Resistin Enhances VCAM-1 Expression and Monocyte Adhesion in Human Osteoarthritis Synovial Fibroblasts by Inhibiting MiR-381 Expression through the PKC, p38, and JNK Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Clinical Samples
2.3. Cell Culture
2.4. Real-Time Quantitative PCR Analysis
2.5. Western Blot Analysis
2.6. Luciferase Assays
2.7. Cell Adhesion Assay
2.8. OA Model and Micro-Computed Tomography (Micro-CT) Assessment
2.9. Micro Computed Tomography (Micro-CT) Imaging
2.10. Micro-CT Analysis Strategy
2.11. Immunohistochemistry (IHC) Staining
2.12. Statistics
3. Results
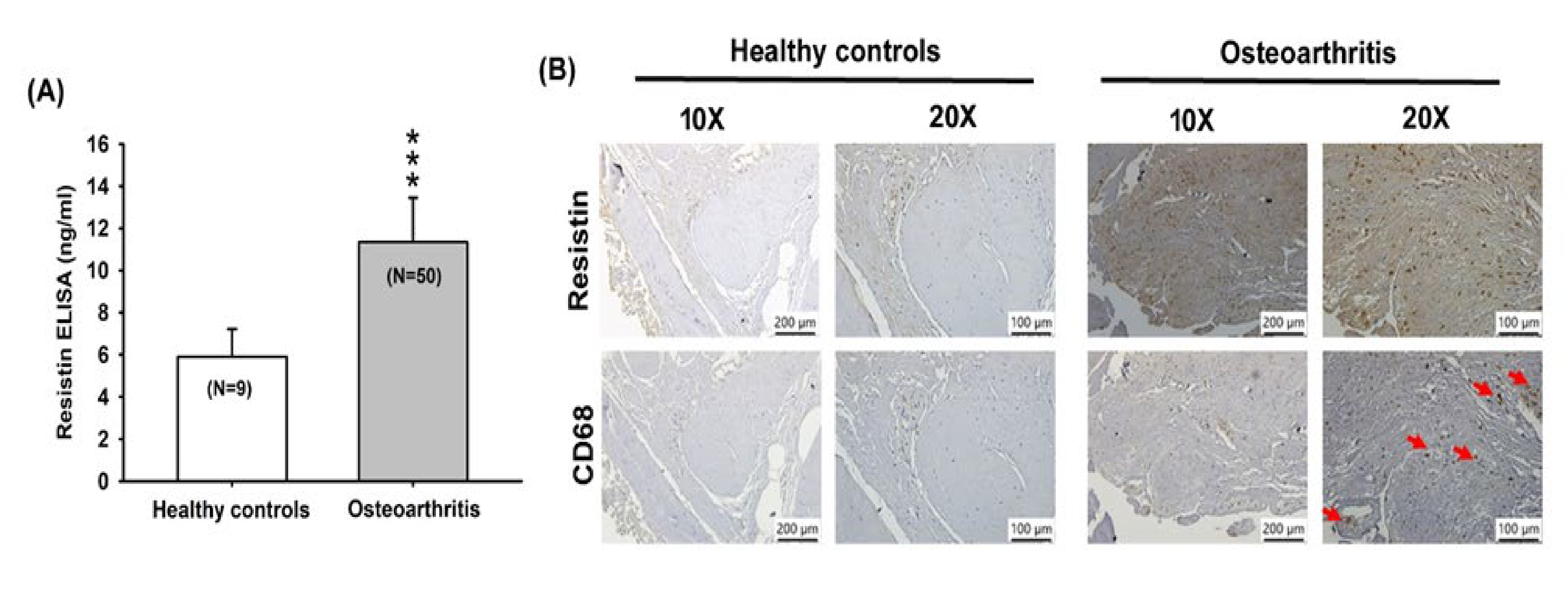
3.1. Resistin Expression is Increased among OA Patients
3.2. Resistin Increases VCAM-1 Expression and Monocyte Adhesion in Human OASFs
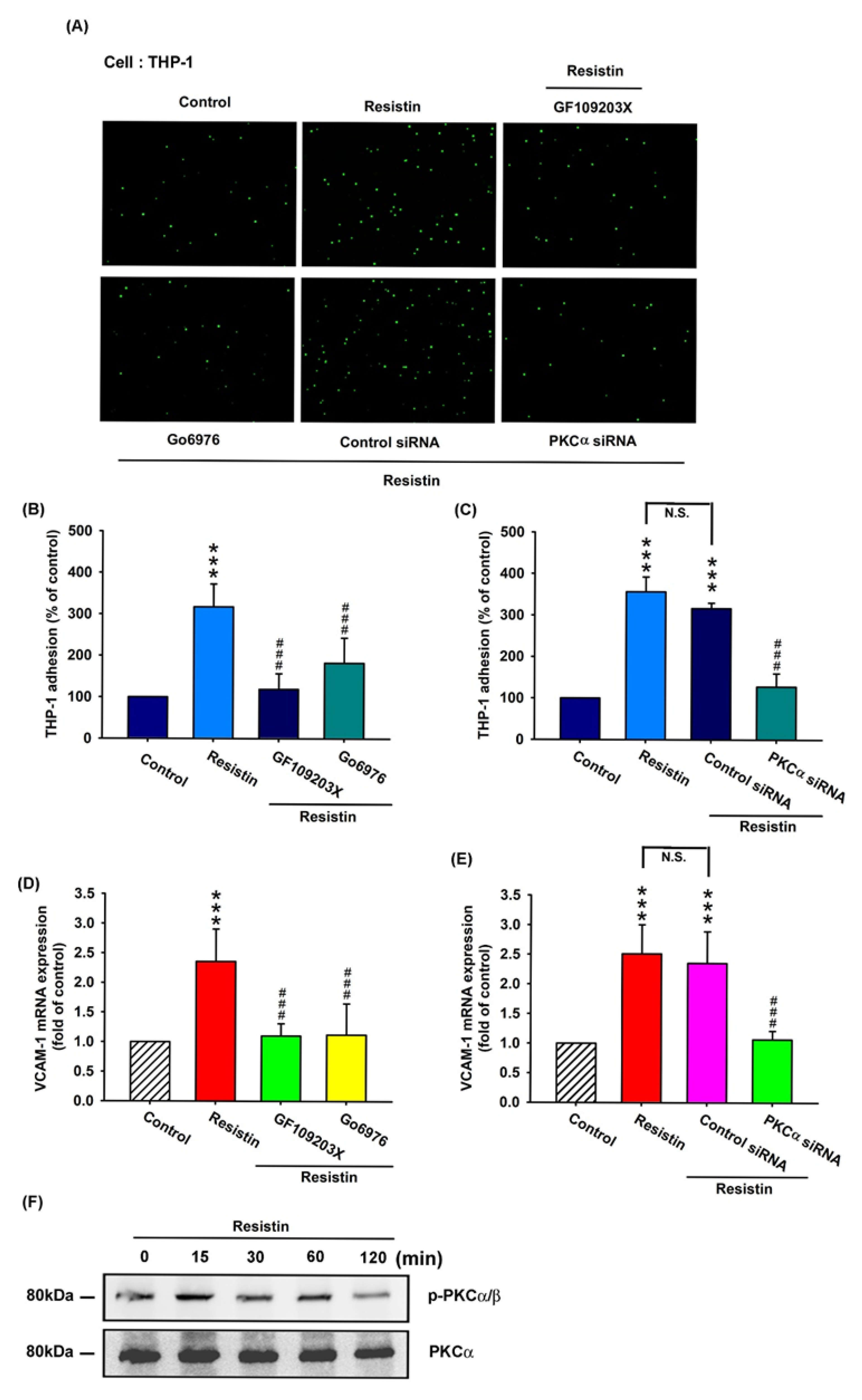
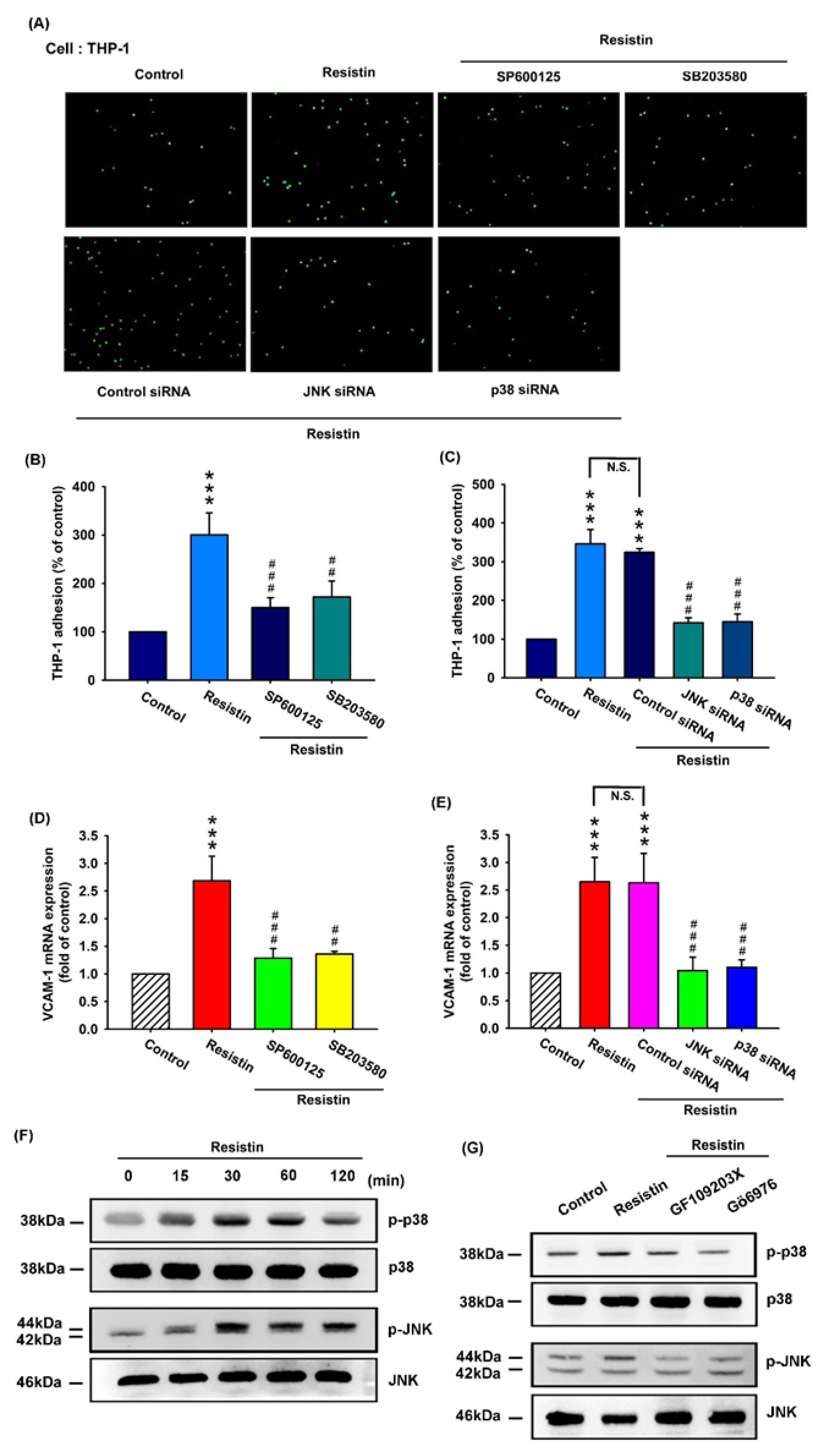
3.3. Resistin Promotes VCAM-1 Expression and Monocyte Adhesion via the PKC and PKC-Dependent p38 and JNK Signaling Pathways
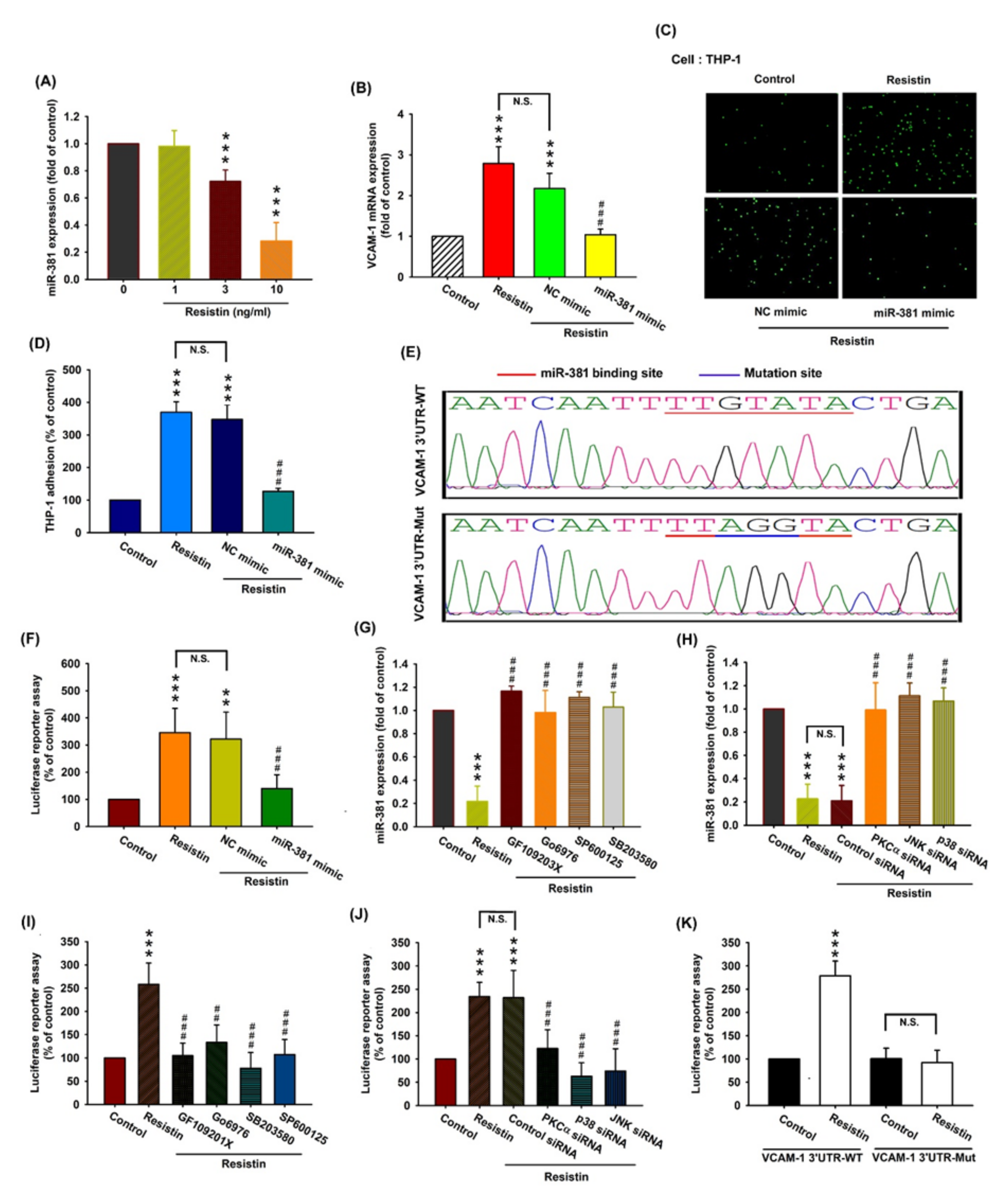
3.4. Resistin Increases VCAM-1 Expression and Monocyte Adhesion via the Inhibition of MiR-381 Synthesis
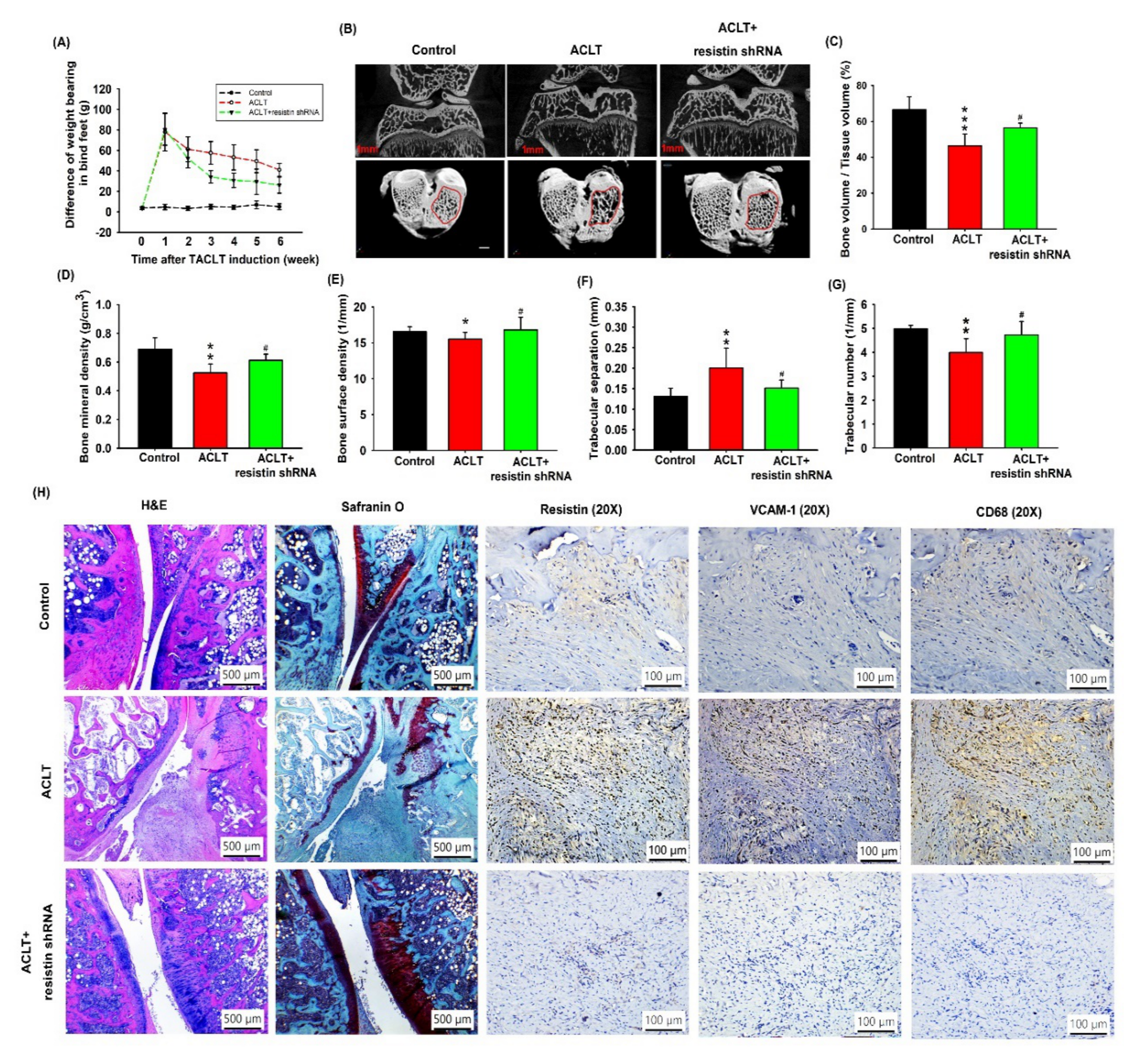
3.5. Transfection with Resistin shRNA Ameliorates the Severity of OA
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuo, S.J.; Liu, S.C.; Huang, Y.L.; Tsai, C.H.; Fong, Y.C.; Hsu, H.C.; Tang, C.H. Tgf-beta1 enhances foxo3 expression in human synovial fibroblasts by inhibiting mir-92a through ampk and p38 pathways. Aging 2019, 11, 4075–4089. [Google Scholar] [CrossRef]
