Non-Human Primate iPSC Generation, Cultivation, and Cardiac Differentiation under Chemically Defined Conditions
Abstract
:1. Introduction
2. Materials and Methods
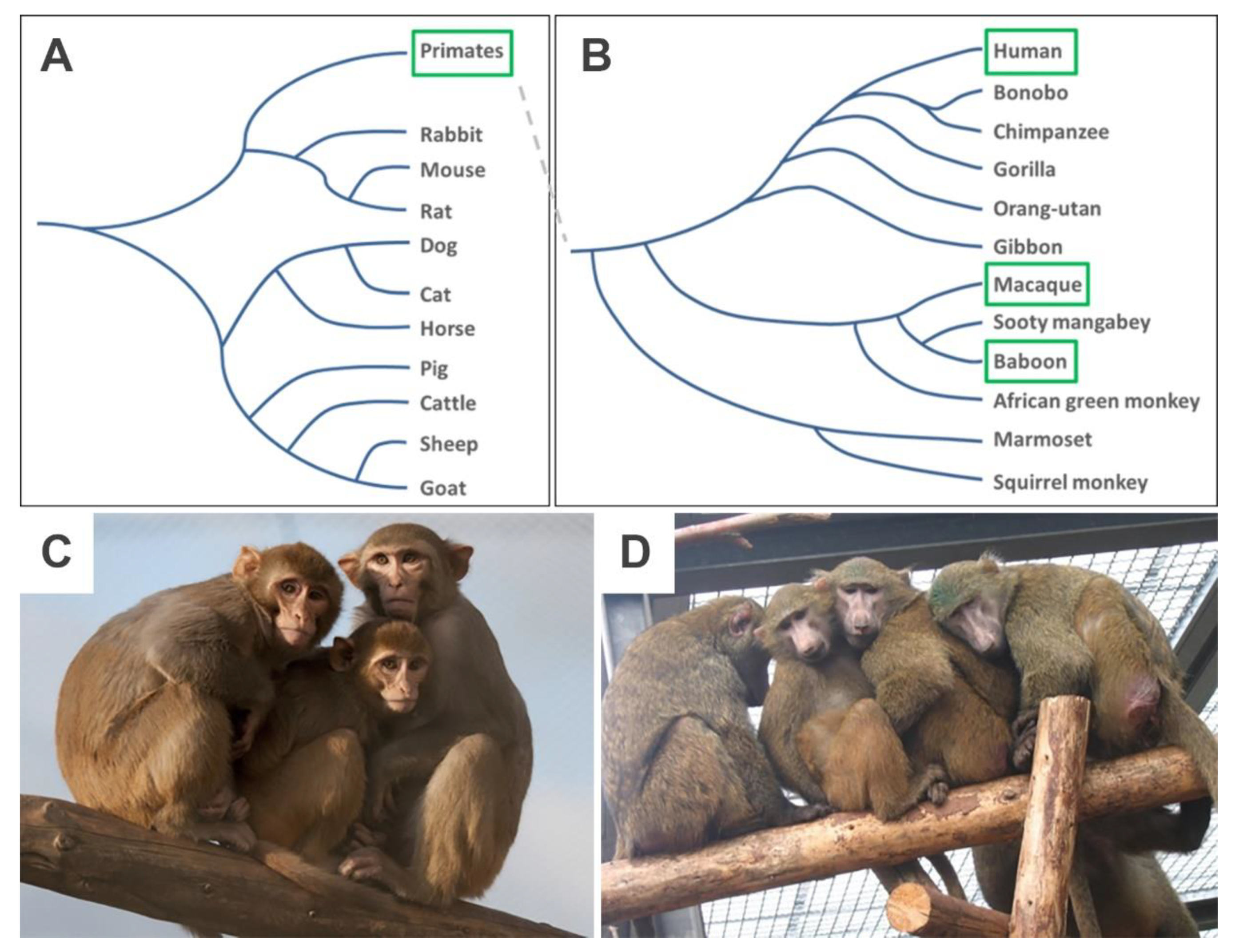
2.1. NHP Species and Ethics Statement
2.2. Isolation and Cultivation of NHP and Human Skin Fibroblasts
2.3. Reprogramming NHP and Human Skin Fibroblasts
2.4. Cultivation of Human and NHP-PSCs
2.5. In Vivo and In Vitro Differentiation
2.6. Cardiac Differentiation
2.7. Immunostaining
2.8. Flow Cytometry
2.9. DNA and RNA Isolation and Polymerase Chain Reaction
2.10. Karyotyping
2.11. Microelectrode Array (MEA) Measurements
2.12. Engineered Heart Muscle (EHM) Generation
2.13. Statistics
3. Results
3.1. NHP and Human Fibroblast Reprogramming
3.2. Long-Term Culture of NHP-PSCs
3.3. NHP-iPSCs Cultured in UPPS Medium Are Pluripotent
3.4. NHP-iPSC-Derived Cardiomyocyte Characterization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Phillips, K.A.; Bales, K.L.; Capitanio, J.P.; Conley, A.; Czoty, P.W.; t Hart, B.A.; Hopkins, W.D.; Hu, S.L.; Miller, L.A.; Nader, M.A.; et al. Why primate models matter. Am. J. Primatol. 2014, 76, 801–827. [Google Scholar] [CrossRef] [Green Version]
- Harding, J.D. Nonhuman Primates and Translational Research: Progress, Opportunities, and Challenges. ILAR J. 2017, 58, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Rogers, J.; Gibbs, R.A. Comparative primate genomics: Emerging patterns of genome content and dynamics. Nat. Rev. Genet. 2014, 15, 347–359. [Google Scholar] [CrossRef]
- Zhang, Y.E.; Long, M. New genes contribute to genetic and phenotypic novelties in human evolution. Curr. Opin. Genet. Dev. 2014, 29, 90–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedges, S.B. The origin and evolution of model organisms. Nat. Rev. Genet. 2002, 3, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Moreira, M.; Halbert, J.; Valloton, D.; Velten, B.; Chen, C.; Shao, Y.; Liechti, A.; Ascencao, K.; Rummel, C.; Ovchinnikova, S.; et al. Gene expression across mammalian organ development. Nature 2019, 571, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Carelli, F.N.; Liechti, A.; Halbert, J.; Warnefors, M.; Kaessmann, H. Repurposing of promoters and enhancers during mammalian evolution. Nat. Commun. 2018, 9, 4066. [Google Scholar] [CrossRef] [Green Version]
- Necsulea, A.; Kaessmann, H. Evolutionary dynamics of coding and non-coding transcriptomes. Nat. Rev. Genet. 2014, 15, 734–748. [Google Scholar] [CrossRef]
- Sarropoulos, I.; Marin, R.; Cardoso-Moreira, M.; Kaessmann, H. Developmental dynamics of lncRNAs across mammalian organs and species. Nature 2019, 571, 510–514. [Google Scholar] [CrossRef]
