Role of microRNA 690 in Mediating Angiotensin II Effects on Inflammation and Endoplasmic Reticulum Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Study
2.2. Cell Cultures
2.3. RNA and MicroRNA Isolation
2.4. mRNA and miRNA Profiling
2.5. Quality Control, Mapping and Assembly
2.6. Data Availability
2.7. Differential Expression Analysis and Prediction of miRNA-mRNA Functional Interactions
2.8. Cell Transfections (Using miRNAs Mimics and Inhibitors)
2.9. Cloning, Construct Preparation and Luciferase Assay
2.10. Western Blotting and ELISA
2.11. Statistical Analyses
3. Results
3.1. Genes and miRNAs are Differentially Expressed in White Adipose Tissue (WAT) When Renin Angiotensin System (RAS) is Overexpressed
3.2. Validation of miR-690 in White Adipose Tissue (WAT) and Identification of Potential Target Genes of miR-690
3.3. Gene Validation of Predicted Targets of miR-690 in White Adipose Tissue (WAT) and Adipocytes
3.4. Regulatory Effect of miR-690 on Potential Target Genes
3.5. Mouse miR-690 Targets MAPKs to Regulate Downstream Signaling Pathways
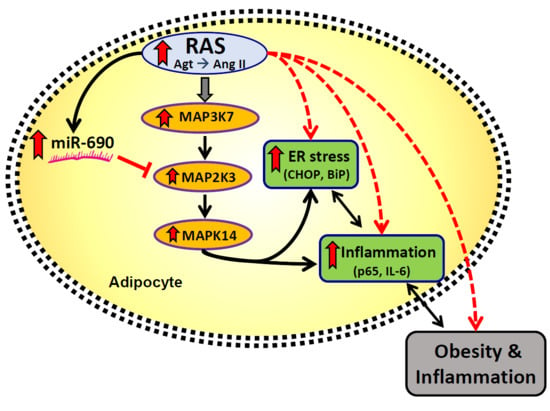
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kratz, M.; Coats, B.R.; Hisert, K.B.; Hagman, D.; Mutskov, V.; Peris, E.; Schoenfelt, K.Q.; Kuzma, J.N.; Larson, I.; Billing, P.S.; et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014, 20, 614–625. [Google Scholar] [CrossRef] [Green Version]
- Kalupahana, N.S.; Massiera, F.; Quignard-Boulange, A.; Ailhaud, G.; Voy, B.H.; Wasserman, D.H.; Moustaid-Moussa, N. Overproduction of Angiotensinogen from Adipose Tissue Induces Adipose Inflammation, Glucose Intolerance, and Insulin Resistance. Obesity 2012, 20, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Menikdiwela, K.R.; Ramalingam, L.; Allen, L.; Scoggin, S.; Kalupahana, N.S.; Moustaid-Moussa, N. Angiotensin II Increases endoplasmic Reticulum stress in Adipose tissue and Adipocytes. Sci. Rep. 2019, 9, 8481. [Google Scholar] [CrossRef] [Green Version]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. Metazoan micrornas. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010, 466, 835. [Google Scholar] [CrossRef] [Green Version]
- Bagga, S.; Bracht, J.; Hunter, S.; Massirer, K.; Holtz, J.; Eachus, R.; Pasquinelli, A.E. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell 2005, 122, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433, 769. [Google Scholar] [CrossRef]
- Gebert, L.F.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takanabe, R.; Ono, K.; Abe, Y.; Takaya, T.; Horie, T.; Wada, H.; Kita, T.; Satoh, N.; Shimatsu, A.; Hasegawa, K. Up-regulated expression of microRNA-143 in association with obesity in adipose tissue of mice fed high-fat diet. Biochem. Biophys. Res. Commun. 2008, 376, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Comas, J.; Moreno-Asso, A.; Moreno-Vedia, J.; Martin, M.; Castano, C.; Marza-Florensa, A.; Bofill-De Ros, X.; Mir-Coll, J.; Montane, J.; Fillat, C.; et al. Stress-Induced MicroRNA-708 Impairs beta-Cell Function and Growth. Diabetes 2017, 66, 3029–3040. [Google Scholar] [CrossRef] [Green Version]
- Strum, J.C.; Johnson, J.H.; Ward, J.; Xie, H.; Feild, J.; Hester, A.; Alford, A.; Waters, K.M. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol. Endocrinol. 2009, 23, 1876–1884. [Google Scholar] [CrossRef] [Green Version]
- Kemp, J.R.; Unal, H.; Desnoyer, R.; Yue, H.; Bhatnagar, A.; Karnik, S.S. Angiotensin II-regulated microRNA 483-3p directly targets multiple components of the renin–angiotensin system. J. Mol. Cell. Cardiol. 2014, 75, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Marques, F.Z.; Campain, A.E.; Tomaszewski, M.; Zukowska-Szczechowska, E.; Yang, Y.H.J.; Charchar, F.J.; Morris, B.J. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension 2011, 58, 1093–1098. [Google Scholar] [CrossRef] [Green Version]
