Phenotyping the Chilling and Freezing Responses of Young Microspore Stage Wheat Spikes Using Targeted Metabolome and Lipidome Profiling
Abstract
:1. Introduction
2. Materials and Methods
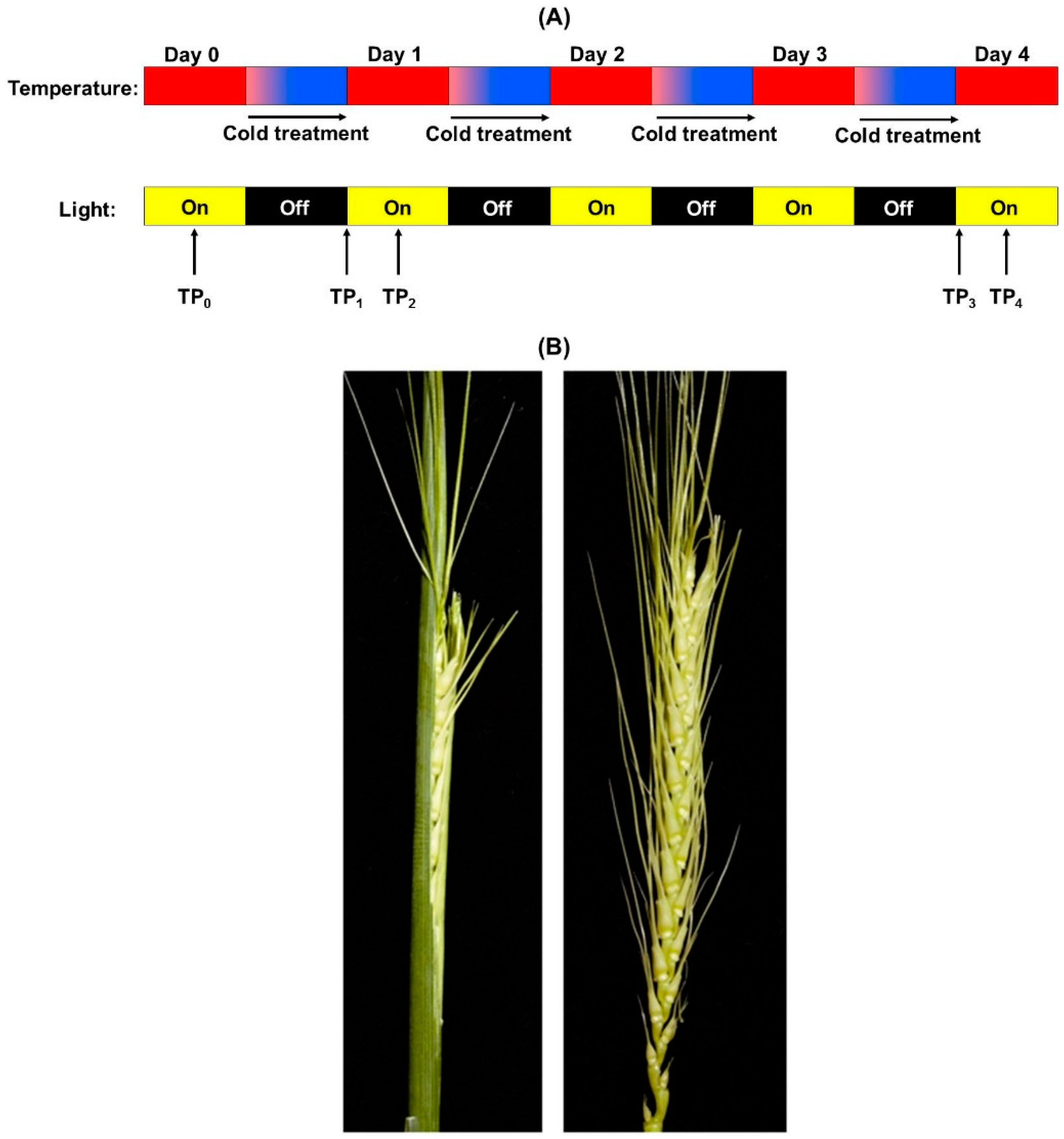
2.1. Controlled Environment Wheat Growth, Cold Treatment, and Tissue Sampling
2.2. Analysis of Sugars, Organic Acids, Amino Acids, and Amines
2.3. Analysis of Lipids
2.4. Processing of Raw Data
2.5. Statistical Analysis of Metabolite and Lipid Data
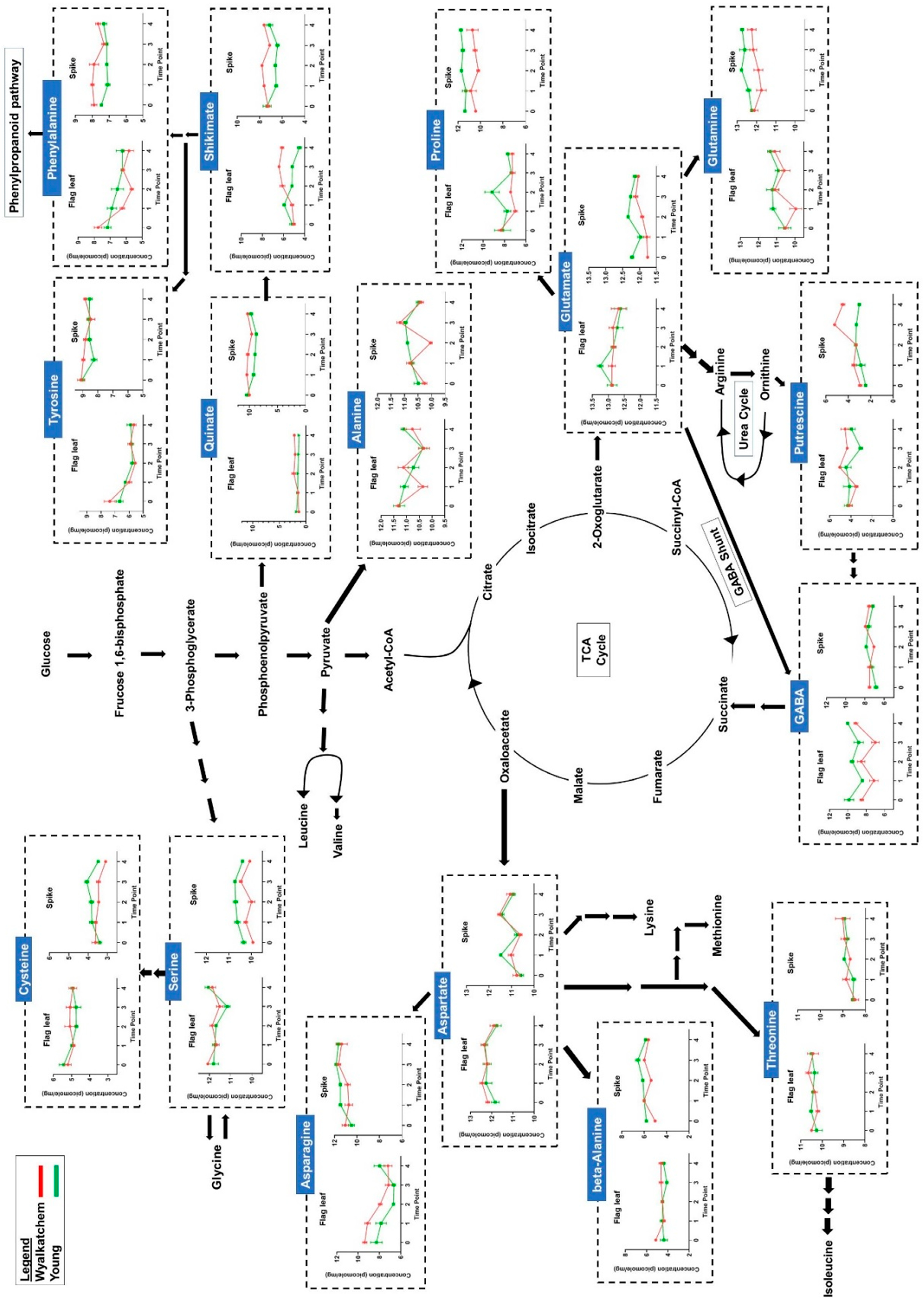
3. Results
3.1. Metabolite and Lipid Profiling Using YM Stage Wheat Spikes
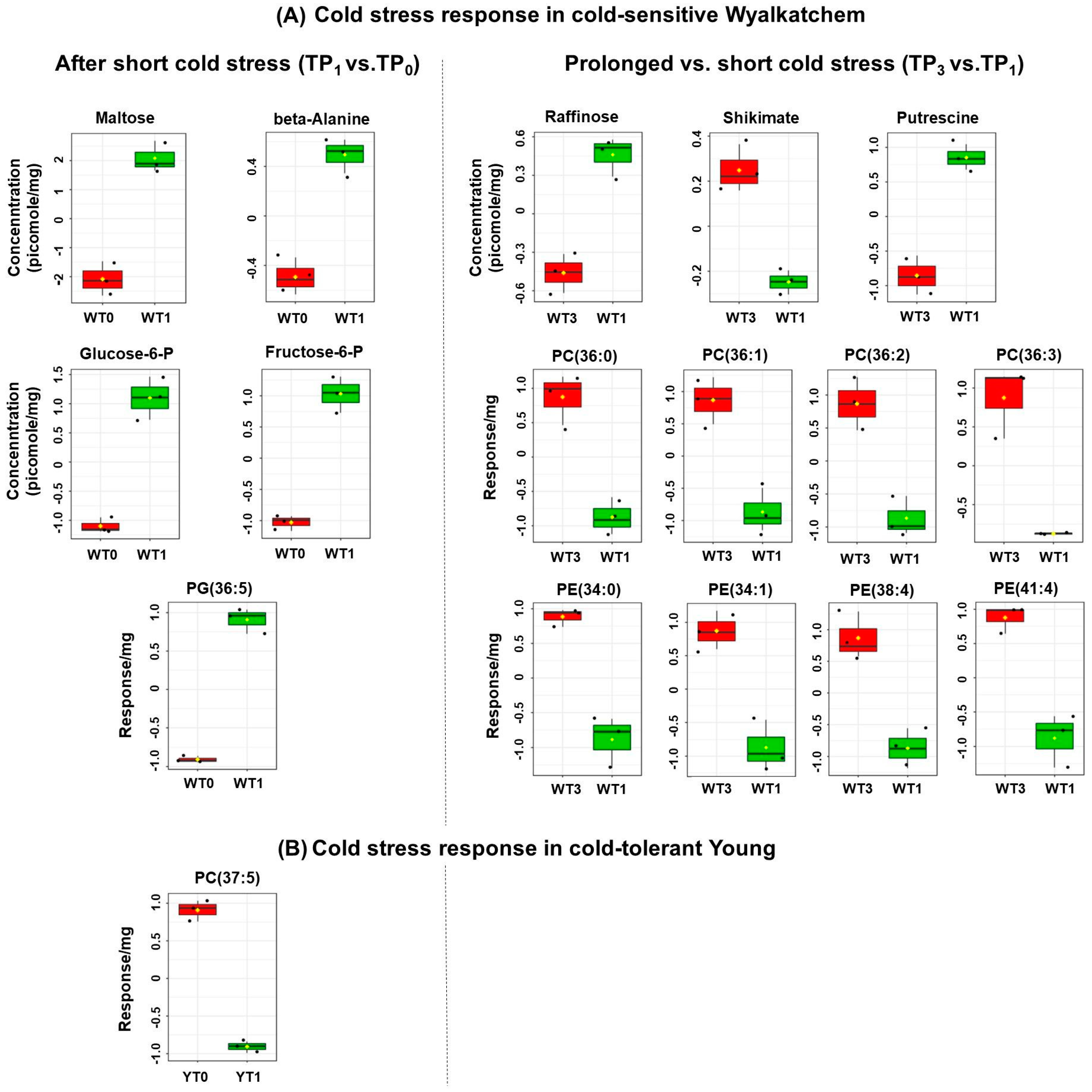
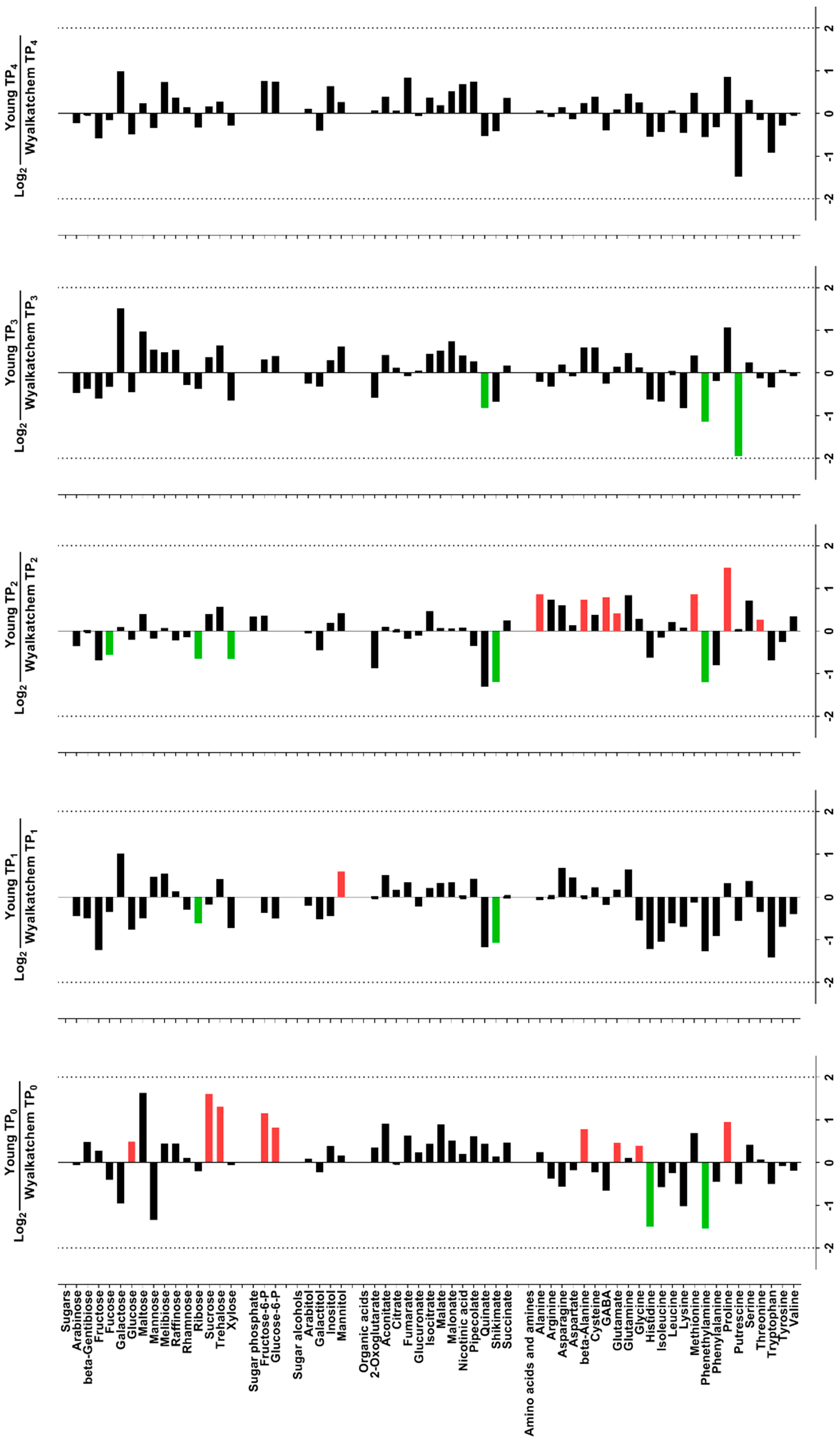
3.2. Cold Treatment Affects Spike Metabolites in Wyalkatchem More than Young Spikes
3.3. Varietal Differences in Metabolite and Lipid Levels before and after Cold Treatment
3.4. Differences in Primary Metabolite Levels between Spikes and Flag Leaves of Wyalkatchem and Young
3.5. Differences in Metabolite and Lipid Levels between Spikes and Flag Leaves of Wyalkatchem and Young
4. Discussion
4.1. Are Wheat Spikes or Flag Leaves Better for Cold Tolerance Phenotyping?
4.2. Spikes of Cold-Tolerant Young Are Potentially Better Prepared for Low Temperature Stress
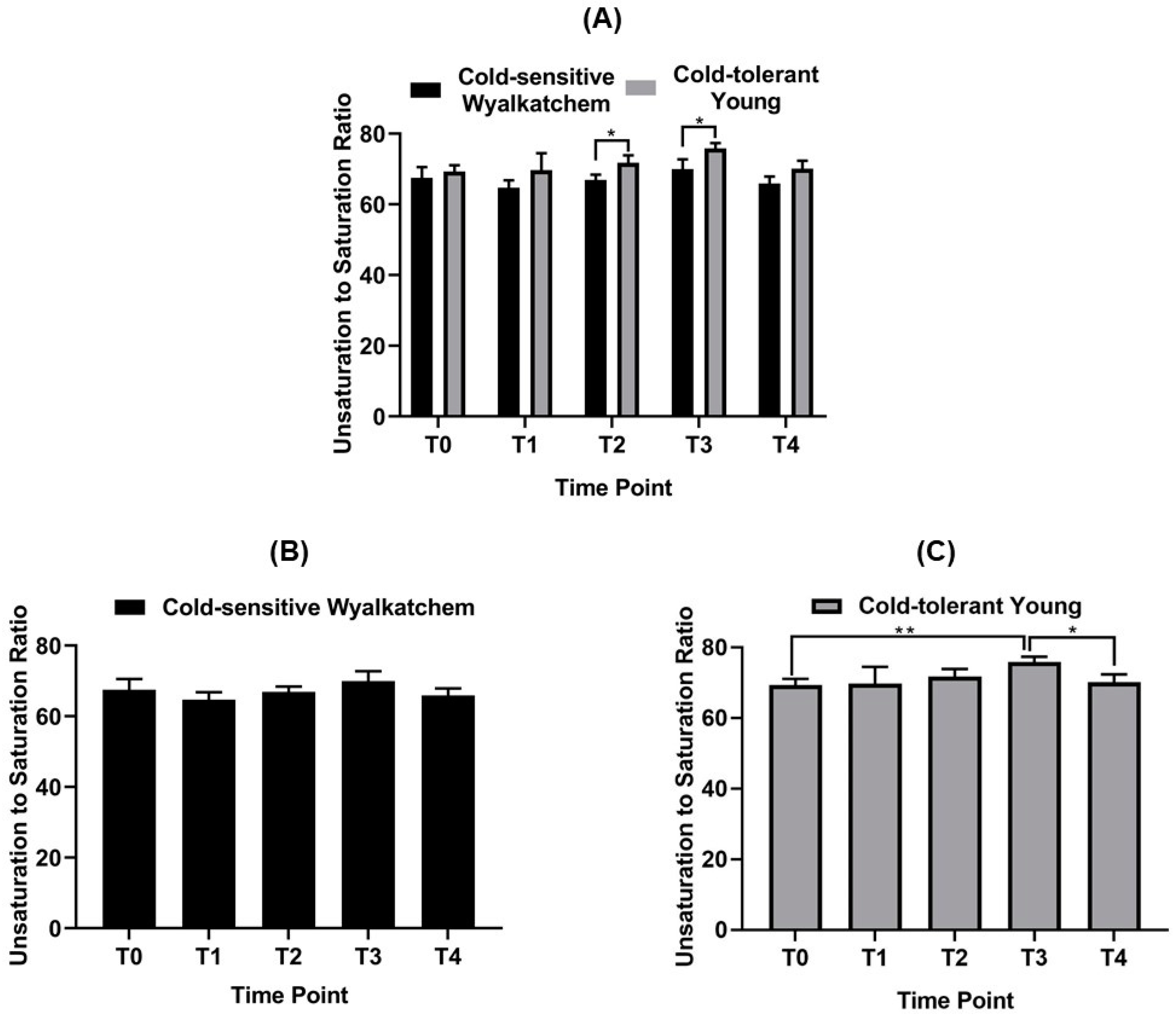
4.3. Lipid Re-Modelling Is Critical for Cold Acclimation in Wheat Spikes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paulsen, G.M.; Heyne, E.G. Grain production of winter wheat after spring freeze injury. Agron. J. 1983, 75, 705–707. [Google Scholar] [CrossRef]
- Boer, R.; Campbell, L.C.; Fletcher, D.J. Characteristics of frost in a major wheat-growing region of Australia. Aust. J. Agric. Res. 1993, 44, 1731–1743. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Hanson, P.J.; Post, W.M.; Kaiser, D.P.; Yang, B.; Nemani, R.; Pallardy, S.G.; Meyers, T. The 2007 Eastern US spring freeze: Increased cold damage in a warming world? BioScience 2008, 58, 253–262. [Google Scholar] [CrossRef]
- Frederiks, T.M.; Christopher, J.T.; Sutherland, M.W.; Borrell, A.K. Post-head-emergence frost in wheat and barley: Defining the problem, assessing the damage, and identifying resistance. J. Exp. Bot. 2015, 66, 3487–3498. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Chapman, S.C.; Christopher, J.T.; Frederiks, T.M.; Chenu, K. Frost trends and their estimated impact on yield in the Australian wheatbelt. J. Exp. Bot. 2015, 66, 3611–3623. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Jiang, D. Spring freeze effect on wheat yield is modulated by winter temperature fluctuations: Evidence from meta-analysis and simulating experiment. J. Agron. Crop Sci. 2014, 201, 288–300. [Google Scholar] [CrossRef]