- Siu, K.K.; Wu, K.T.; Ko, J.Y.; Wang, F.S.; Chou, W.Y.; Wang, C.J.; Kuo, S.J. Effects of computer-assisted navigation versus the conventional technique for total knee arthroplasty on levels of plasma thrombotic markers: A prospective study. Biomed. Eng. Online 2019, 18, 99. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Hou, S.M.; Tsai, C.H.; Huang, C.Y.; Hsu, C.J.; Tang, C.H. Ccn4 induces vascular cell adhesion molecule-1 expression in human synovial fibroblasts and promotes monocyte adhesion. Biochim. Et Biophys. Acta 2013, 1833, 966–975. [Google Scholar] [CrossRef]
- Schett, G.; Kiechl, S.; Bonora, E.; Zwerina, J.; Mayr, A.; Axmann, R.; Weger, S.; Oberhollenzer, F.; Lorenzini, R.; Willeit, J. Vascular cell adhesion molecule 1 as a predictor of severe osteoarthritis of the hip and knee joints. Arthritis Rheum. 2009, 60, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, L.; Pantsulaia, I.; Kobyliansky, E. Association between vascular cell adhesion molecule 1 and radiographic hand osteoarthritis. Clin. Exp. Rheumatol. 2011, 29, 544–546. [Google Scholar] [PubMed]
- Zhao, C.W.; Gao, Y.H.; Song, W.X.; Liu, B.; Ding, L.; Dong, N.; Qi, X. An update on the emerging role of resistin on the pathogenesis of osteoarthritis. Mediat. Inflamm. 2019, 2019, 1532164. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, A.; Vuolteenaho, K.; Moilanen, T.; Moilanen, E. Resistin as a factor in osteoarthritis: Synovial fluid resistin concentrations correlate positively with interleukin 6 and matrix metalloproteinases mmp-1 and mmp-3. Scand. J. Rheumatol. 2014, 43, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Kuo, S.J.; Liu, S.C.; Wang, S.W.; Tsai, C.H.; Fong, Y.C.; Tang, C.H. Apelin affects the progression of osteoarthritis by regulating vegf-dependent angiogenesis and mir-150-5p expression in human synovial fibroblasts. Cells 2020, 9, 594. [Google Scholar] [CrossRef]
