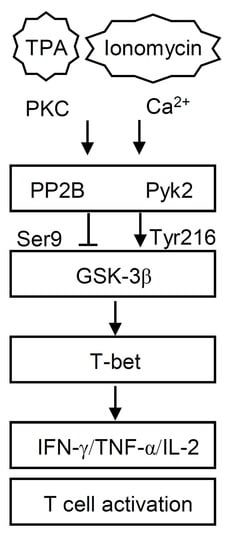
Glycogen Synthase Kinase-3β Facilitates Cytokine Production in 12-O-Tetradecanoylphorbol-13-Acetate/Ionomycin-Activated Human CD4+ T Lymphocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Mice and Thymocyte Isolation
2.3. Human T Lymphocyte Isolation
2.4. Cell Culture
2.5. Immunostaining
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Cytotoxicity Assay
2.8. Western Blotting
2.9. Short Hairpin RNA (shRNA)
2.10. Calcineurin Cellular Activity Assay
2.11. Fluorescence Imaging
2.12. RNA Interference
2.13. Animal Treatment
2.14. Statistical Analysis
3. Results
3.1. 12-O-Tetradecanoylphorbol-13-Acetate (TPA) and Ionomycin (T/I) Treatment Induces Cytokine Production in Different T Lymphocyte Lineages
3.2. GSK-3β Regulates T/I-activated Cytokine Production
3.3. T/I Treatment Induces GSK-3β-Mediated T Cell-Associated Cytokine Production through the Pyk2-Regulated Pathway
3.4. PP2B Regulates T/I-Induced GSK-3β Activation and Cytokine Production
3.5. T/I Treatment Induces GSK-3β-Regulated T-bet Nuclear Translocation Followed by T-bet-Mediated Cytokine Production
3.6. Pharmacological Inhibition of GSK-3 Reduces Mortality and Suppresses Cytokine production in T/I-Treated Mice
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Wilkinson, B.; Downey, J.S.; Rudd, C.E. T-cell signalling and immune system disorders. Expert Rev. Mol. Med. 2005, 7, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Paul, W.E. Heterogeneity and plasticity of T helper cells. Cell Res. 2010, 20, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations (*). Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Spolski, R.; Liao, W.; Leonard, W.J. Complex interactions of transcription factors in mediating cytokine biology in T cells. Immunol. Rev. 2014, 261, 141–156. [Google Scholar] [CrossRef] [Green Version]
- Gaud, G.; Lesourne, R.; Love, P.E. Regulatory mechanisms in T cell receptor signalling. Nat. Rev. Immunol. 2018, 18, 485–497. [Google Scholar] [CrossRef]
- Brownlie, R.J.; Zamoyska, R. T cell receptor signalling networks: Branched, diversified and bounded. Nat. Rev. Immunol. 2013, 13, 257–269. [Google Scholar] [CrossRef]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T cell activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef]
- Wang, H.; Brown, J.; Martin, M. Glycogen synthase kinase 3: A point of convergence for the host inflammatory response. Cytokine 2011, 53, 130–140. [Google Scholar] [CrossRef] [Green Version]
- Beurel, E.; Michalek, S.M.; Jope, R.S. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 2010, 31, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.; Woodgett, J.R. Glycogen Synthase Kinase 3: A Kinase for All Pathways? Curr. Top. Dev. Biol. 2017, 123, 277–302. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Lee, Y.I.; Seo, M.; Kim, S.Y.; Lee, J.E.; Youn, H.D.; Kim, Y.S.; Juhnn, Y.S. Calcineurin dephosphorylates glycogen synthase kinase-3 beta at serine-9 in neuroblast-derived cells. J. Neurochem. 2009, 111, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Seo, M.; Kim, Y.; Kim, S.Y.; Kang, U.G.; Kim, Y.S.; Juhnn, Y.S. Membrane depolarization induces the undulating phosphorylation/dephosphorylation of glycogen synthase kinase 3beta, and this dephosphorylation involves protein phosphatases 2A and 2B in SH-SY5Y human neuroblastoma cells. J. Biol. Chem. 2005, 280, 22044–22052. [Google Scholar] [CrossRef] [Green Version]
- Beals, C.R.; Sheridan, C.M.; Turck, C.W.; Gardner, P.; Crabtree, G.R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 1997, 275, 1930–1934. [Google Scholar] [CrossRef]