- Brennan, F.R.; Cavagnaro, J.; McKeever, K.; Ryan, P.C.; Schutten, M.M.; Vahle, J.; Weinbauer, G.F.; Marrer-Berger, E.; Black, L.E. Safety testing of monoclonal antibodies in non-human primates: Case studies highlighting their impact on human risk assessment. MAbs 2018, 10, 1–17. [Google Scholar] [CrossRef]
- Buckley, L.A.; Chapman, K.; Burns-Naas, L.A.; Todd, M.D.; Martin, P.L.; Lansita, J.A. Considerations regarding nonhuman primate use in safety assessment of biopharmaceuticals. Int. J. Toxicol. 2011, 30, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Chellman, G.J.; Bussiere, J.L.; Makori, N.; Martin, P.L.; Ooshima, Y.; Weinbauer, G.F. Developmental and reproductive toxicology studies in nonhuman primates. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2009, 86, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Faqi, A.S. A critical evaluation of developmental and reproductive toxicology in nonhuman primates. Syst. Biol. Reprod. Med. 2012, 58, 23–32. [Google Scholar] [CrossRef]
- Li, T.; Ai, Z.; Ji, W. Primate stem cells: Bridge the translation from basic research to clinic application. Sci. China Life Sci. 2019, 62, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Orsi, A.; Rees, D.; Andreini, I.; Venturella, S.; Cinelli, S.; Oberto, G. Overview of the marmoset as a model in nonclinical development of pharmaceutical products. Regul. Toxicol. Pharmacol. 2011, 59, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badin, R.A.; Bugi, A.; Williams, S.; Vadori, M.; Michael, M.; Jan, C.; Nassi, A.; Lecourtois, S.; Blancher, A.; Cozzi, E.; et al. MHC matching fails to prevent long-term rejection of iPSC-derived neurons in non-human primates. Nat. Commun. 2019, 10, 4357. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.J.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef]
- Florence Wianny, J.V. Transplantation in the nonhuman primate MPTP model of Parkinson’s disease: Update and perspectives. Primate Biol. 2017, 4, 185–213. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, T.; Morizane, A.; Doi, D.; Magotani, H.; Onoe, H.; Hayashi, T.; Mizuma, H.; Takara, S.; Takahashi, R.; Inoue, H.; et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 2017, 548, 592–596. [Google Scholar] [CrossRef]
- Kriks, S.; Shim, J.W.; Piao, J.; Ganat, Y.M.; Wakeman, D.R.; Xie, Z.; Carrillo-Reid, L.; Auyeung, G.; Antonacci, C.; Buch, A.; et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011, 480, 547–551. [Google Scholar] [CrossRef]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K.; et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Vermilyea, S.C.; Emborg, M.E. The role of nonhuman primate models in the development of cell-based therapies for Parkinson’s disease. J. Neural. Transm. (Vienna) 2018, 125, 365–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zou, C.; Fu, L.; Wang, B.; An, J.; Song, G.; Wu, J.; Tang, X.; Li, M.; Zhang, J.; et al. Autologous iPSC-derived dopamine neuron transplantation in a nonhuman primate Parkinson’s disease model. Cell Discov. 