- Min, X.-L.; Wang, T.-y.; Cao, Y.; Liu, J.; Li, J.-T.; Wang, T.-h. MicroRNAs: A novel promising therapeutic target for cerebral ischemia/reperfusion injury? Neural Regen. Res. 2015, 10, 1799. [Google Scholar]
- Jeppesen, P.L.; Christensen, G.L.; Schneider, M.; Nossent, A.Y.; Jensen, H.B.; Andersen, D.C.; Eskildsen, T.; Gammeltoft, S.; Hansen, J.L.; Sheikh, S.P. Angiotensin II type 1 receptor signalling regulates microRNA differentially in cardiac fibroblasts and myocytes. Br. J. Pharmacol. 2011, 164, 394–404. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.T.; Liu, M.Y. Daily sesame oil supplementation attenuates local renin-angiotensin system via inhibiting MAPK activation and oxidative stress in cardiac hypertrophy. J. Nutr. Biochem. 2017, 42, 108–116. [Google Scholar] [CrossRef]
- Min, L.J.; Mogi, M.; Li, J.M.; Iwanami, J.; Iwai, M.; Horiuchi, M. Aldosterone and angiotensin II synergistically induce mitogenic response in vascular smooth muscle cells. Circ. Res. 2005, 97, 434–442. [Google Scholar] [CrossRef]
- Wang, X.; Ron, D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science 1996, 272, 1347–1349. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Lee, A.S. Requirement of the p38 mitogen-activated protein kinase signalling pathway for the induction of the 78 kDa glucose-regulated protein/immunoglobulin heavy-chain binding protein by azetidine stress: Activating transcription factor 6 as a target for stress-induced phosphorylation. Biochem. J. 2002, 366, 787–795. [Google Scholar] [PubMed] [Green Version]
- Raingeaud, J.; Whitmarsh, A.J.; Barrett, T.; Derijard, B.; Davis, R.J. MKK3-and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 1996, 16, 1247–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulivi, V.; Giannoni, P.; Gentili, C.; Cancedda, R.; Descalzi, F. p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes. J. Cell. Biochem. 2008, 104, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Fukuda, K.; Sato, T.; Kawaguchi, H.; Suematsu, M.; Matsuda, S.; Koyasu, S.; Matsui, H.; Yamauchi-Takihara, K.; Harada, M. ERK and p38 MAPK, but not NF-κB, are critically involved in reactive oxygen species–mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ. Res. 2001, 89, 661–669. [Google Scholar] [CrossRef]
- Kiribayashi, K.; Masaki, T.; Naito, T.; Ogawa, T.; Ito, T.; Yorioka, N.; Kohno, N. Angiotensin II induces fibronectin expression in human peritoneal mesothelial cells via ERK1/2 and p38 MAPK. Kidney Int. 2005, 67, 1126–1135. [Google Scholar] [CrossRef] [Green Version]
- Watkins, S.J.; Borthwick, G.M.; Oakenfull, R.; Robson, A.; Arthur, H.M. Angiotensin II-induced cardiomyocyte hypertrophy in vitro is TAK1-dependent and Smad2/3-independent. Hypertens. Res. 2012, 35, 393. [Google Scholar] [CrossRef] [Green Version]
- Hegde, V.L.; Tomar, S.; Jackson, A.; Rao, R.; Yang, X.; Singh, U.P.; Singh, N.P.; Nagarkatti, P.S.; Nagarkatti, M. Distinct microRNA expression profile and targeted biological pathways in functional myeloid-derived suppressor cells induced by Δ9-tetrahydrocannabinol in vivo regulation of CCAAT/enhancer-binding protein α by microRNA-690. J. Biol. Chem. 2013, 288, 36810–36826. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Geng, Q.; Pan, Q.; Liu, Z.; Ding, S.; Xiang, Q.; Sun, F.; Wang, C.; Huang, Y.; Hong, A. MiR-690, a Runx2-targeted miRNA, regulates osteogenic differentiation of C2C12 myogenic progenitor cells by targeting NF-kappaB p65. Cell Biosci. 2016, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Bioinformatics: Cambridge, UK, 2010; Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 6 October 2011).