- Stankunas, K.; Graef, I.A.; Neilson, J.R.; Park, S.H.; Crabtree, G.R. Signaling through calcium, calcineurin, and NF-AT in lymphocyte activation and development. Cold Spring Harb. Symp. Quant. Biol. 1999, 64, 505–516. [Google Scholar] [CrossRef]
- Altman, A.; Mally, M.I.; Isakov, N. Phorbol ester synergizes with Ca2+ ionophore in activation of protein kinase C (PKC)alpha and PKC beta isoenzymes in human T cells and in induction of related cellular functions. Immunology 1992, 76, 465–471. [Google Scholar]
- Lin, Y.S.; Chen, K.H.; Kuo, C.F.; Huang, K.J.; Wu, J.J. Induction of thymocyte apoptosis in mice by Yersinia enterocolitica products. J. Med. Microbiol. 1998, 47, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.Y.; Chen, C.L.; Tsai, C.C.; Huang, W.C.; Tseng, P.C.; Lin, Y.S.; Chen, S.H.; Wong, T.W.; Choi, P.C.; Lin, C.F. Inhibiting glycogen synthase kinase-3 decreases 12-O-tetradecanoylphorbol-13-acetate-induced interferon-gamma-mediated skin inflammation. J. Pharmacol. Exp. Ther. 2012, 343, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Huang, W.C.; Wang, C.Y.; Tsai, C.C.; Chen, C.L.; Chang, Y.T.; Kai, J.I.; Lin, C.F. Inhibiting glycogen synthase kinase-3 reduces endotoxaemic acute renal failure by down-regulating inflammation and renal cell apoptosis. Br. J. Pharmacol. 2009, 157, 1004–1013. [Google Scholar] [CrossRef] [Green Version]
- Beurel, E. Regulation of inflammation and T cells by glycogen synthase kinase-3: Links to mood disorders. Neuroimmunomodulation 2014, 21, 140–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neilson, J.; Stankunas, K.; Crabtree, G.R. Monitoring the duration of antigen-receptor occupancy by calcineurin/glycogen-synthase-kinase-3 control of NF-AT nuclear shuttling. Curr. Opin. Immunol. 2001, 13, 346–350. [Google Scholar] [CrossRef]
- Hartigan, J.A.; Xiong, W.C.; Johnson, G.V. Glycogen synthase kinase 3beta is tyrosine phosphorylated by PYK2. Biochem. Biophys. Res. Commun. 2001, 284, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Meng, F.; Fu, X.; Song, B.; Yan, X.; Zhang, G. N-methyl-D-aspartate receptor and L-type voltage-gated Ca2+ channel activation mediate proline-rich tyrosine kinase 2 phosphorylation during cerebral ischemia in rats. Neurosci. Lett. 2004, 355, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. The structure and regulation of protein phosphatases. Annu. Rev. Biochem. 1989, 58, 453–508. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Luo, C.; Hogan, P.G. Transcription factors of the NFAT family: Regulation and function. Annu. Rev. Immunol. 1997, 15, 707–747. [Google Scholar] [CrossRef]
- Lugo-Villarino, G.; Maldonado-Lopez, R.; Possemato, R.; Penaranda, C.; Glimcher, L.H. T-bet is required for optimal production of IFN-gamma and antigen-specific T cell activation by dendritic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 7749–7754. [Google Scholar] [CrossRef] [Green Version]
- Neal, J.W.; Clipstone, N.A. Glycogen synthase kinase-3 inhibits the DNA binding activity of NFATc. J. Biol. Chem. 2001, 276, 3666–3673. [Google Scholar] [CrossRef] [Green Version]
- Ohteki, T.; Parsons, M.; Zakarian, A.; Jones, R.G.; Nguyen, L.T.; Woodgett, J.R.; Ohashi, P.S. Negative regulation of T cell proliferation and interleukin 2 production by the serine threonine kinase GSK-3. J. Exp. Med. 2000, 192, 99–104. [Google Scholar] [CrossRef]
- Serfling, E.; Avots, A.; Neumann, M. The architecture of the interleukin-2 promoter: A reflection of T lymphocyte activation. Biochim. Biophys. Acta 1995, 1263, 181–200. [Google Scholar] [CrossRef]