- Swingler, T.E.; Niu, L.; Smith, P.; Paddy, P.; Le, L.; Barter, M.J.; Young, D.A.; Clark, I.M. The function of micrornas in cartilage and osteoarthritis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 40–47. [Google Scholar]
- Makki, M.S.; Haseeb, A.; Haqqi, T.M. Microrna-9 promotion of interleukin-6 expression by inhibiting monocyte chemoattractant protein-induced protein 1 expression in interleukin-1beta-stimulated human chondrocytes. Arthritis Rheumatol. 2015, 67, 2117–2128. [Google Scholar] [CrossRef]
- Kim, J.E.; Song, D.H.; Kim, S.H.; Jung, Y.; Kim, S.J. Development and characterization of various osteoarthritis models for tissue engineering. PLoS ONE 2018, 13, e0194288. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Hsu, C.J.; Tsai, C.H.; Huang, C.Y.; Wang, S.W.; Tang, C.H. Resistin promotes angiogenesis in endothelial progenitor cells through inhibition of microrna206: Potential implications for rheumatoid arthritis. Stem Cells 2015, 33, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.J.; Lin, C.Y.; Tsai, C.H.; Huang, Y.L.; Tang, C.H. Glucose suppresses il-1beta-induced mmp-1 expression through the fak, mek, erk, and ap-1 signaling pathways. Environ. Toxicol. 2018, 33, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chao, C.C.; Chen, P.C.; Liu, P.I.; Yang, Y.C.; Su, C.M.; Huang, W.C.; Tang, C.H. Thrombospondin enhances rankl-dependent osteoclastogenesis and facilitates lung cancer bone metastasis. Biochem. Pharmacol. 2019, 166, 23–32. [Google Scholar] [CrossRef]
- Liu, J.F.; Lee, C.W.; Tsai, M.H.; Tang, C.H.; Chen, P.C.; Lin, L.W.; Lin, C.Y.; Lu, C.H.; Lin, Y.F.; Yang, S.H.; et al. Thrombospondin 2 promotes tumor metastasis by inducing matrix metalloproteinase-13 production in lung cancer cells. Biochem. Pharmacol. 2018, 155, 537–546. [Google Scholar] [CrossRef]