- Creighton, C.J.; Reid, J.G.; Gunaratne, P.H. Expression profiling of microRNAs by deep sequencing. Brief. Bioinform. 2009, 10, 490–497. [Google Scholar] [CrossRef]
- Du, P.; Kibbe, W.A.; Lin, S.M. Lumi: A pipeline for processing Illumina microarray. Bioinformatics 2008, 24, 1547–1548. [Google Scholar] [CrossRef] [Green Version]
- Smyth, G. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 12, 3. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, 68–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.-T.; Lee, C.-Y.; Lai, L.-C.; Tsai, M.-H.; Lu, T.-P.; Chuang, E.Y. anamiR: Integrated analysis of MicroRNA and gene expression profiling. BMC Bioinform. 2019, 20, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Zuo, Z.; Cai, G.; Kang, S.; Gao, X.; Li, T. miRecords: An integrated resource for microRNA–target interactions. Nucleic Acids Res. 2009, 37, D105–D110. [Google Scholar] [CrossRef]
- Paraskevopoulou, M.D.; Georgakilas, G.; Kostoulas, N.; Vlachos, I.S.; Vergoulis, T.; Reczko, M.; Filippidis, C.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-microT web server v5. 0: Service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013, 41, W169–W173. [Google Scholar] [CrossRef] [Green Version]
- Gaidatzis, D.; Van Nimwegen, E.; Hausser, J.; Zavolan, M. Inference of miRNA targets using evolutionary conservation and pathway analysis. BMC Bioinform. 2007, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Basseri, S.; Lhoták, Š.; Sharma, A.M.; Austin, R.C. The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response. J. Lipid Res. 2009, 50, 2486–2501. [Google Scholar] [CrossRef] [Green Version]
- Kutmon, M.; Riutta, A.; Nunes, N.; Hanspers, K.; Willighagen, E.L.; Bohler, A.; Mélius, J.; Waagmeester, A.; Sinha, S.R.; Miller, R. WikiPathways: Capturing the full diversity of pathway knowledge. Nucleic Acids Res. 2016, 44, D488–D494. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Moustaid-Moussa, N. The renin-angiotensin system: A link between obesity, inflammation and insulin resistance. Obes. Rev. 2012, 13, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, L.; Menikdiwela, K.; LeMieux, M.; Dufour, J.M.; Kaur, G.; Kalupahana, N.; Moustaid-Moussa, N. The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim Biophys Acta 2017, 1863, 1106–1114. [Google Scholar] [CrossRef]
- Wu, G.; Jose, P.A.; Zeng, C. Noncoding RNAs in the regulatory network of hypertension. Hypertension 2018, 72, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; MacKenzie, S.M.; Alvarez-Madrazo, S.; Diver, L.A.; Lin, J.; Stewart, P.M.; Fraser, R.; Connell, J.M.; Davies, E. MicroRNA-24 is a novel regulator of aldosterone and cortisol production in the human adrenal cortex. Hypertension 2013, 62, 572–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sõber, S.; Laan, M.; Annilo, T. MicroRNAs miR-124 and miR-135a are potential regulators of the mineralocorticoid receptor gene (NR3C2) expression. Biochem. Biophys. Res. Commun. 2010, 391, 727–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceolotto, G.; Papparella, I.; Bortoluzzi, A.; Strapazzon, G.; Ragazzo, F.; Bratti, P.; Fabricio, A.S.; Squarcina, E.; Gion, M.; Palatini, P. Interplay between miR-155, AT1R A1166C polymorphism, and AT1R expression in young untreated hypertensives. Am. J. Hypertens. 2011, 24, 241–246. [Google Scholar] [CrossRef]
