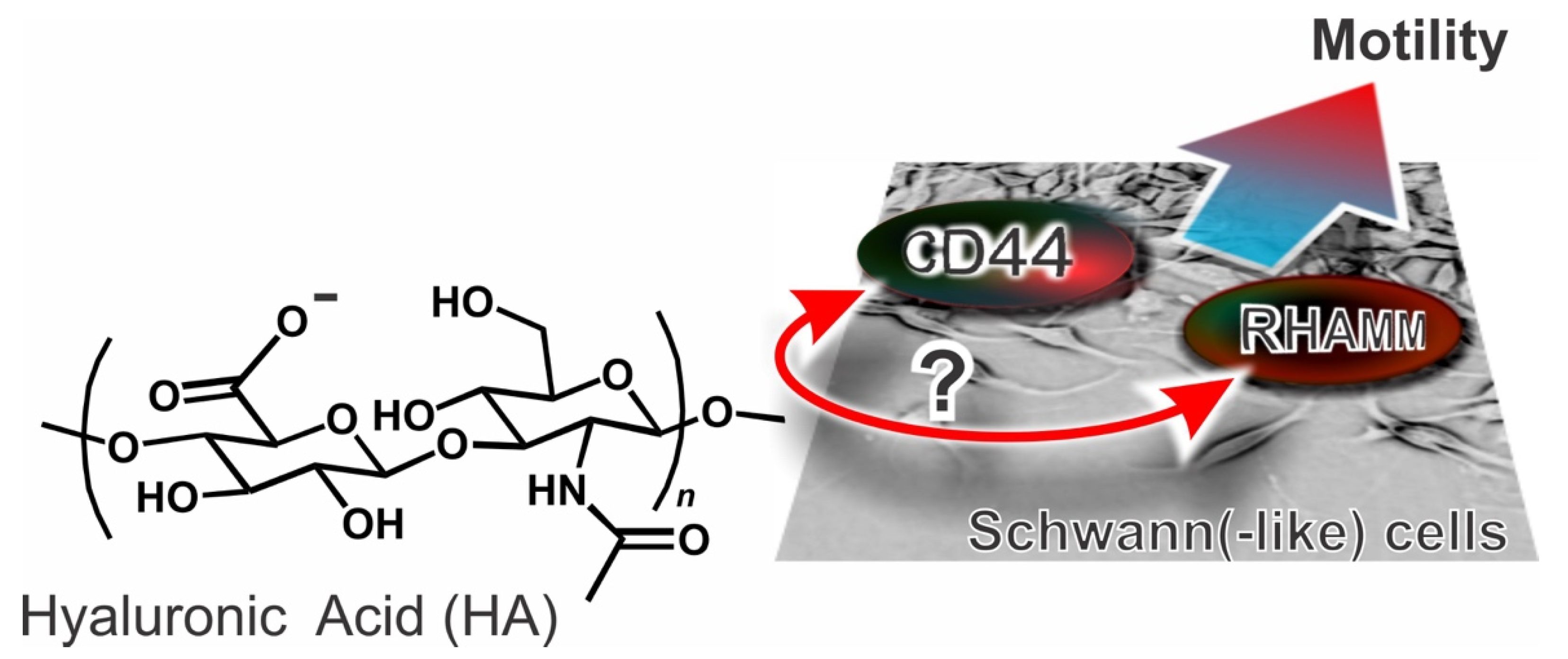
Hyaluronic Acid (HA) Receptors and the Motility of Schwann Cell(-Like) Phenotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Section
2.2. Cell Culture
2.2.1. Undifferentiated Adipose-Derived Mesenchymal Stem Cells (uAD-MSCs)
2.2.2. uAD-SC Differentiation into a Schwann Cell Phenotype (dAD-MSC)
2.3. Flow Cytometry
2.4. Scratch Wound Assay (SWA)
2.5. Statistical Analysis
3. Results and Discussion
3.1. HA and HA Receptors Are Abundant in Peripheral Nerves
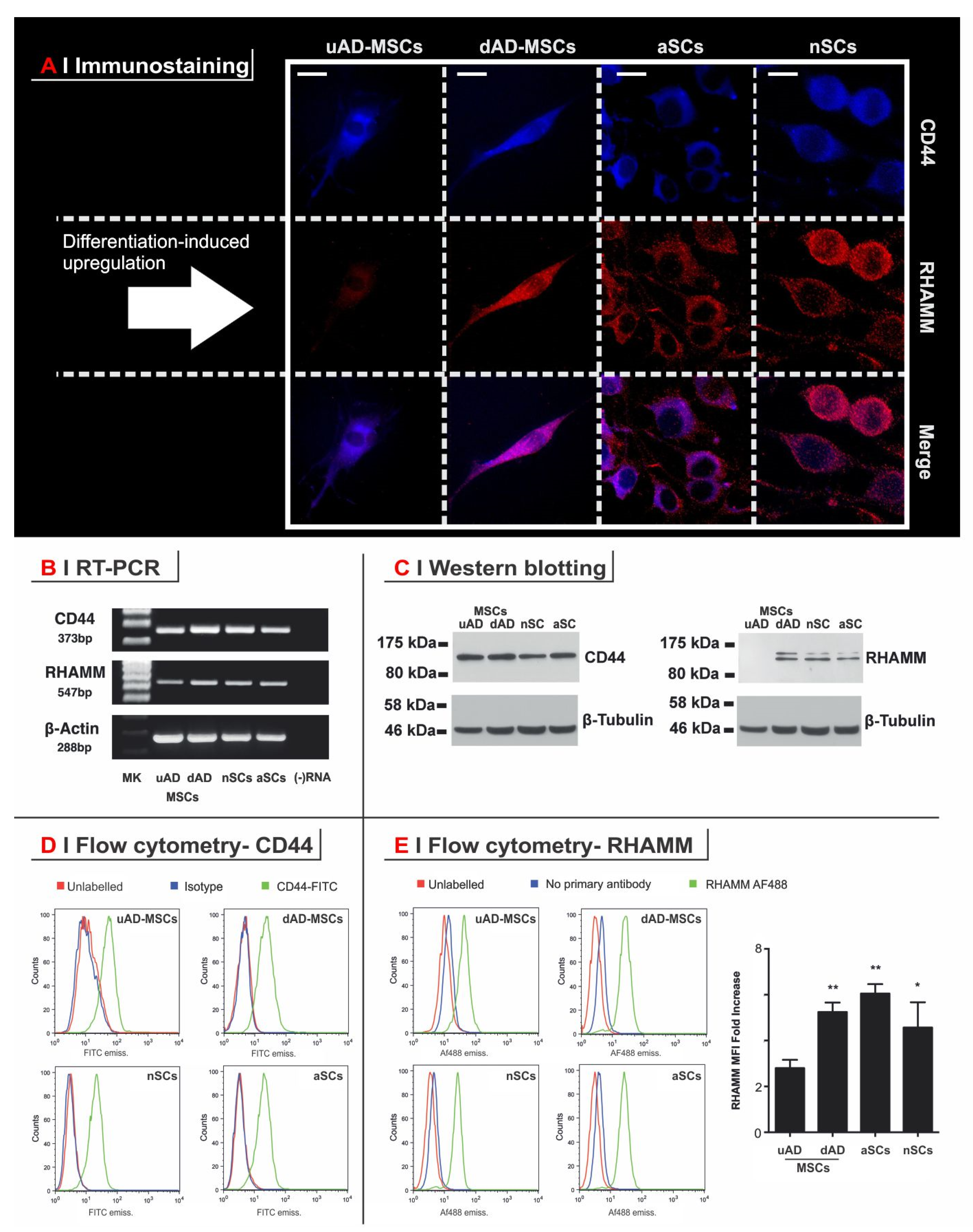
3.2. RHAMM but Not CD44 Is Upregulated in the Differentiation of Progenitor Cells (uAD-MSCs) to a Schwann-Like Phenotype (dAD-MSCs)
3.3. HA Receptors Affect Independently Both Motility and Morphology of Schwann (-Like) Cells
- (a)
- The supplementation of the cell culture medium with HA (234 kDa, 0.2 mg/mL; empty circle in all panels of Figure 3A) doubled the scratch closure rate for dAD-MSCs, nSCs, and aSCs, i.e., all the cell RHAMM(+) types. uAD-MSCs presented a modest (about 30%) but not statistically significant increase.
- (b)
- Anti-RHAMM almost completely immobilized the three RHAMM(+) types, but not uAD-MSCs, whose motility was unaffected by this blocking antibody (Figure 3A, left panel). Interestingly, supplementation of HA + anti-RHAMM had on uAD-MSCs the same modest stimulatory effect seen for HA alone, whereas had no effect on the other cell types. These data are in agreement with the substantial absence of RHAMM in uAD-MSCs and support the hypothesis of RHAMM as a gatekeeper for HA-mediated motility effects.
- (c)
- The effect of anti-CD44 was largely different; this blocking antibody abrogated the motility of uAD-MSC, appreciably reduced that of dAD-MSCs and aSCs (20–30% of initial motility) and had marginal influence on nSCs, but with largely variable results. Since the overall levels of CD44 (all standard isoform) did not appear to be much different among the cell types, the scarce sensitivity of nSCs to anti-CD44 may be due to a specific post-translational modification of this receptor. In any case, the key observation is that also CD44 blockage reduced motility, but through mechanisms quite different from RHAMM blockage. It is noteworthy that the RHAMM(+) cell types had some gain in wound closing speed in the presence of HA, also in the presence of anti-CD44, although the effect was marginal in comparison to the non-blocked controls.
- (d)
- Anti-CD44 and anti-RHAMM together had an additive (if not synergic) effect; all cell types were immobilized by their combination, independent of the presence of HA.
- (a)
- Anti-RHAMM did not appreciably bind to uAD-MSCs, thereby confirming the RHAMM(low) nature of these cells and supporting the lack of effects of the antibody treatment. Correspondingly, the cells preserved an elongated/stretched morphology.