2015, 1, 15012. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Chen, H.; Xiao, D.; Yang, H.; Itzhaki, I.; Qin, X.; Chour, T.; Aguirre, A.; Lehmann, K.; Kim, Y.; et al. Comparison of Non-human Primate versus Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Treatment of Myocardial Infarction. Stem Cell Rep. 2018, 10, 422–435. [Google Scholar] [CrossRef] [Green Version]
- Hakala, H.; Rajala, K.; Ojala, M.; Panula, S.; Areva, S.; Kellomaki, M.; Suuronen, R.; Skottman, H. Comparison of biomaterials and extracellular matrices as a culture platform for multiple, independently derived human embryonic stem cell lines. Tissue Eng. Part A 2009, 15, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.G.; Winkler, T.; Wu, C.; Guo, V.; Pittaluga, S.; Nicolae, A.; Donahue, R.E.; Metzger, M.E.; Price, S.D.; Uchida, N.; et al. Path to the clinic: Assessment of iPSC-based cell therapies in vivo in a nonhuman primate model. Cell Rep. 2014, 7, 1298–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakai, R.; Ohnuki, M.; Kuroki, K.; Ito, H.; Hirai, H.; Kitajima, R.; Fujimoto, T.; Nakagawa, M.; Enard, W.; Imamura, M. Derivation of induced pluripotent stem cells in Japanese macaque (Macaca fuscata). Sci. Rep. 2018, 8, 12187. [Google Scholar] [CrossRef] [Green Version]
- Navara, C.S.; Chaudhari, S.; McCarrey, J.R. Optimization of culture conditions for the derivation and propagation of baboon (Papio anubis) induced pluripotent stem cells. PLoS ONE 2018, 13, e0193195. [Google Scholar] [CrossRef] [Green Version]
- Navara, C.S.; Hornecker, J.; Grow, D.; Chaudhari, S.; Hornsby, P.J.; Ichida, J.K.; Eggan, K.; McCarrey, J.R. Derivation of induced pluripotent stem cells from the baboon: A nonhuman primate model for preclinical testing of stem cell therapies. Cell. Reprogram. 2013, 15, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Wunderlich, S.; Kircher, M.; Vieth, B.; Haase, A.; Merkert, S.; Beier, J.; Gohring, G.; Glage, S.; Schambach, A.; Curnow, E.C.; et al. Primate iPS cells as tools for evolutionary analyses. Stem Cell Res. 2014, 12, 622–629. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Debowski, K.; Warthemann, R.; Lentes, J.; Salinas-Riester, G.; Dressel, R.; Langenstroth, D.; Gromoll, J.; Sasaki, E.; Behr, R. Non-viral generation of marmoset monkey iPS cells by a six-factor-in-one-vector approach. PLoS ONE 2015, 10, e0118424. [Google Scholar] [CrossRef]