- Eskildsen, T.; Jeppesen, P.; Schneider, M.; Nossent, A.; Sandberg, M.; Hansen, P.; Jensen, C.; Hansen, M.; Marcussen, N.; Rasmussen, L. Angiotensin II regulates microRNA-132/-212 in hypertensive rats and humans. Int. J. Mol. Sci. 2013, 14, 11190–11207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambros, V.; Bartel, B.; Bartel, D.P.; Burge, C.B.; Carrington, J.C.; Chen, X.M.; Dreyfuss, G.; Eddy, S.R.; Griffiths-Jones, S.; Marshall, M.; et al. A uniform system for microRNA annotation. RNA A Publ. RNA Soc. 2003, 9, 277–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, E.C.; Tomancak, P.; Williams, R.W.; Rubin, G.M. Computational identification of Drosophila microRNA genes. Genome Biol. 2003, 4, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsen, T.W. Mechanisms of microRNA-mediated gene regulation in animal cells. TRENDS Genet. 2007, 23, 243–249. [Google Scholar] [CrossRef]
- Meerson, A.; Traurig, M.; Ossowski, V.; Fleming, J.; Mullins, M.; Baier, L. Human adipose microRNA-221 is upregulated in obesity and affects fat metabolism downstream of leptin and TNF-α. Diabetologia 2013, 56, 1971–1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiriet, M. Mitogen-Activated Protein Kinase Module. In Intracellular Signaling Mediators in the Circulatory and Ventilatory Systems; Springer: New York, NY, USA, 2013; pp. 311–378. [Google Scholar]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.T.; Yang, D.D.; Wysk, M.; Gatti, E.; Mellman, I.; Davis, R.J.; Flavell, R.A. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 1999, 18, 1845–1857. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.S.; Park, Y.C.; Kim, J.H.; Kim, H.R.; Yu, T.; Byeon, S.E.; Unsworth, L.D.; Lee, J.; Cho, J.Y. Nanostructured, self-assembling peptide K5 blocks TNF-α and PGE2 production by suppression of the AP-1/p38 pathway. Mediat. Inflamm. 2012, 2012, 489810. [Google Scholar] [CrossRef] [Green Version]
- Saccani, S.; Pantano, S.; Natoli, G. p38-Dependent marking of inflammatory genes for increased NF-κB recruitment. Nat. Immunol. 2002, 3, 69–75. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.-S.; Rhee, M.H.; Sung, G.-H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef] [Green Version]
- Yin, F.; Wang, Y.-Y.; Du, J.-H.; Li, C.; Lu, Z.-Z.; Han, C.; Zhang, Y.-Y. Noncanonical cAMP pathway and p38 MAPK mediate β2-adrenergic receptor-induced IL-6 production in neonatal mouse cardiac fibroblasts. J. Mol. Cell. Cardiol. 2006, 40, 384–393. [Google Scholar] [CrossRef]
- Ushio-Fukai, M.; Alexander, R.W.; Akers, M.; Griendling, K.K. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II Role in vascular smooth muscle cell hypertrophy. J. Biol. Chem. 1998, 273, 15022–15029. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimian, T.; Li, M.W.; Lemarié, C.A.; Simeone, S.M.C.; Pagano, P.J.; Gaestel, M.; Paradis, P.S.; Wassmann, S.; Schiffrin, E.L. Mitogen-Activated Protein Kinase–Activated Protein Kinase 2 in Angiotensin II–Induced Inflammation and Hypertension: Regulation of Oxidative Stress. Hypertension 2011, 57, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranganathan, A.C.; Zhang, L.; Adam, A.P.; Aguirre-Ghiso, J.A. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase–like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 2006, 66, 1702–1711. [Google Scholar] [CrossRef] [Green Version]