- Crimp, S.; Bakar, K.S.; Kokic, P.; Jin, H.; Nicholls, N.; Howden, M. Bayesian space–time model to analyse frost risk for agriculture in Southeast Australia. Int. J. Climatol. 2015, 35, 2092–2108. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cai, J.; Liu, F.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Wheat plants exposed to winter warming are more susceptible to low temperature stress in the spring. Plant Growth Regul. 2015, 77, 11–19. [Google Scholar] [CrossRef]
- Marcellos, H.; Single, W.V. Temperatures in wheat during radiation frost. Aust. J. Exp. Agric. 1975, 15, 818–822. [Google Scholar] [CrossRef]
- March, T.; Knights, S.; Biddulph, B.; Ogbonnaya, F.; Maccallum, R.; Belford, R. The GRDC National Frost Initiative. Available online: https://grdc.com.au/Research-and-Development/GRDC-Update-Papers/2015/02/The-GRDC-National-Frost-Initiative (accessed on 4 April 2020).
- Frederiks, T.M.; Christopher, J.T.; Harvey, G.L.; Sutherland, M.W.; Borrell, A.K. Current and emerging screening methods to identify post-head-emergence frost adaptation in wheat and barley. J. Exp. Bot. 2012, 63, 5405–5416. [Google Scholar] [CrossRef] [Green Version]
- Single, W.V. Studies on frost injury to wheat. III. Screening of varieties for resistance to ear and stem frosting. Aust. J. Agric. Res. 1966, 17, 601–610. [Google Scholar] [CrossRef]
- Fowler, D.B.; Breton, G.; Limin, A.E.; Mahfoozi, S.; Sarhan, F. Photoperiod and temperature interactions regulate low-temperature-induced gene expression in barley. Plant Physiol. 2001, 127, 1676–1681. [Google Scholar] [CrossRef]
- Limin, A.E.; Fowler, D.B. Low-temperature tolerance and genetic potential in wheat (Triticum aestivum L.): Response to photoperiod, vernalization, and plant development. Planta 2006, 224, 360–366. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Wong, H.L.; Shimamoto, K. Sending ROS on a bullet train. Sci. Signal. 2009, 2, pe60. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Vágújfalvi, A.; Kerepesi, I.; Galiba, G.; Tischner, T.; Sutka, J. Frost hardiness depending on carbohydrate changes during cold acclimation in wheat. Plant Sci. 1999, 144, 85–92. [Google Scholar] [CrossRef]
- Uemura, M.; Warren, G.; Steponkus, P.L. Freezing sensitivity in the sfr4 mutant of Arabidopsis is due to low sugar content and is manifested by loss of osmotic responsiveness. Plant Physiol. 2003, 131, 1800–1807. [Google Scholar] [CrossRef] [Green Version]