- Lee, H.P.; Chen, P.C.; Wang, S.W.; Fong, Y.C.; Tsai, C.H.; Tsai, F.J.; Chung, J.G.; Huang, C.Y.; Yang, J.S.; Hsu, Y.M.; et al. Plumbagin suppresses endothelial progenitor cell-related angiogenesis in vitro and in vivo. J. Funct. Foods 2019, 52, 537–544. [Google Scholar] [CrossRef]
- Lee, H.P.; Wang, S.W.; Wu, Y.C.; Lin, L.W.; Tsai, F.J.; Yang, J.S.; Li, T.M.; Tang, C.H. Soya-cerebroside inhibits vegf-facilitated angiogenesis in endothelial progenitor cells. Food Agric. Immunol. 2020, 31, 193–204. [Google Scholar] [CrossRef]
- Lee, H.P.; Wang, S.W.; Wu, Y.C.; Tsai, C.H.; Tsai, F.J.; Chung, J.G.; Huang, C.Y.; Yang, J.S.; Hsu, Y.M.; Yin, M.C.; et al. Glucocerebroside reduces endothelial progenitor cell-induced angiogenesis. Food Agric. Immunol. 2019, 30, 1033–1045. [Google Scholar] [CrossRef]
- Su, C.M.; Tang, C.H.; Chi, M.J.; Lin, C.Y.; Fong, Y.C.; Liu, Y.C.; Chen, W.C.; Wang, S.W. Resistin facilitates vegf-c-associated lymphangiogenesis by inhibiting mir-186 in human chondrosarcoma cells. Biochem. Pharm. 2018, 154, 234–242. [Google Scholar] [CrossRef]
- Wu, K.M.; Hsu, Y.M.; Ying, M.C.; Tsai, F.J.; Tsai, C.H.; Chung, J.G.; Yang, J.S.; Tang, C.H.; Cheng, L.Y.; Su, P.H.; et al. High-density lipoprotein ameliorates palmitic acid-induced lipotoxicity and oxidative dysfunction in h9c2 cardiomyoblast cells via ros suppression. Nutr. Metab. 2019, 16, 36. [Google Scholar] [CrossRef]
- Kuo, S.J.; Yang, W.H.; Liu, S.C.; Tsai, C.H.; Hsu, H.C.; Tang, C.H. Transforming growth factor beta1 enhances heme oxygenase 1 expression in human synovial fibroblasts by inhibiting microrna 519b synthesis. PLoS ONE 2017, 12, e0176052. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Chiou, P.C.; Chen, P.C.; Liu, P.Y.; Huang, W.C.; Chao, C.C.; Tang, C.H. Melatonin reduces lung cancer stemness through inhibiting of plc, erk, p38, beta-catenin, and twist pathways. Environ. Toxicol. 2019, 34, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Mohan, G.; Perilli, E.; Kuliwaba, J.S.; Humphries, J.M.; Parkinson, I.H.; Fazzalari, N.L. Application of in vivo micro-computed tomography in the temporal characterisation of subchondral bone architecture in a rat model of low-dose monosodium iodoacetate-induced osteoarthritis. Arthritis Res. Ther. 2011, 13, R210. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.Y.; Tsai, C.H.; Liu, S.C.; Huang, C.C.; Lin, T.H.; Yang, Y.Z.; Tang, C.H. Noggin inhibits il-1beta and bmp-2 expression, and attenuates cartilage degeneration and subchondral bone destruction in experimental osteoarthritis. Cells 2020, 9, 927. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Chiang, Y.C.; Huang, C.Y.; Hsu, C.J.; Fong, Y.C.; Tang, C.H. Osteopontin promotes oncostatin m production in human osteoblasts: Implication of rheumatoid arthritis therapy. J. Immunol 2015, 195, 3355–3364. [Google Scholar] [CrossRef]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The oarsi histopathology initiative-recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S24–S34. [Google Scholar] [CrossRef]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The oarsi histopathology initiative-recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S17–S23. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, D.; Bai, X. Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 2020, 28, 555–561. [Google Scholar] [CrossRef]
- Gao, J.; Chang Chua, C.; Chen, Z.; Wang, H.; Xu, X.; R, C.H.; McMullen, J.R.; Shioi, T.; Izumo, S.; Chua, B.H. Resistin, an adipocytokine, offers protection against acute myocardial infarction. J. Mol. Cell. Cardiol. 2007, 43, 601–609. [Google Scholar] [CrossRef]
