TLR2 and Dectin-1 Signaling in Mouse Hematopoietic Stem and Progenitor Cells Impacts the Ability of the Antigen Presenting Cells They Produce to Activate CD4 T Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Microbial Components and Fungal Cell Preparation
2.3. Isolation of Hematopoietic Stem and Progenitor Cells and APC Differentiation
2.4. APC and CD4 T Cell Co-Culture
2.5. Antibodies and Flow Cytometry
2.6. Cytokine Measurements
2.7. Statistics
3. Results
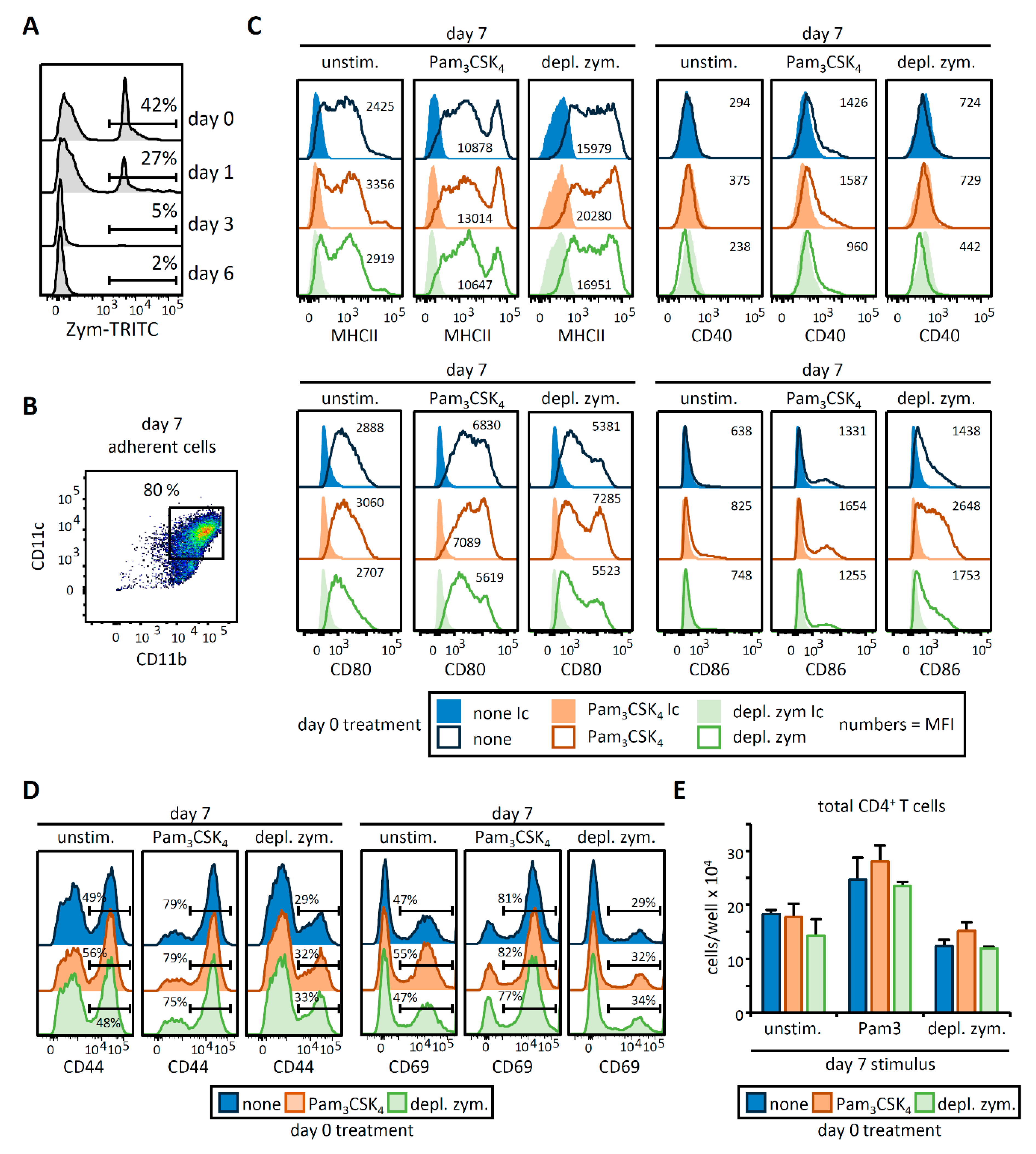
3.1. HSPC Exposure to Pam3CSK4 or Depleted Zymosan Alters MHCII Levels, Co-Stimulatory Molecule Expression, and Cytokine Release by the APCs Derived from Them In Vitro
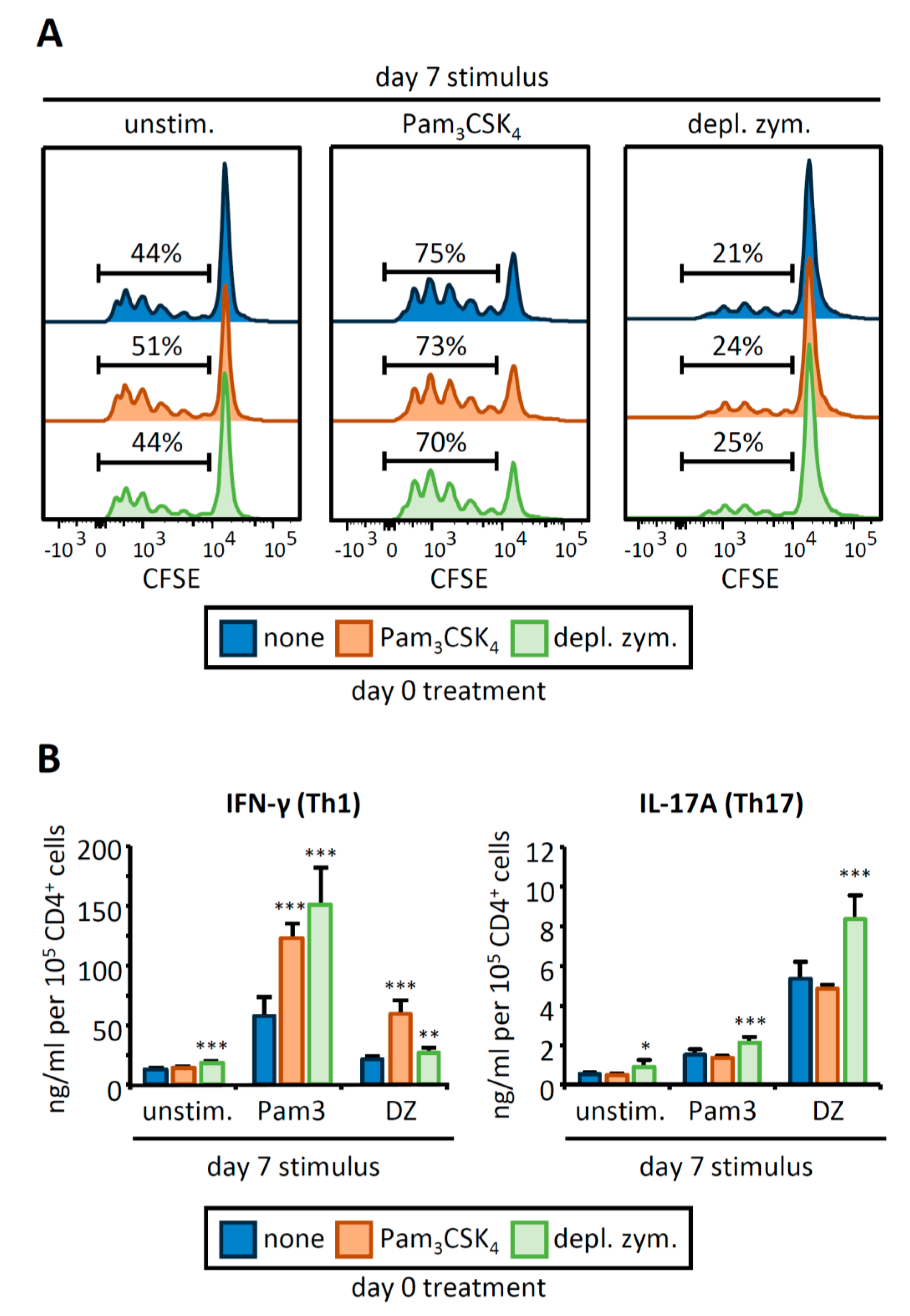
3.2. OVA-Loaded APCs Derived from HSPCs Exposed to Pam3CSK4 or Depleted Zymosan Prime Altered OVA-Specific CD4 T Cell Responses
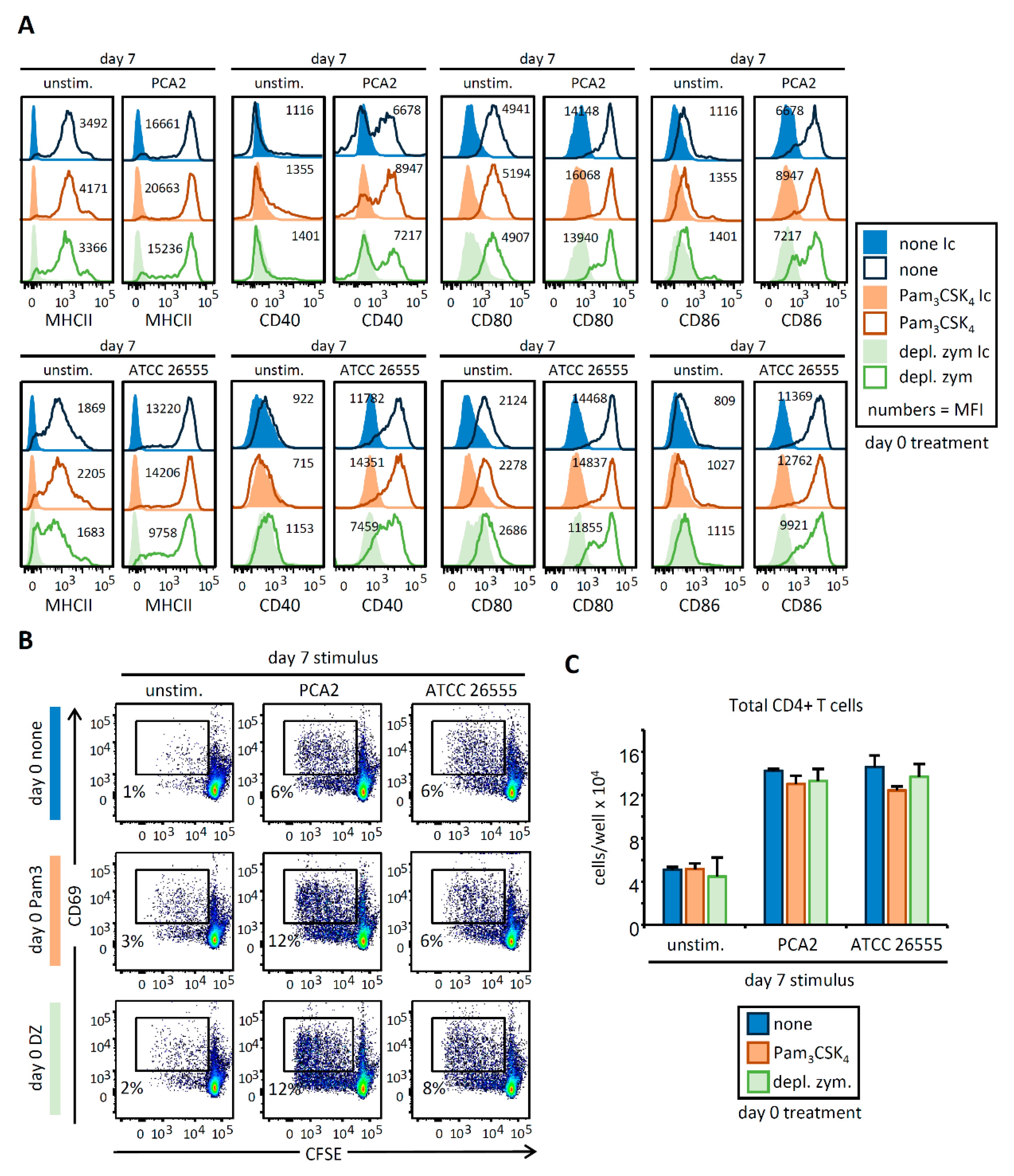
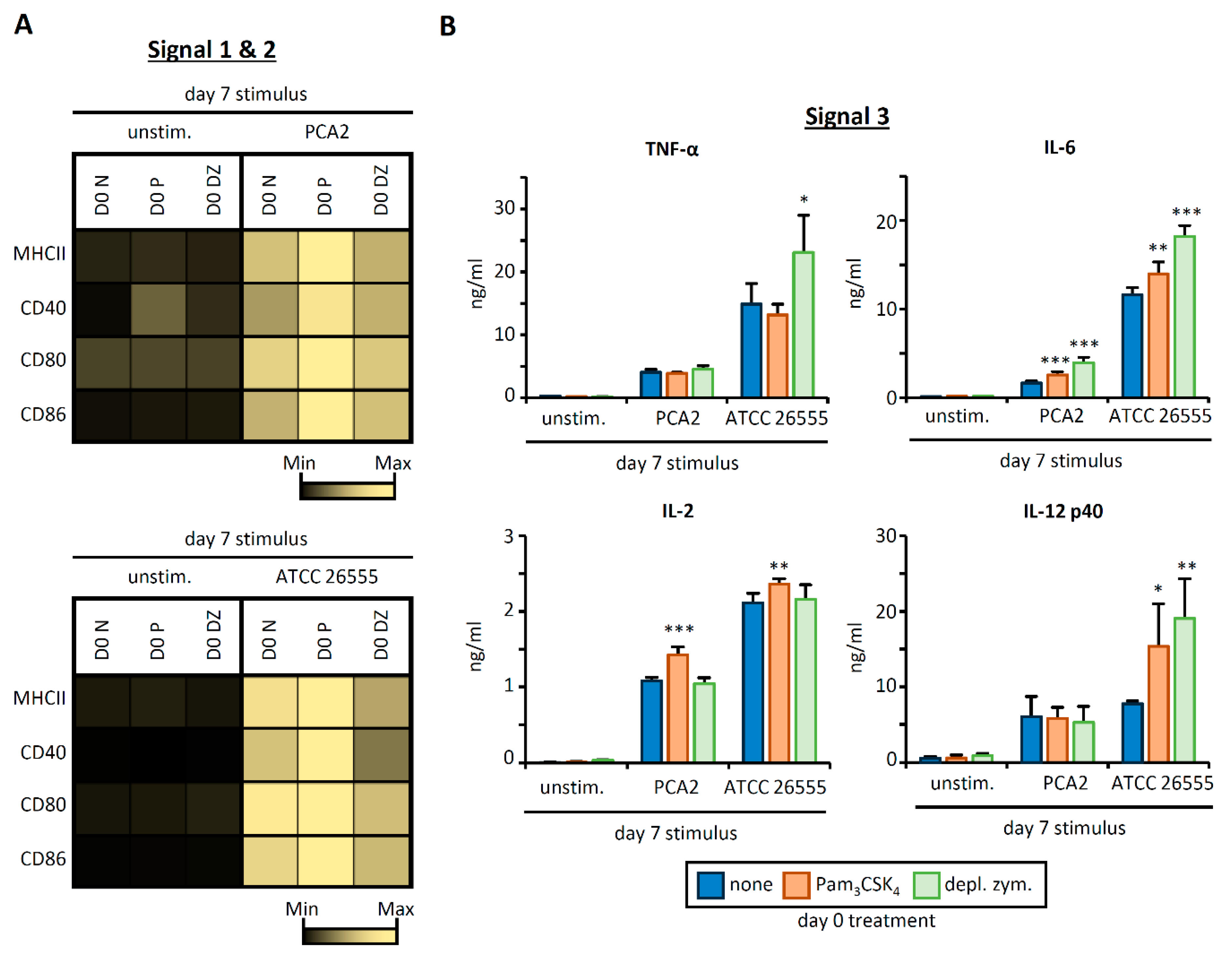
3.3. HSPC Exposure to Pam3CSK4 or Depleted Zymosan Alters MHCII Levels, Co-Stimulatory Molecule Expression, and Cytokine Release by APCs Derived from Them In Vitro after C. albicans Yeast Stimulation
3.4. C. albicans Stimulation of APCs Derived from HSPCs Exposed to Pam3CSK4 or Depleted Zymosan Enhances Th1 and Th17 Responses in a Strain-Dependent Manner