- Tomioka, I.; Maeda, T.; Shimada, H.; Kawai, K.; Okada, Y.; Igarashi, H.; Oiwa, R.; Iwasaki, T.; Aoki, M.; Kimura, T.; et al. Generating induced pluripotent stem cells from common marmoset (Callithrix jacchus) fetal liver cells using defined factors, including Lin28. Genes Cells 2010, 15, 959–969. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Hong, H.; Torres, A.; Malloy, K.E.; Choudhury, G.R.; Kim, J.; Daadi, M.M. Standards for Deriving Nonhuman Primate-Induced Pluripotent Stem Cells, Neural Stem Cells and Dopaminergic Lineage. Int. J. Mol. Sci. 2018, 19, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Kahland, T.S. Modifying the Common Marmoset Monkey (Callithrix jacchus) Genome: Transgenesis and Targeted Gene Modification In Vivo and In Vitro. Ph.D. Thesis, Georg-August-Universität Göttingen, Göttingen, Germany, 2015. [Google Scholar]
- Langin, M.; Mayr, T.; Reichart, B.; Michel, S.; Buchholz, S.; Guethoff, S.; Dashkevich, A.; Baehr, A.; Egerer, S.; Bauer, A.; et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 2018, 564, 430–433. [Google Scholar] [CrossRef]
- Liu, Y.W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018, 36, 597–605. [Google Scholar] [CrossRef]
- Weber, B.; Scherman, J.; Emmert, M.Y.; Gruenenfelder, J.; Verbeek, R.; Bracher, M.; Black, M.; Kortsmit, J.; Franz, T.; Schoenauer, R.; et al. Injectable living marrow stromal cell-based autologous tissue engineered heart valves: First experiences with a one-step intervention in primates. Eur. Heart J. 2011, 32, 2830–2840. [Google Scholar] [CrossRef] [Green Version]
- Okita, K.; Matsumura, Y.; Sato, Y.; Okada, A.; Morizane, A.; Okamoto, S.; Hong, H.; Nakagawa, M.; Tanabe, K.; Tezuka, K.; et al. A more efficient method to generate integration-free human iPS cells. Nat. Methods 2011, 8, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Tiburcy, M.; Hudson, J.E.; Balfanz, P.; Schlick, S.; Meyer, T.; Chang Liao, M.L.; Levent, E.; Raad, F.; Zeidler, S.; Wingender, E.; et al. Defined Engineered Human Myocardium with Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation 2017, 135, 1832–1847. [Google Scholar] [CrossRef]
- Tohyama, S.; Hattori, F.; Sano, M.; Hishiki, T.; Nagahata, Y.; Matsuura, T.; Hashimoto, H.; Suzuki, T.; Yamashita, H.; Satoh, Y.; et al. Distinct Metabolic Flow Enables Large-Scale Purification of Mouse and Human Pluripotent Stem Cell-Derived Cardiomyocytes. Cell Stem Cell 2013, 12, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, E.; Suplicki, M.M.; Behr, R. Primordial germ cells do not migrate along nerve fibres in marmoset monkey and mouse embryos. Reproduction 2018, 157, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Polo, I.S.; Stauske, M.; Becker, A.; Bartels, I.; Dressel, R.; Behr, R. Baboon induced pluripotent stem cell generation by piggyBac transposition of reprogramming factors. Primate Biol. 2019, 6, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Kalishman, J.; Golos, T.G.; Durning, M.; Harris, C.P.; Becker, R.A.; Hearn, J.P. Isolation of a primate embryonic stem cell line. Proc. Natl. Acad. Sci. USA 1995, 92, 7844–7848. [Google Scholar] [CrossRef] [Green Version]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.; et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef] [Green Version]