- Bouche, N.; Fait, A.; Zik, M.; Fromm, H. The root-specific glutamate decarboxylase (GAD1) is essential for sustaining GABA levels in Arabidopsis. Plant Mol. Biol. 2004, 55, 315–325. [Google Scholar] [CrossRef]
- Burg, M.B.; Ferraris, J.D. Intracellular Organic Osmolytes: Function and regulation. J. Biol. Chem. 2008, 283, 7309–7313. [Google Scholar] [CrossRef] [Green Version]
- Nahar, K.; Hasanuzzaman, M.; Fujita, M. Roles of osmolytes in plant adaptation to drought and salinity. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R.A., Khan, N., Eds.; Springer: New Delhi, India, 2016; pp. 37–68. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 2004, 1666, 142–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Ruelland, E.; Zachowski, A. How plants sense temperature. Environ. Exp. Bot. 2010, 69, 225–232. [Google Scholar] [CrossRef]
- Keurentjes, J.J. Genetical metabolomics: Closing in on phenotypes. Curr. Opin. Plant Biol. 2009, 12, 223–230. [Google Scholar] [CrossRef]
- Weckwerth, W. Green systems biology—From single genomes, proteomes and metabolomes to ecosystems research and biotechnology. J. Proteom. 2011, 75, 284–305. [Google Scholar] [CrossRef] [Green Version]
- Radomiljac, J.D.; Whelan, J.; van der Merwe, M. Coordinating metabolite changes with our perception of plant abiotic stress responses: Emerging views revealed by integrative-omic analyses. Metabolites 2013, 3, 761–786. [Google Scholar] [CrossRef] [Green Version]
- Cheong, B.E.; Ho, W.W.H.; Biddulph, B.; Wallace, X.; Rathjen, T.; Rupasinghe, T.W.T.; Roessner, U.; Dolferus, R. Phenotyping reproductive stage chilling and frost tolerance in wheat using targeted metabolome and lipidome profiling. Metab. Off. J. Metab. Soc. 2019, 15, 144. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.R.; Rao, I.M.; Merchant, A. Source-sink relationships in crop plants and their influence on yield development and nutritional quality. Front. Plant Sci. 2018, 9, 1889. [Google Scholar] [CrossRef] [Green Version]
- Zinselmeier, C.; Lauer, M.J.; Boyer, J.S. Reversing drought-induced losses in grain yield—Sucrose maintains embryo growth in maize. Crop Sci. 1995, 35, 1390–1400. [Google Scholar] [CrossRef]
- Zinselmeier, C.; Westgate, M.; Schussler, J.; Jones, R. Low water potential disrupts carbohydrate metabolism in maize (Zea mays L.) ovaries. Plant Physiol. 1995, 107, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Clément, C.; Burrus, M.; Audran, J.-C. Floral organ growth and carbohydrate content during pollen development in Lilium. Am. J. Bot. 1996, 83, 459–469. [Google Scholar] [CrossRef]