- Tsai, H.C.; Cheng, S.P.; Han, C.K.; Huang, Y.L.; Wang, S.W.; Lee, J.J.; Lai, C.T.; Fong, Y.C.; Tang, C.H. Resistin enhances angiogenesis in osteosarcoma via the mapk signaling pathway. Aging 2019, 11, 9767–9777. [Google Scholar] [CrossRef]
- Taipaleenmaki, H. Regulation of bone metabolism by micrornas. Curr. Osteoporos. Rep. 2018, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Gao, W.; Wu, Z.; Zhou, T.; Qiu, X.; Wang, X.; Lian, C.; Peng, Y.; Liang, A.; Qiu, J.; et al. Melatonin rescued interleukin 1beta-impaired chondrogenesis of human mesenchymal stem cells. Stem Cell Res. 2018, 9, 162. [Google Scholar]
- Liu, S.C.; Tsai, C.H.; Wu, T.Y.; Tsai, C.H.; Tsai, F.J.; Chung, J.G.; Huang, C.Y.; Yang, J.S.; Hsu, Y.M.; Yin, M.C.; et al. Soya-cerebroside reduces il-1 beta-induced mmp-1 production in chondrocytes and inhibits cartilage degradation: Implications for the treatment of osteoarthritis. Food Agric. Immunol. 2019, 30, 620–632. [Google Scholar] [CrossRef]
- Lavigne, P.; Benderdour, M.; Shi, Q.; Lajeunesse, D.; Fernandes, J.C. Involvement of icam-1 in bone metabolism: A potential target in the treatment of bone diseases? Expert Opin. Biol. Ther. 2005, 5, 313–320. [Google Scholar] [CrossRef]
- Song, Y.Z.; Guan, J.; Wang, H.J.; Ma, W.; Li, F.; Xu, F.; Ding, L.B.; Xie, L.; Liu, B.; Liu, K.; et al. Possible involvement of serum and synovial fluid resistin in knee osteoarthritis: Cartilage damage, clinical, and radiological links. J. Clin. Lab. Anal. 2016, 30, 437–443. [Google Scholar] [CrossRef]