- Kattman, S.J.; Witty, A.D.; Gagliardi, M.; Dubois, N.C.; Niapour, M.; Hotta, A.; Ellis, J.; Keller, G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 2011, 8, 228–240. [Google Scholar] [CrossRef] [Green Version]
- Lian, X.; Bao, X.; Zilberter, M.; Westman, M.; Fisahn, A.; Hsiao, C.; Hazeltine, L.B.; Dunn, K.K.; Kamp, T.J.; Palecek, S.P. Chemically defined, albumin-free human cardiomyocyte generation. Nat. Methods 2015, 12, 595–596. [Google Scholar] [CrossRef]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef] [Green Version]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [Green Version]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Announcement of Physician-Initiated Clinical Trials for Parkinson’s Disease. Available online: https://www.cira.kyoto-u.ac.jp/e/pressrelease/news/180730-170000.html (accessed on 30 July 2018).
- Revealed: Two Men in China Were First to Receive Pioneering Stem-Cell Treatment for Heart Disease. Available online: https://www.nature.com/articles/d41586-020-01285-w (accessed on 13 May 2020).
- Wu, Y.; Mishra, A.; Qiu, Z.; Farnsworth, S.; Tardif, S.D.; Hornsby, P.J. Nonhuman primate induced pluripotent stem cells in regenerative medicine. Stem Cells Int. 2012, 2012, 767195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deleidi, M.; Hargus, G.; Hallett, P.; Osborn, T.; Isacson, O. Development of histocompatible primate-induced pluripotent stem cells for neural transplantation. Stem Cells 2011, 29, 1052–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhu, F.; Yong, J.; Zhang, P.; Hou, P.; Li, H.; Jiang, W.; Cai, J.; Liu, M.; Cui, K.; et al. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell 2008, 3, 587–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchetto, M.C.N.; Narvaiza, I.; Denli, A.M.; Benner, C.; Lazzarini, T.A.; Nathanson, J.L.; Paquola, A.C.M.; Desai, K.N.; Herai, R.H.; Weitzman, M.D.; et al. Differential L1 regulation in pluripotent stem cells of humans and apes. Nature 2013, 503, 525–529. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Mishra, A.; Tardif, S.D.; Hornsby, P.J. Generation of induced pluripotent stem cells from newborn marmoset skin fibroblasts. Stem Cell Res. 2010, 4, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, B.; Trobridge, G.D.; Zhang, X.; Watts, K.L.; Ramakrishnan, A.; Wohlfahrt, M.; Adair, J.E.; Kiem, H.P. Efficient generation of nonhuman primate induced pluripotent stem cells. Stem Cells Dev. 2011, 20, 795–807. [Google Scholar] [CrossRef]