- Clément, C.; Laporte, P.; Audran, J.C. The loculus content and tapetum during pollen development in Lilium. Sex. Plant Reprod. 1998, 11, 94–106. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Pellegrineschi, A.; Skovmand, B. Sink-limitation to yield and biomass: A summary of some investigations in spring wheat. Ann. Appl. Biol. 2005, 146, 39–49. [Google Scholar] [CrossRef]
- Castro, A.J.; Clément, C. Sucrose and starch catabolism in the anther of Lilium during its development: A comparative study among the anther wall, locular fluid and microspore/pollen fractions. Planta 2007, 225, 1573–1582. [Google Scholar] [CrossRef]
- Oliver, S.N.; Van Dongen, J.T.; Alfred, S.C.; Mamun, E.A.; Zhao, X.; Saini, H.S.; Fernandes, S.F.; Blanchard, C.L.; Sutton, B.G.; Geigenberger, P.; et al. Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility. Plant Cell Environ. 2005, 28, 1534–1551. [Google Scholar] [CrossRef]
- Ji, X.; Shiran, B.; Wan, J.; Lewis, D.C.; Jenkins, C.L.D.; Condon, A.G.; Richards, R.A.; Dolferus, R. Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant Cell Environ. 2010, 33, 926–942. [Google Scholar] [CrossRef]
- Dias, D.A.; Hill, C.B.; Jayasinghe, N.S.; Atieno, J.; Sutton, T.; Roessner, U. Quantitative profiling of polar primary metabolites of two chickpea cultivars with contrasting responses to salinity. J. Chromatogr. B 2015, 1000, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Boughton, B.A.; Callahan, D.L.; Silva, C.; Bowne, J.; Nahid, A.; Rupasinghe, T.; Tull, D.L.; McConville, M.J.; Bacic, A.; Roessner, U. Comprehensive profiling and quantitation of amine group containing metabolites. Anal. Chem. 2011, 83, 7523–7530. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metab. Off. J. Metab. Soc. 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.11–14.10.91. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Marcelis, L.F. Sink strength as a determinant of dry matter partitioning in the whole plant. J. Exp. Bot. 1996, 47, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dedaldechamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thevenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [Green Version]
- Thakur, P.; Kumar, S.; Malika, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Lokhande, V.H.; Suprasanna, P. Prospects of halophytes in understanding and managing abiotic stress yolerance. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 29–56. [Google Scholar] [CrossRef]