- Chen, L.; Rao, A.; Harrison, S.C. Signal integration by transcription-factor assemblies: Interactions of NF-AT1 and AP-1 on the IL-2 promoter. Cold Spring Harb. Symp. Quant. Biol. 1999, 64, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.; Loh, C.; Rao, A. Transcriptional regulation of the IL-2 gene. Curr. Opin. Immunol. 1995, 7, 333–342. [Google Scholar] [CrossRef]
- Penix, L.A.; Sweetser, M.T.; Weaver, W.M.; Hoeffler, J.P.; Kerppola, T.K.; Wilson, C.B. The proximal regulatory element of the interferon-gamma promoter mediates selective expression in T cells. J. Biol. Chem. 1996, 271, 31964–31972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falvo, J.V.; Tsytsykova, A.V.; Goldfeld, A.E. Transcriptional control of the TNF gene. Curr. Dir. Autoimmun. 2010, 11, 27–60. [Google Scholar] [CrossRef] [Green Version]
- Harris, D.P.; Goodrich, S.; Gerth, A.J.; Peng, S.L.; Lund, F.E. Regulation of IFN-gamma production by B effector 1 cells: Essential roles for T-bet and the IFN-gamma receptor. J. Immunol. 2005, 174, 6781–6790. [Google Scholar] [CrossRef] [Green Version]
- Jenner, R.G.; Townsend, M.J.; Jackson, I.; Sun, K.; Bouwman, R.D.; Young, R.A.; Glimcher, L.H.; Lord, G.M. The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc. Natl. Acad. Sci. USA 2009, 106, 17876–17881. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Chong, M.M.; Littman, D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity 2009, 30, 646–655. [Google Scholar] [CrossRef] [Green Version]
- Orbach, A.; Bassan-Levin, T.; Dan, P.; Hihinashvilli, B.; Marx, S. Utilizing glycogen synthase kinase-3beta as a marker for the diagnosis of graft-versus-host disease. Transplant. Proc. 2013, 45, 2051–2055. [Google Scholar] [CrossRef]
- Hill, L.; Alousi, A.; Kebriaei, P.; Mehta, R.; Rezvani, K.; Shpall, E. New and emerging therapies for acute and chronic graft versus host disease. Ther. Adv. Hematol. 2018, 9, 21–46. [Google Scholar] [CrossRef] [Green Version]
- Vaeth, M.; Bauerlein, C.A.; Pusch, T.; Findeis, J.; Chopra, M.; Mottok, A.; Rosenwald, A.; Beilhack, A.; Berberich-Siebelt, F. Selective NFAT targeting in T cells ameliorates GvHD while maintaining antitumor activity. Proc. Natl. Acad. Sci. USA 2015, 112, 1125–1130. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Yu, S.; Tanyi, J.L.; Lu, Y.; Woodgett, J.R.; Mills, G.B. Convergence of multiple signaling cascades at glycogen synthase kinase 3: Edg receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a protein kinase C-dependent intracellular pathway. Mol. Cell. Biol. 2002, 22, 2099–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-C.; Tsai, C.-K.; Tseng, P.-C.; Lin, C.-F.; Chen, C.-L. Glycogen Synthase Kinase-3β Facilitates Cytokine Production in 12-O-Tetradecanoylphorbol-13-Acetate/Ionomycin-Activated Human CD4+ T Lymphocytes. Cells 2020, 9, 1424. https://doi.org/10.3390/cells9061424
Tsai C-C, Tsai C-K, Tseng P-C, Lin C-F, Chen C-L. Glycogen Synthase Kinase-3β Facilitates Cytokine Production in 12-O-Tetradecanoylphorbol-13-Acetate/Ionomycin-Activated Human CD4+ T Lymphocytes. Cells. 2020; 9(6):1424. https://doi.org/10.3390/cells9061424
Chicago/Turabian StyleTsai, Cheng-Chieh, Chin-Kun Tsai, Po-Chun Tseng, Chiou-Feng Lin, and Chia-Ling Chen. 2020. "Glycogen Synthase Kinase-3β Facilitates Cytokine Production in 12-O-Tetradecanoylphorbol-13-Acetate/Ionomycin-Activated Human CD4+ T Lymphocytes" Cells 9, no. 6: 1424. https://doi.org/10.3390/cells9061424
APA StyleTsai, C.-C., Tsai, C.-K., Tseng, P.-C., Lin, C.-F., & Chen, C.-L. (2020). Glycogen Synthase Kinase-3β Facilitates Cytokine Production in 12-O-Tetradecanoylphorbol-13-Acetate/Ionomycin-Activated Human CD4+ T Lymphocytes. Cells, 9(6), 1424. https://doi.org/10.3390/cells9061424