- Yada, R.C.; Hong, S.G.; Lin, Y.; Winkler, T.; Dunbar, C.E. Rhesus Macaque iPSC Generation and Maintenance. Curr. Protoc. Stem Cell Biol. 2017, 41, 4A-11. [Google Scholar] [CrossRef] [Green Version]
- Carey, B.W.; Markoulaki, S.; Hanna, J.; Saha, K.; Gao, Q.; Mitalipova, M.; Jaenisch, R. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc. Natl. Acad. Sci. USA 2009, 106, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Weltner, J.; Anisimov, A.; Alitalo, K.; Otonkoski, T.; Trokovic, R. Induced pluripotent stem cell clones reprogrammed via recombinant adeno-associated virus-mediated transduction contain integrated vector sequences. J. Virol. 2012, 86, 4463–4467. [Google Scholar] [CrossRef] [Green Version]
- Grow, D.A.; Simmons, D.V.; Gomez, J.A.; Wanat, M.J.; McCarrey, J.R.; Paladini, C.A.; Navara, C.S. Differentiation and Characterization of Dopaminergic Neurons From Baboon Induced Pluripotent Stem Cells. Stem Cells Transl. Med. 2016, 5, 1133–1144. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, H.; Bai, S.; Huo, W.; Ma, Y. Differentiation and characterization of rhesus monkey atrial and ventricular cardiomyocytes from induced pluripotent stem cells. Stem Cell Res. 2017, 20, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gulbranson, D.R.; Hou, Z.; Bolin, J.M.; Ruotti, V.; Probasco, M.D.; Smuga-Otto, K.; Howden, S.E.; Diol, N.R.; Propson, N.E.; et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 2011, 8, 424–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Okamura, D.; Li, M.; Suzuki, K.; Luo, C.; Ma, L.; He, Y.; Li, Z.; Benner, C.; Tamura, I.; et al. An alternative pluripotent state confers interspecies chimaeric competency. Nature 2015, 521, 316–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Li, T.G.; Hao, J.; Hu, J.; Wang, J.; Simmons, H.; Miura, S.; Mishina, Y.; Zhao, G.Q. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc. Natl. Acad. Sci. USA 2004, 101, 6027–6032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Wu, J.; Ye, S.; Tai, C.I.; Zhou, X.; Yan, H.; Li, P.; Pera, M.; Ying, Q.L. Modulation of beta-catenin function maintains mouse epiblast stem cell and human embryonic stem cell self-renewal. Nat. Commun. 2013, 4, 2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Name | Vendor | Catalogue | Dilution |
|---|---|---|---|
| α-feto protein | Dako | A0008 | 1:100 |
| α-actinin | Sigma-Aldrich | A7811 | 1:1000 |
| β-tubulin III | Sigma-Aldrich | T8660 | 1:1000 |
| Connexin 43 (Cx43) | Abcam | ab11370 | 1:1000 |
| Cardiac troponin I (cTNI) | Abcam | ab47003 | 1:200 |
| Cardiac troponin T (cTNT) 1 | Miltenyi Biotec | 130-106-687 | 1:10 |
| Cardiac troponin T (cTNT) 2 | Thermo Fisher | MS295PABX | 1:200 |
| LIN28 | R&D Systems | AF3757 | 1:300 |
| Myosin light chain 2a (MLC2a) | Synaptic Systems | 311-011 | 1:200 |