- Mayer, R.R.; Cherry, J.H.; Rhodes, D. Effects of heat shock on amino acid metabolism of cowpea cells. Plant Physiol. 1990, 94, 796–810. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Lehmann, S.; Funck, D.; Szabados, L.; Rentsch, D. Proline metabolism and transport in plant development. Amino Acids 2010, 39, 949–962. [Google Scholar] [CrossRef] [Green Version]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, F.; Guy, C.L. beta-Amylase induction and the protective role of maltose during temperature shock. Plant Physiol. 2004, 135, 1674–1684. [Google Scholar] [CrossRef] [Green Version]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef] [Green Version]
- Broeckling, C.D.; Huhman, D.V.; Farag, M.A.; Smith, J.T.; May, G.D.; Mendes, P.; Dixon, R.A.; Sumner, L.W. Metabolic profiling of Medicago truncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism. J. Exp. Bot. 2005, 56, 323–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, A.D.; Rathinasabapathi, B.; Chamberlin, B.; Gage, D.A. Comparative physiological evidence that β-alanine betaine and choline-O-sulfate act as compatible osmolytes in halophytic Limonium species. Plant Physiol. 1991, 97, 1199–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, M.; Licausi, F.; Araujo, W.L.; Nunes-Nesi, A.; Sodek, L.; Fernie, A.R.; van Dongen, J.T. Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol. 2010, 152, 1501–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, M.; Sodek, L.; Licausi, F.; Hameed, M.W.; Dornelas, M.C.; van Dongen, J.T. Analysis of alanine aminotransferase in various organs of soybean (Glycine max) and in dependence of different nitrogen fertilisers during hypoxic stress. Amino Acids 2010, 39, 1043–1053. [Google Scholar] [CrossRef] [Green Version]
- Hanson, A.D.; Rathinasabapathi, B.; Rivoal, J.; Burnet, M.; Dillon, M.O.; Gage, D.A. Osmoprotective compounds in the Plumbaginaceae: A natural experiment in metabolic engineering of stress tolerance. Proc. Natl. Acad. Sci. USA 1994, 91, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Morsy, M.R.; Jouve, L.; Hausman, J.F.; Hoffmann, L.; Stewart, J.M. Alteration of oxidative and carbohydrate metabolism under abiotic stress in two rice (Oryza sativa L.) genotypes contrasting in chilling tolerance. J. Plant Physiol. 2007, 164, 157–167. [Google Scholar] [CrossRef]