- Tedesco, S.; De Majo, F.; Kim, J.; Trenti, A.; Trevisi, L.; Fadini, G.P.; Bolego, C.; Zandstra, P.W.; Cignarella, A.; Vitiello, L. Convenience versus biological significance: Are pma-differentiated thp-1 cells a reliable substitute for blood-derived macrophages when studying in vitro polarization? Front. Pharmacol. 2018, 9, 71. [Google Scholar] [CrossRef]
- de Lange-Brokaar, B.J.; Ioan-Facsinay, A.; van Osch, G.J.; Zuurmond, A.M.; Schoones, J.; Toes, R.E.; Huizinga, T.W.; Kloppenburg, M. Synovial inflammation, immune cells and their cytokines in osteoarthritis: A review. Osteoarthr. Cartil. 2012, 20, 1484–1499. [Google Scholar] [CrossRef]
- Bondeson, J.; Blom, A.B.; Wainwright, S.; Hughes, C.; Caterson, B.; van den Berg, W.B. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010, 62, 647–657. [Google Scholar] [CrossRef]
- Kennedy, A.; Fearon, U.; Veale, D.J.; Godson, C. Macrophages in synovial inflammation. Front. Immunol. 2011, 2, 52. [Google Scholar] [CrossRef]
- Zuniga, M.C.; Raghuraman, G.; Hitchner, E.; Weyand, C.; Robinson, W.; Zhou, W. Pkc-epsilon and tlr4 synergistically regulate resistin-mediated inflammation in human macrophages. Atherosclerosis 2017, 259, 51–59. [Google Scholar] [CrossRef]
- Chen, H.T.; Tsou, H.K.; Chen, J.C.; Shih, J.M.; Chen, Y.J.; Tang, C.H. Adiponectin enhances intercellular adhesion molecule-1 expression and promotes monocyte adhesion in human synovial fibroblasts. PLoS ONE 2014, 9, e92741. [Google Scholar] [CrossRef]
- Hsu, C.J.; Wu, M.H.; Chen, C.Y.; Tsai, C.H.; Hsu, H.C.; Tang, C.H. Amp-activated protein kinase activation mediates ccl3-induced cell migration and matrix metalloproteinase-2 expression in human chondrosarcoma. Cell Commun. Signal. CCS 2013, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Nugent, M. Micrornas: Exploring new horizons in osteoarthritis. Osteoarthr. Cartil. 