| NANOG | Cell Signalling | 4903 | 1:400 |
| SALL4 | Abcam | ab57577 | 1:200 |
| Smooth muscle actin | Sigma-Aldrich | A2547 | 1:1000 |
| SOX9 | Merck | AB5535 | 1:1000 |
| SSEA4 | Abcam | ab16287 | 1:200 |
| Titin | Merck | MAB1553 | 1:50 |
| TRA-1-60 | Abcam | ab16288 | 1:200 |
| Alexa555-goat-α-mouse IgG | Thermo Fisher | A21424 | 1:1000 |
| Alexa488-goat-α-mouse IgG | Thermo Fisher | A11029 | 1:1000 |
| Alexa488-goat-α-mouse IgG/IgM | Thermo Fisher | A10680 | 1:1000 |
| Alexa488-donkey-α-goat IgG | Thermo Fisher | A11055 | 1:1000 |
| Alexa488-donkey-α-rabbit IgG | Thermo Fisher | A21206 | 1:1000 |
| Cultivation Media | Mesodermal Induction | Cardiac Induction | Reference |
|---|---|---|---|
| Day 0–5: RPMI 1640 Day ≥ 6: RPMI 1640 + B27 | 1 µM CHIR99021 (48 h) or 2.5 µM CHIR99021 (48 h) or 4 µM CHIR99021 (48 h) or 5 µM CHIR99021 (48 h) | 5 μM IWP-2 (48 h) or 5 μM IWR-1 (48 h) | Modified after Lian et al., 2015 |
| Day 0–7: RPMI 1640 + L-ascorbic acid 2-phosphate (690 µM) + recombinant human albumin (0.5 mg/mL) Day ≥ 8: RPMI 1640 + B27 | 1 µM CHIR99021 (48 h) or 2.5 µM CHIR99021 (48 h) or 4 µM CHIR99021 (48 h) or 5 µM CHIR99021 (48 h) | 5 μM IWP-2 (48 h) or 5 μM IWR-1 (48 h) | Modified after Burridge et al., 2014 |
| Day 0–6: RPMI 1640 + B27 (minus insulin) Day ≥ 7: RPMI 1640 + B27 | 1 µM CHIR99021 (24 h) or 3 µM CHIR99021 (24 h) or 4 µM CHIR99021 (24 h) or 8 µM CHIR99021 (24 h) or9 µM CHIR99021 (24 h) or 10 µM CHIR99021 (24 h) | 5 μM IWP-2 (48 h) or 5 μM IWR-1 (48 h) | Modified after Lian et al., 2013 |
| Day 0–6: IMDM + B27 (minus insulin) Day ≥ 7: RPMI 1640 + B27 | 1 µM CHIR99021 (24 h) or 3 µM CHIR99021 (24 h) or 4 µM CHIR99021 (24 h) or 8 µM CHIR99021 (24 h) or 9 µM CHIR99021 (24 h) or 10 µM CHIR99021 (24 h) | 5 μM IWP-2 (48 h) or 5 μM IWR-1 (48 h) | Modified after Lian et al., 2013 |
| Day 0–6: RPMI 1640 + B27 (minus insulin) + sodium pyruvate (1 mM) + L-ascorbic acid 2-phosphate (200 µM) Day ≥ 7: RPMI 1640 + B27 + L-ascorbic acid 2-phosphate (200 µM) | 1 µM CHIR99021 + 9 ng/mL activin A + 5 ng/mL BMP4 (36 h) | 5 μM IWR-1 (96 h) | Modified after Tiburcy et al., 2017 |
| Name | Sequence | F (bp) 1 | T (°C) 2 | C 3 |
|---|---|---|---|---|
| WPRE (1) | for: 5′-GCT ATT GCT TCC CGT ATG GC-3′ rev: 5′-CAA AGG GAG ATC CGA CTC GT-3′ | 470 | 54 | 32 |
| EBNA-LoxP (2) | for: 5′-AAG AGG AGG GGT CCC GAG A-3′ rev: 5′-GCC AAT GCA ACT TGG ACG TT-3′ | 555 | 61 | 32 |
| OriP1 (3) | for: 5′-GGT TCA CTA CCC TCG TGG AAT-3′ rev: 5′-CGG GGC AGT GCA TGT AAT-3′ | 592 | 57 | 32 |
| OriP2 (4) | for: 5′-GGT GAC TGT GTG CAG CTT TG-3′ rev: 5′-GGA GCT GAG TGA CGT GAC AA-3′ | 416 | 54 | 32 |
| β-actin4 | for: 5′-GAC CTG ACT GAC TAC CTC ATG-3′ rev: 5′-GGT AGT TTC GTG GAT GCC ACA-3′ | 379/380 | 61 | 32 |