- Folgado, R.; Panis, B.; Sergeant, K.; Renaut, J.; Swennen, R.; Hausman, J.F. Unravelling the effect of sucrose and cold pretreatment on cryopreservation of potato through sugar analysis and proteomics. Cryobiology 2015, 71, 432–441. [Google Scholar] [CrossRef]
- Llanes, A.; Bertazza, G.; Palacio, G.; Luna, V. Different sodium salts cause different solute accumulation in the halophyte Prosopis strombulifera. Plant Biol. 2013, 15 (Suppl. 1), 118–125. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sharkey, T.D. The importance of maltose in transitory starch breakdown. Plant Cell Environ. 2006, 29, 353–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, J.; Bryant, G. Freezing, drying, and/or vitrification of membrane-solute-water systems. Cryobiology 1999, 39, 103–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambroise, V.; Legay, S.; Guerriero, G.; Hausman, J.-F.; Cuypers, A.; Sergeant, K. The roots of plant frost hardiness and tolerance. Plant Cell Physiol. 2019, 61, 3–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Chan, Z. Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J. Integr. Plant Biol. 2014, 56, 114–121. [Google Scholar] [CrossRef]
- Knaupp, M.; Mishra, K.B.; Nedbal, L.; Heyer, A.G. Evidence for a role of raffinose in stabilizing photosystem II during freeze-thaw cycles. Planta 2011, 234, 477–486. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Beck, E.H.; Fettig, S.; Knake, C.; Hartig, K.; Bhattarai, T. Specific and unspecific responses of plants to cold and drought stress. J. Biosci. 2007, 32, 501–510. [Google Scholar] [CrossRef]
- Ren, M.; Phoon, C.K.; Schlame, M. Metabolism and function of mitochondrial cardiolipin. Prog. Lipid Res. 2014, 55, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Wada, H.; Gombos, Z. Modes of fatty-acid desaturation in cyanobacteria. Plant Cell Physiol. 1992, 33, 933–941. [Google Scholar] [CrossRef]
- Welti, R.; Shah, J.; Li, W.; Li, M.; Chen, J.; Burke, J.J.; Fauconnier, M.L.; Chapman, K.; Chye, M.L.; Wang, X. Plant lipidomics: Discerning biological function by profiling plant complex lipids using mass spectrometry. Front. Biosci. 2007, 12, 2494–2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zheng, Q.; Shen, W.; Cram, D.; Fowler, D.B.; Wei, Y.; Zou, J. Understanding the biochemical basis of temperature-induced lipid pathway adjustments in plants. Plant Cell 2015, 27, 86–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, M.; Harwood, J.L. Effect of drought on volatile production by the lipoxygenase pathway in olive fruit. Biochem. Soc. Trans. 1997, 25, 499S. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.; Quartin, V.; Ramalho, J.; Nunes, M. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J. Plant Physiol. 2003, 160, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Moon, B.Y.; Higashi, S.; Gombos, Z.; Murata, N. Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. Proc. Natl. Acad. Sci. USA 1995, 92, 6219–6223. [Google Scholar] [CrossRef] [Green Version]
- Welti, R.; Li, W.; Li, M.; Sang, Y.; Biesiada, H.; Zhou, H.E.; Rajashekar, C.B.; Williams, T.D.; Wang, X. Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.D.; Wang, R.P.; Zhang, F.J.; Tao, F.Q.; Li, W.Q. Lipid profiling and tolerance to low-temperature stress in Thellungiella salsuginea in comparison with Arabidopsis thaliana. Biol. Plant. 2013, 57, 149–153. [Google Scholar] [CrossRef]
- Vance, D.E.; Vance, J.E. CHAPTER 8—Phospholipid biosynthesis in eukaryotes. In Biochemistry of Lipids, Lipoproteins and Membranes, 5th ed.; Vance, D.E., Vance, J.E., Eds.; Elsevier: San Diego, CA, USA, 2008; pp. 213–244. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Mechanisms of temperature adaptation in poikilotherms. FEBS Lett. 2006, 580, 5477–5483. [Google Scholar] [CrossRef] [Green Version]
- Ruelland, E.; Cantrel, C.; Gawer, M.; Kader, J.C.; Zachowski, A. Activation of phospholipases C and D is an early response to a cold exposure in Arabidopsis suspension cells. Plant Physiol. 2002, 130, 999–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, N.; Yamaya, J. Temperature-dependent phase behavior of phosphatidylglycerols from chilling-sensitive and chilling-resistant plants. Plant Physiol. 1984, 74, 1016–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bargmann, B.O.; Munnik, T. The role of phospholipase D in plant stress responses. Curr. Opin. Plant Biol. 2006, 9, 515–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testerink, C.; Munnik, T. Phosphatidic acid: A multifunctional stress signaling lipid in plants. Trends Plant Sci. 2005, 10, 368–375. [Google Scholar] [CrossRef]
- Guo, L.; Mishra, G.; Taylor, K.; Wang, X. Phosphatidic acid binds and stimulates Arabidopsis sphingosine kinases. J. Biol. Chem. 2011, 286, 13336–13345. [Google Scholar] [CrossRef] [Green Version]
- Barbaglia, A.M.; Hoffmann-Benning, S. Long-distance lipid signaling and its role in plant development and stress response. Subcell Biochem. 2016, 86, 339–361. [Google Scholar] [CrossRef]