2016, 24, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Al-Modawi, R.N.; Brinchmann, J.E.; Karlsen, T.A. Multi-pathway protective effects of micrornas on human chondrocytes in an in vitro model of osteoarthritis. Mol. Nucleic Acids 2019, 17, 776–790. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-C.; Lin, C.-Y.; Kuo, S.-J.; Liu, S.-C.; Lu, Y.-C.; Chen, Y.-L.; Wang, S.-W.; Tang, C.-H. Resistin Enhances VCAM-1 Expression and Monocyte Adhesion in Human Osteoarthritis Synovial Fibroblasts by Inhibiting MiR-381 Expression through the PKC, p38, and JNK Signaling Pathways. Cells 2020, 9, 1369. https://doi.org/10.3390/cells9061369
Chen W-C, Lin C-Y, Kuo S-J, Liu S-C, Lu Y-C, Chen Y-L, Wang S-W, Tang C-H. Resistin Enhances VCAM-1 Expression and Monocyte Adhesion in Human Osteoarthritis Synovial Fibroblasts by Inhibiting MiR-381 Expression through the PKC, p38, and JNK Signaling Pathways. Cells. 2020; 9(6):1369. https://doi.org/10.3390/cells9061369
Chicago/Turabian StyleChen, Wei-Cheng, Chih-Yang Lin, Shu-Jui Kuo, Shan-Chi Liu, Yung-Chang Lu, Yen-Ling Chen, Shih-Wei Wang, and Chih-Hsin Tang. 2020. "Resistin Enhances VCAM-1 Expression and Monocyte Adhesion in Human Osteoarthritis Synovial Fibroblasts by Inhibiting MiR-381 Expression through the PKC, p38, and JNK Signaling Pathways" Cells 9, no. 6: 1369. https://doi.org/10.3390/cells9061369
APA StyleChen, W.-C., Lin, C.-Y., Kuo, S.-J., Liu, S.-C., Lu, Y.-C., Chen, Y.-L., Wang, S.-W., & Tang, C.-H. (2020). Resistin Enhances VCAM-1 Expression and Monocyte Adhesion in Human Osteoarthritis Synovial Fibroblasts by Inhibiting MiR-381 Expression through the PKC, p38, and JNK Signaling Pathways. Cells, 9(6), 1369. https://doi.org/10.3390/cells9061369