| KLF4 (exo) | for: 5′-TTC ATC GAC GAG GCT AAG CG-3′ rev: 5′-TCA CTG ACA GCC ATG GTG AA-3′ | 812 | 53 | 30 |
| OCT4 (exo) | for: 5′-TGA TCC TCG GAC CTG GCT AA-3′ rev: 5′-TCCCCGAAGCTTGAATTCGC-3′ | 1021 | 54 | 30 |
| OCT4 (endo) | for: 5′-GAG AAG GAG AAG CTG GAG CAA-3′ rev: 5′-ACA TCC TTC TCG AGC CCA A-3′ | 841 | 53 | 30 |
| LIN28 (exo) | for: 5′-ACT CAA ACT GGC TGG GGA TG-3′ rev: 5′-TTC AAG CTC CGG AAC CCT TC-3′ | 327 | 54 | 30 |
| LIN28 (endo) | for: 5′-GGG TGT TCT GTA TTG GGA GTG-3′ rev: 5′-GCA CCC TAT TCC CAC TTT CTC-3′ | 371 | 61 | 30 |
| NANOG | for: 5′-CAG AGA TAC CTC AGC CTC CAG-3′ rev: 5′-CTT CAG GTT GCA TGT TCG T-3′ | 562 | 54 | 30 |
| SOX2 (endo) | for: 5′-GGT AGG AGC TTT GCA GGA AGT-3′ rev: 5′-CCA ACG ATG TCA ACC TGC ATG-3′ | 428 | 61 | 30 |
| β-actin5 | for: 5′-TGG ATG ATG ATA TCG CCG CGC T-3′ rev: 5′-GGG CCT CGG TCA GCA GCA CGG-3′ | 324 | 61 | 20 |
| Condition # | Culture Media | Supplements |
|---|---|---|
| 1 | Essential 8 | - |
| 2 | Essential 8 | 2.5 µM IWR-1 |
| 3 | Essential 8 | 2.5 µM IWR-1 25 ng/mL BMP4 |
| 4 | Essential 8 | 2.5 µM IWR-1 3 µM CHIR99021 |
| 5 | StemMACS iPS-Brew XF | - |
| 6 | StemMACS iPS-Brew XF | 2.5 µM IWR-1 |
| 7 | StemMACS iPS-Brew XF | 2.5 µM IWR-1 25 ng/mL BMP4 |
| 8 | StemMACS iPS-Brew XF | 2.5 µM IWR-1 3 µM CHIR99021 |
| 9 | StemMACS iPS-Brew XF | 1 µM IWR-1 0.5 µM CHIR99021 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stauske, M.; Rodriguez Polo, I.; Haas, W.; Knorr, D.Y.; Borchert, T.; Streckfuss-Bömeke, K.; Dressel, R.; Bartels, I.; Tiburcy, M.; Zimmermann, W.-H.; et al. Non-Human Primate iPSC Generation, Cultivation, and Cardiac Differentiation under Chemically Defined Conditions. Cells 2020, 9, 1349. https://doi.org/10.3390/cells9061349
Stauske M, Rodriguez Polo I, Haas W, Knorr DY, Borchert T, Streckfuss-Bömeke K, Dressel R, Bartels I, Tiburcy M, Zimmermann W-H, et al. Non-Human Primate iPSC Generation, Cultivation, and Cardiac Differentiation under Chemically Defined Conditions. Cells. 2020; 9(6):1349. https://doi.org/10.3390/cells9061349
Chicago/Turabian StyleStauske, Michael, Ignacio Rodriguez Polo, Wadim Haas, Debbra Yasemin Knorr, Thomas Borchert, Katrin Streckfuss-Bömeke, Ralf Dressel, Iris Bartels, Malte Tiburcy, Wolfram-Hubertus Zimmermann, and et al. 2020. "Non-Human Primate iPSC Generation, Cultivation, and Cardiac Differentiation under Chemically Defined Conditions" Cells 9, no. 6: 1349. https://doi.org/10.3390/cells9061349
APA StyleStauske, M., Rodriguez Polo, I., Haas, W., Knorr, D. Y., Borchert, T., Streckfuss-Bömeke, K., Dressel, R., Bartels, I., Tiburcy, M., Zimmermann, W.-H., & Behr, R. (2020). Non-Human Primate iPSC Generation, Cultivation, and Cardiac Differentiation under Chemically Defined Conditions. Cells, 9(6), 1349. https://doi.org/10.3390/cells9061349