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Nagai, Y.; Garrett, K.P.; Ohta, S.; Bahrun, U.; Kouro, T.; Akira, S.; Takatsu, K.; Kincade, P.W. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 2006, 24, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, A.; Megías, J.; O’Connor, J.E.; Gozalbo, D.; Gil, M.L. Candida albicans induces selective development of macrophages and monocyte derived dendritic cells by a TLR2 dependent signalling. PLoS ONE 2011, 6, e24761. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, A.; Goodridge, H.S.; Gozalbo, D.; Gil, M.L. TLRs control hematopoiesis during infection. Eur. J. Immunol. 2013, 43, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, A.; Hassanzadeh-Kiabi, N.; Ng, M.Y.; Megías, J.; Subramanian, A.; Liu, G.Y.; Underhill, D.M.; Gil, M.L.; Goodridge, H.S. Detection of a TLR2 agonist by hematopoietic stem and progenitor cells impacts the function of the macrophages they produce. Eur. J. Immunol. 2013, 43, 2114–2125. [Google Scholar] [CrossRef] [PubMed]
- Megías, J.; Martínez, A.; Yáñez, A.; Goodridge, H.S.; Gozalbo, D.; Gil, M.L. TLR2, TLR4 and Dectin-1 signalling in hematopoietic stem and progenitor cells determines the antifungal phenotype of the macrophages they produce. Microbes Infect. 2016, 18, 354–363. [Google Scholar] [CrossRef]