- Olsen, P.H.; Ambros, V. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev. Biol. 1999, 216, 671–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seggerson, K.; Tang, L.; Moss, E.G. Two genetic circuits repress the Caenorhabditis elegans heterochronic gene lin-28 after translation initiation. Dev. Biol. 2002, 243, 215–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichhorn, S.W.; Guo, H.; McGeary, S.E.; Rodriguez-Mias, R.A.; Shin, C.; Baek, D.; Hsu, S.-H.; Ghoshal, K.; Villén, J.; Bartel, D.P. mRNA destabilization is the dominant effect of mammalian microRNAs by the time substantial repression ensues. Mol. Cell 2014, 56, 104–115. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Song, C.; Zhou, X.; Han, X.; Li, J.; Wang, Z.; Shang, H.; Liu, Y.; Cao, H. Mitochondria associated microRNA expression profiling of heart failure. BioMed Res. Int. 2017, 2017, 4042509. [Google Scholar] [CrossRef]
- Sun, J.; Madan, R.; Karp, C.L.; Braciale, T.J. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 2009, 15, 277. [Google Scholar] [CrossRef]
- He, F.; Xiao, Z.; Yao, H.; Li, S.; Feng, M.; Wang, W.; Liu, Z.; Liu, Z.; Wu, J. The protective role of microRNA-21 against coxsackievirus B3 infection through targeting the MAP2K3/P38 MAPK signaling pathway. J. Transl. Med. 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Felekkis, K.; Touvana, E.; Stefanou, C.; Deltas, C. microRNAs: A newly described class of encoded molecules that play a role in health and disease. Hippokratia 2010, 14, 236. [Google Scholar]
- Kertesz, M.N.; Iovino, U. Unnerstall, U. Gaul, and E. Segal. The role of site accessibility in microRNA target recognition. Nat. Genet. 2007, 39, 1278. [Google Scholar] [CrossRef]
- Arvani, S.; Markert, A.; Loeschcke, A.; Jaeger, K.-E.; Drepper, T. A T7 RNA polymerase-based toolkit for the concerted expression of clustered genes. J. Biotechnol. 2012, 159, 162–171. [Google Scholar] [CrossRef]
- Studier, F.W.; Moffatt, B.A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 1986, 189, 113–130. [Google Scholar] [CrossRef]
- Chitnis, N.; Clark, P.M.; Kamoun, M.; Stolle, C.; Brad Johnson, F.; Monos, D.S. An expanded role for HLA genes: HLA-B encodes a microRNA that regulates IgA and other immune response transcripts. Front. Immunol. 2017, 8, 583. [Google Scholar] [CrossRef] [PubMed]
- Makowski, L.; Brittingham, K.C.; Reynolds, J.M.; Suttles, J.; Hotamisligil, G.S. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity macrophage expression of aP2 impacts peroxisome proliferator-activated receptor γ and IκB kinase activities. J. Biol. Chem. 2005, 280, 12888–12895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wen, Y.; Lv, L.-L.; Liu, H.; Tang, R.-N.; Ma, K.-L.; Liu, B.-C. Involvement of endoplasmic reticulum stress in angiotensin II-induced NLRP3 inflammasome activation in human renal proximal tubular cells in vitro. Acta Pharmacol. Sin. 2015, 36, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Sahar, S.; Dwarakanath, R.S.; Reddy, M.A.; Lanting, L.; Todorov, I.; Natarajan, R. Angiotensin II enhances interleukin-18 mediated inflammatory gene expression in vascular smooth muscle cells: A novel cross-talk in the pathogenesis of atherosclerosis. Circ. Res. 2005, 96, 1064–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauter, N.S.; Thienel, C.; Plutino, Y.; Kampe, K.; Dror, E.; Traub, S.; Timper, K.; Bédat, B.; Pattou, F.; Kerr-Conte, J. Angiotensin II induces interleukin-1β–mediated islet inflammation and β-cell dysfunction independently of vasoconstrictive effects. Diabetes 2015, 64, 1273–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Signaling Pathway | Positive (Genes) | Measured (Genes) | Percentage (%) | Z Score | p-Value |
