda, O.; Takahashi, E.; Tanabe, T. Characterization of the human gene (PTGS2) encoding prostaglandin-endoperoxide synthase 2. Eur. J. Biochem. 1994, 221, 889–897. [Google Scholar] [CrossRef]
- Srivastava, T.; Ju, W.; Milne, G.L.; Rezaiekhaligh, M.H.; Staggs, V.S.; Alon, U.S.; Sharma, R.; Zhou, J.; El-Meanawy, A.; McCarthy, E.T.; et al. Urinary prostaglandin E2 is a biomarker of early adaptive hyperfiltration in solitary functioning kidney. Prostaglandins Lipid Mediat. 2020, 146, 106403. [Google Scholar] [CrossRef]
- Needleman, P.; Turk, J.; Jakschik, B.A.; Morrison, A.R.; Lefkowith, J.B. Arachidonic acid metabolism. Annu. Rev. Biochem. 1986, 55, 69–102. [Google Scholar] [CrossRef]
- Schulz, A.; Weiss, J.; Schlesener, M.; Hansch, J.; Wehland, M.; Wendt, N.; Kossmehl, P.; Sietmann, A.; Grimm, D.; Stoll, M.; et al. Development of overt proteinuria in the Munich Wistar Fromter rat is suppressed by replacement of chromosome 6 in a consomic rat strain. J. Am. Soc. Nephrol. 2007, 18, 113–121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fassi, A.; Sangalli, F.; Maffi, R.; Colombi, F.; Mohamed, E.I.; Brenner, B.M.; Remuzzi, G.; Remuzzi, A. Progressive glomerular injury in the MWF rat is predicted by inborn nephron deficit. J. Am. Soc. Nephrol. 1998, 9, 1399–1406. [Google Scholar] [PubMed]
- Remuzzi, A.; Puntorieri, S.; Mazzoleni, A.; Remuzzi, G. Sex related differences in glomerular ultrafiltration and proteinuria in Munich-Wistar rats. Kidney Int. 1988, 34, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Macconi, D.; Bonomelli, M.; Benigni, A.; Plati, T.; Sangalli, F.; Longaretti, L.; Conti, S.; Kawachi, H.; Hill, P.; Remuzzi, G.; et al. Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am. J. Pathol. 2006, 168, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Ijpelaar, D.H.; Schulz, A.; Koop, K.; Schlesener, M.; Bruijn, J.A.; Kerjaschki, D.; Kreutz, R.; de Heer, E. Glomerular hypertrophy precedes albuminuria and segmental loss of podoplanin in podocytes in Munich-Wistar-Frömter rats. Am. J. Physiol. Ren. Physiol. 2008, 294, F758–F767. [Google Scholar] [CrossRef]
- van Es, N.; Schulz, A.; Ijpelaar, D.; van der Wal, A.; Kuhn, K.; Schutten, S.; Kossmehl, P.; Nyengaard, J.R.; de Heer, E.; Kreutz, R. Elimination of severe albuminuria in aging hypertensive rats by exchange of 2 chromosomes in double-consomic rats. Hypertension 2011, 58, 219–224. [Google Scholar] [CrossRef][Green Version]
- Boerries, M.; Grahammer, F.; Eiselein, S.; Buck, M.; Meyer, C.; Goedel, M.; Bechtel, W.; Zschiedrich, S.; Pfeifer, D.; Laloe, D.; et al. Molecular fingerprinting of the podocyte reveals novel gene and protein regulatory networks. Kidney Int. 2013, 83, 1052–1064. [Google Scholar] [CrossRef]
- Schell, C.; Baumhakl, L.; Salou, S.; Conzelmann, A.C.; Meyer, C.; Helmstadter, M.; Wrede, C.; Grahammer, F.; Eimer, S.; Kerjaschki, D.; et al. N-wasp is required for stabilization of podocyte foot processes. J. Am. Soc. Nephrol. 2013, 24, 713–721. [Google Scholar] [CrossRef]
- Staffel, J.; Valletta, D.; Federlein, A.; Ehm, K.; Volkmann, R.; Fuchsl, A.M.; Witzgall, R.; Kuhn, M.; Schweda, F. Natriuretic Peptide Receptor Guanylyl Cyclase-A in Podocytes is Renoprotective but Dispensable for Physiologic Renal Function. J. Am. Soc. Nephrol. 2017, 28, 260–277. [Google Scholar] [CrossRef]
- Koehler, S.; Kuczkowski, A.; Kuehne, L.; Jungst, C.; Hoehne, M.; Grahammer, F.; Eddy, S.; Kretzler, M.; Beck, B.B.; Hohfeld, J.; et al. Proteome Analysis of Isolated Podocytes Reveals Stress Responses in Glomerular Sclerosis. J. Am. Soc. Nephrol. 2020, 31, 544–559. [Google Scholar] [CrossRef]
- Sharma, R.; Waller, A.P.; Agrawal, S.; Wolfgang, K.J.; Luu, H.; Shahzad, K.; Isermann, B.; Smoyer, W.E.; Nieman, M.T.; Kerlin, B.A. Thrombin-Induced Podocyte Injury Is Protease-Activated Receptor Dependent. J. Am. Soc. Nephrol. 2017, 28, 2618–2630. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| hGAPDH | gagtcaacggatttggtcgt | gatctcgctcctggaagatg |
| hCOX2 | tgatgattgcccgactcccttg | tgaaagctggccctcgcttatg |
| hPTGER1 | ttcggcctccaccttctttg | cgcagtaggatgtacacccaag |
| hPTGER2 | gacggaccacctcattctcc | tccgacaacagaggactgaac |
| hPTGER3 | tctccgctcctgataatgatg | atctttccaaatggtcgctc |
| hPTGER4 | ttactcattgccacctccct | agtcaaaggacatcttctgcca |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangelsen, E.; Rothe, M.; Schulz, A.; Kourpa, A.; Panáková, D.; Kreutz, R.; Bolbrinker, J. Concerted EP2 and EP4 Receptor Signaling Stimulates Autocrine Prostaglandin E2 Activation in Human Podocytes. Cells 2020, 9, 1256. https://doi.org/10.3390/cells9051256
Mangelsen E, Rothe M, Schulz A, Kourpa A, Panáková D, Kreutz R, Bolbrinker J. Concerted EP2 and EP4 Receptor Signaling Stimulates Autocrine Prostaglandin E2 Activation in Human Podocytes. Cells. 2020; 9(5):1256. https://doi.org/10.3390/cells9051256
Chicago/Turabian StyleMangelsen, Eva, Michael Rothe, Angela Schulz, Aikaterini Kourpa, Daniela Panáková, Reinhold Kreutz, and Juliane Bolbrinker. 2020. "Concerted EP2 and EP4 Receptor Signaling Stimulates Autocrine Prostaglandin E2 Activation in Human Podocytes" Cells 9, no. 5: 1256. https://doi.org/10.3390/cells9051256
APA StyleMangelsen, E., Rothe, M., Schulz, A., Kourpa, A., Panáková, D., Kreutz, R., & Bolbrinker, J. (2020). Concerted EP2 and EP4 Receptor Signaling Stimulates Autocrine Prostaglandin E2 Activation in Human Podocytes. Cells, 9(5), 1256. https://doi.org/10.3390/cells9051256