- Wolf, A.A.; Yáñez, A.; Barman, P.K.; Goodridge, H.S. The ontogeny of monocyte subsets. Front. Immunol. 2019, 10, 1642. [Google Scholar] [CrossRef] [PubMed]
- Mitroulis, I.; Ruppova, K.; Wang, B.; Chen, L.S.; Grzybek, M.; Grinenko, T.; Eugster, A.; Troullinaki, M.; Palladini, A.; Kourtzelis, I.; et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 2018, 172, 147–161. [Google Scholar] [CrossRef]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonça, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.C.; et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 2018, 172, 176–190. [Google Scholar] [CrossRef]
- De Laval, B.; Maurizio, J.; Kandalla, P.K.; Brisou, G.; Simonnet, L.; Huber, C.; Gimenez, G.; Matcovitch-Natan, O.; Reinhardt, S.; David, E.; et al. C/EBPβ-dependent epigenetic memory induces trained immunity in hematopoietic stem cells. Cell Stem Cell 2020, 26, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Ibrahim, A.S.; Xu, X.; Farber, J.M.; Avanesian, V.; Baquir, B.; Fu, Y.; French, S.W.; Edwards, J.E.; Spellberg, B. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009, 5, e1000703. [Google Scholar] [CrossRef]
- Hassanzadeh-Kiabi, N.; Yáñez, A.; Dang, I.; Martins, G.A.; Underhill, D.M.; Goodridge, H.S. Autocrine type I IFN signaling in dendritic cells stimulated with fungal β-glucans or lipopolysaccharide promotes CD8 T cell activation. J. Immunol. 2017, 198, 375–382. [Google Scholar] [CrossRef] [PubMed]
- LeibundGut-Landmann, S.; Gross, O.; Robinson, M.J.; Osorio, F.; Slack, E.C.; Tsoni, S.V.; Schweighoffer, E.; Tybulewicz, V.; Brown, G.D.; Ruland, J.; et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 2007, 8, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Sieling, P.A.; Chung, W.; Duong, B.T.; Godowski, P.J.; Modlin, R.L. Toll-like receptor 2 ligands as adjuvants for human Th1 responses. J. Immunol. 2003, 170, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A. The case for an expanded concept of trained immunity. mBio 2018, 9, e00570-18. [Google Scholar] [CrossRef]
- Mulder, W.J.M.; Ochando, J.; Joosten, L.A.B.; Fayad, Z.A.; Netea, M.G. Therapeutic targeting of trained immunity. Nat. Rev. Drug Discov. 2019, 18, 553–566. [Google Scholar] [CrossRef]
- Martínez, A.; Bono, C.; Megías, J.; Yáñez, A.; Gozalbo, D.; Gil, M.L. Systemic candidiasis and TLR2 agonist exposure impact the antifungal response of hematopoietic stem and progenitor cells. Front. Cell. Infect. Microbiol. 2018, 8, 309. [Google Scholar] [CrossRef]