|---|---|---|---|---|---|
| IL-3 | 14 | 83 | 16.87 | 2.4 | 0.015 |
| IL-6 | 12 | 72 | 16.67 | 2.18 | 0.028 |
| Delta-Notch | 10 | 53 | 18.87 | 2.42 | 0.015 |
| IL-2 | 10 | 54 | 18.52 | 2.35 | 0.014 |
| IL-5 | 9 | 52 | 17.31 | 2 | 0.024 |
| Inflammatory Response | 5 | 19 | 26.32 | 2.56 | 0.014 |
| Oxidative Stress | 5 | 20 | 25.00 | 2.42 | 0.019 |
| Mitochondrial Gene Expression | 4 | 16 | 25.00 | 2.17 | 0.032 |
| p38 MAPK | 5 | 24 | 20.83 | 1.95 | 0.047 |
| IL-9 | 4 | 18 | 22.22 | 1.89 | 0.05 |
| Signaling Pathway | Hits | Score | Expected Score | p-Value | Genes |
|---|---|---|---|---|---|
| IL-3 | 39 | 39 | 23.98 | 0.0049 | Atf2, Bcl2, Birc5, Bmx, Cdc42, Creb1, Crkl, Dnm1, Foxo1, Gab2, Grb2, Gsk3b, Inpp5d, Jak2, Kcnip3, Kras, Mapk1, Mapk14, Mapk7, Mapk8, Mmp2, Mras, Pak1, Pik3ca, Pik3r1, Prkca, Prkcb, Ptpn11, Rac1, Rapgef1, Rps6kb2, Rxra, Shc1, Socs2, Sos1, Src, Stat3 |
| IL-5 | 25 | 25 | 16.54 | 0.0533 | Atf2, Btk, Crkl, Ctnnb1, Dnm2, Foxo3, Grb2, Gsk3b, Il2rb, Jak2, Kras, Mapk1, Mapk14, Pik3cg, Pik3r1, Prkcb, Ptpn11, Rac1, Rapgef1, Rps6ka1, Sdcbp, Shc1, Shc2, Stat3, Syk |
| IL-6 | 33 | 33 | 23.74 | 0.0711 | Ar, Bmx, Btk, Cd40, Crebbp, Eif4e, Erbb3, Foxo1, Foxo3, Foxo4, Gab2, Grb2, Gsk3b, Il6st, Inpp5d, Jak2, Map3k7, Mapk1, Mapk14, Mapk8, Mapt, Pik3r1, Plcg1, Ppp2r1b, Ppp2r2c, Ptpn11, Rac1, Rb1, Rps6kb1, Sgk1, Shc1, Sos1, Stat3 |
| IL-9 | 7 | 7 | 5.75 | 0.4644 | Grb2, Irs1, Mapk1, Pik3r1, Ptpn11, Shc1, Stat3 |
| Inflammatory Response | 10 | 10 | 7.19 | 0.2825 | Cd28, Cd40, Cd80, Fn1, Il2ra, Il2rb, Lamb2, Lamc1, Lamc2, Thbs1 |
| Mitochondrial Gene Expression | 10 | 10 | 4.55 | 0.0320 | Camk4, Creb1, Gabpb2, Hcfc1, Nrf1, Ppargc1a, Ppargc1b, Ppp3ca, Sp1, Tfam |
| Oxidative Stress | 6 | 6 | 6.71 | 0.5370 | Maoa, Mapk14, Mgst1, Nqo1, Sod2, Sp1 |
| p38 MAPK | 14 | 14 | 8.63 | 0.0949 | Atf2, Cdc42, Creb1, Grb2, Map3k1, Map3k5, Map3k7, Map3k9, Mapk14, Mknk1, Rac1, Ripk1, Shc1, Tgfbr1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menikdiwela, K.R.; Ramalingam, L.; Abbas, M.M.; Bensmail, H.; Scoggin, S.; Kalupahana, N.S.; Palat, A.; Gunaratne, P.; Moustaid-Moussa, N. Role of microRNA 690 in Mediating Angiotensin II Effects on Inflammation and Endoplasmic Reticulum Stress. Cells 2020, 9, 1327. https://doi.org/10.3390/cells9061327
Menikdiwela KR, Ramalingam L, Abbas MM, Bensmail H, Scoggin S, Kalupahana NS, Palat A, Gunaratne P, Moustaid-Moussa N. Role of microRNA 690 in Mediating Angiotensin II Effects on Inflammation and Endoplasmic Reticulum Stress. Cells. 2020; 9(6):1327. https://doi.org/10.3390/cells9061327
Chicago/Turabian StyleMenikdiwela, Kalhara R., Latha Ramalingam, Mostafa M. Abbas, Halima Bensmail, Shane Scoggin, Nishan S. Kalupahana, Asha Palat, Preethi Gunaratne, and Naima Moustaid-Moussa. 2020. "Role of microRNA 690 in Mediating Angiotensin II Effects on Inflammation and Endoplasmic Reticulum Stress" Cells 9, no. 6: 1327. https://doi.org/10.3390/cells9061327