- Pyke, K.A. Plastid division and development. Plant Cell 1999, 11, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Pogson, B.J.; Ganguly, D.; Albrecht-Borth, V. Insights into chloroplast biogenesis and development. Biochim. Biophys. Acta—Bioenerg. 2015, 1847, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Vothknecht, U.C.; Westhoff, P. Biogenesis and origin of thylakoid membranes. Biochim. Biophys. Acta—Mol. Cell Res. 2001, 1541, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Dörmann, P. Galactolipids in plant membranes. eLS 2013. [Google Scholar] [CrossRef]
- Routaboul, J.M.; Fischer, S.F.; Browse, J. Trienoic fatty acids are required to maintain chloroplast function at low temperatures. Plant Physiol. 2000, 124, 1697–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quartacci, M.F.; Pinzino, C.; Sgherri, C.L.M.; Navari-Izzo, F. Lipid composition and protein dynamics in thylakoids of two wheat cultivars differently sensitive to drought. Plant Physiol. 1995, 108, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navari-Izzo, F.; Ricci, F.; Vazzana, C.; Quartacci, M.F. Unusual composition of thylakoid membranes of the resurrection plant Boea hygroscopica: Changes in lipids upon dehydration and rehydration. Physiol. Plant. 1995, 94, 135–142. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheong, B.E.; Onyemaobi, O.; Wing Ho Ho, W.; Biddulph, T.B.; Rupasinghe, T.W.T.; Roessner, U.; Dolferus, R. Phenotyping the Chilling and Freezing Responses of Young Microspore Stage Wheat Spikes Using Targeted Metabolome and Lipidome Profiling. Cells 2020, 9, 1309. https://doi.org/10.3390/cells9051309
Cheong BE, Onyemaobi O, Wing Ho Ho W, Biddulph TB, Rupasinghe TWT, Roessner U, Dolferus R. Phenotyping the Chilling and Freezing Responses of Young Microspore Stage Wheat Spikes Using Targeted Metabolome and Lipidome Profiling. Cells. 2020; 9(5):1309. https://doi.org/10.3390/cells9051309
Chicago/Turabian StyleCheong, Bo Eng, Olive Onyemaobi, William Wing Ho Ho, Thomas Ben Biddulph, Thusitha W. T. Rupasinghe, Ute Roessner, and Rudy Dolferus. 2020. "Phenotyping the Chilling and Freezing Responses of Young Microspore Stage Wheat Spikes Using Targeted Metabolome and Lipidome Profiling" Cells 9, no. 5: 1309. https://doi.org/10.3390/cells9051309
APA StyleCheong, B. E., Onyemaobi, O., Wing Ho Ho, W., Biddulph, T. B., Rupasinghe, T. W. T., Roessner, U., & Dolferus, R. (2020). Phenotyping the Chilling and Freezing Responses of Young Microspore Stage Wheat Spikes Using Targeted Metabolome and Lipidome Profiling. Cells, 9(5), 1309. https://doi.org/10.3390/cells9051309