- Sánchez-Ramón, S.; Conejero, L.; Netea, M.G.; Sancho, D.; Palomares, Ó.; Subiza, J.L. Trained immunity-based vaccines: A new paradigm for the development of broad-spectrum anti-infectious formulations. Front. Immunol. 2018, 9, 2936. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Marshall, F.A.; Wilson, E.H.; Houston, K.M.; Liew, F.Y.; Harnett, M.M.; Harnett, W. In vivo exposure of murine dendritic cell and macrophage bone marrow progenitors to the phosphorylcholine-containing filarial nematode glycoprotein ES-62 polarizes their differentiation to an anti-inflammatory phenotype. Immunology 2004, 113, 491–498. [Google Scholar] [CrossRef]
- Goodridge, H.S. Editorial: Bone marrow progenitors share their experiences with their offspring. J. Leukoc. Biol. 2014, 95, 201–204. [Google Scholar] [CrossRef]
- Lutz, M.B.; Strobl, H.; Schuler, G.; Romani, N. GM-CSF monocyte-derived cells and Langerhans cells as part of the dendritic cell family. Front. Immunol. 2017, 8, 1388. [Google Scholar] [CrossRef]
- Ginhoux, F.; Guilliams, M.; Naik, S.H. Editorial: Dendritic cell and macrophage nomenclature and classification. Front. Immunol. 2016, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.R.; Jung, D.; Gu, G.J.; Seok, S.H. GM-CSF grown bone marrow derived cells are composed of phenotypically different dendritic cells and macrophages. Mol. Cells 2016, 39, 734–741. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, A.; Bono, C.; Gozalbo, D.; Goodridge, H.S.; Gil, M.L.; Yáñez, A. TLR2 and Dectin-1 Signaling in Mouse Hematopoietic Stem and Progenitor Cells Impacts the Ability of the Antigen Presenting Cells They Produce to Activate CD4 T Cells. Cells 2020, 9, 1317. https://doi.org/10.3390/cells9051317
Martínez A, Bono C, Gozalbo D, Goodridge HS, Gil ML, Yáñez A. TLR2 and Dectin-1 Signaling in Mouse Hematopoietic Stem and Progenitor Cells Impacts the Ability of the Antigen Presenting Cells They Produce to Activate CD4 T Cells. Cells. 2020; 9(5):1317. https://doi.org/10.3390/cells9051317
Chicago/Turabian StyleMartínez, Alba, Cristina Bono, Daniel Gozalbo, Helen S. Goodridge, M. Luisa Gil, and Alberto Yáñez. 2020. "TLR2 and Dectin-1 Signaling in Mouse Hematopoietic Stem and Progenitor Cells Impacts the Ability of the Antigen Presenting Cells They Produce to Activate CD4 T Cells" Cells 9, no. 5: 1317. https://doi.org/10.3390/cells9051317
APA StyleMartínez, A., Bono, C., Gozalbo, D., Goodridge, H. S., Gil, M. L., & Yáñez, A. (2020). TLR2 and Dectin-1 Signaling in Mouse Hematopoietic Stem and Progenitor Cells Impacts the Ability of the Antigen Presenting Cells They Produce to Activate CD4 T Cells. Cells, 9(5), 1317. https://doi.org/10.3390/cells9051317