- (b)
- Curiously, anti-CD44 clearly bound (and immobilized) uAD-MSCs did not appear to cause any significant morphological alteration to them. This would appear to indicate that the anti-CD44 effect on these cells may be due to specific signaling rather than to a decreased adhesion to substrate.
- (c)
- The RHAMM(high) cell types showed similar effects upon anti-RHAMM and anti-CD44 treatment: the elongated/stretched phenotype was lost and the cells acquired a more round morphology. It is noteworthy, however, that the two antibodies had virtually identical effects on the motility of the two cells, but dAD-MSCs were round already after 1 h of exposure, while aSCs required a longer time (effect apparent at 24 h); due to the different kinetics of the reduction in adhesion, also in this case, we are tempted to ascribe the effects on motility to a signaling cascade triggered by the receptor binding rather than to loss of adhesion.
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nichols, C.M.; Brenner, M.J.; Fox, I.K.; Tung, T.H.; Hunter, D.A.; Rickman, S.R.; Mackinnon, S.E. Effect of motor versus sensory nerve grafts on peripheral nerve regeneration. Exp. Neurol 2004, 190, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cebral, R.; Silva-Correia, J.; Reis, R.L.; Silva, T.H.; Oliveira, J.M. Peripheral Nerve Injury: Current Challenges, Conventional Treatment Approaches, and New Trends in Biomaterials-Based Regenerative Strategies. ACS Biomater. Sci. Eng. 2017, 3, 3098–3122. [Google Scholar] [CrossRef]
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J.; Reid, A.J. Peripheral nerve regeneration: Experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015, 82–83, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Pfister, L.A.; Papaloizos, M.; Merkle, H.P.; Gander, B. Nerve conduits and growth factor delivery in peripheral nerve repair. J. Peripher. Nerv. Syst. 2007, 12, 65–82. [Google Scholar] [CrossRef]
- Young, R.C.; Wiberg, M.; Terenghi, G. Poly-3-hydroxybutyrate (PHB): A resorbable conduit for long-gap repair in peripheral nerves. Brit. J. Plast. Surg. 2002, 55, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Mohanna, P.N.; Terenghi, G.; Wiberg, M. Composite PHB-GGF conduit for long nerve gap repair: A long-term evaluation. Scan. J. Plast. Recons. 2005, 39, 129–137. [Google Scholar] [CrossRef]
- Schlosshauer, B.; Dreesmann, L.; Schaller, H.E.; Sinis, N. Synthetic nerve guide implants in humans: A comprehensive survey. Neurosurgery 2006, 59, 740–747. [Google Scholar] [CrossRef]
- Jones, S.; Eisenberg, H.M.; Jia, X.F. Advances and Future Applications of Augmented Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2016, 17, 17. [Google Scholar] [CrossRef]
- Liu, S.W.; Sandner, B.; Schackel, T.; Nicholson, L.; Chtarto, A.; Tenenbaum, L.; Puttagunta, R.; Muller, R.; Weidner, N.; Blesch, A. Regulated viral BDNF delivery in combination with Schwann cells promotes axonal regeneration through capillary alginate hydrogels after spinal cord injury. Acta Biomater. 2017, 60, 167–180. [Google Scholar] [CrossRef]
- Tajdaran, K.; Gordon, T.; Wood, M.D.; Shoichet, M.S.; Borschel, G.H. A glial cell line-derived neurotrophic factor delivery system enhances nerve regeneration across acellular nerve allografts. Acta Biomater. 2016, 29, 62–70. [Google Scholar] [CrossRef]
- Ching, R.C.; Wiberg, M.; Kingham, P.J. Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res. Ther. 2018, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Rosen, J.M. The role of undifferentiated adipose-derived stem cells in peripheral nerve repair. Neural Regen. Res. 2018, 13, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Schaffler, A.; Buchler, C. Concise review: Adipose tissue-derived stromal cells—Basic and clinical implications for novel cell-based therapies. Stem Cells 2007, 25, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Kingham, P.J.; Kolar, M.K.; Novikova, L.N.; Novikov, L.N.; Wiberg, M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 2014, 23, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Madura, T.; Sakai, Y.; Yano, K.; Terenghi, G.; Hosokawa, K. Glial differentiation of human adipose-derived stem cells: Implications for cell-based transplantation therapy. Neuroscience 2013, 236, 55–65. [Google Scholar] [CrossRef]
- Faroni, A.; Smith, R.J.; Lu, L.; Reid, A.J. Human Schwann-like cells derived from adipose-derived mesenchymal stem cells rapidly de-differentiate in the absence of stimulating medium. Eur. J. Neurosci. 2016, 43, 417–430. [Google Scholar] [CrossRef]
- Di Summa, P.G.; Kingham, P.J.; Raffoul, W.; Wiberg, M.; Terenghi, G.; Kalbermatten, D.F. Adipose-derived stem cells enhance peripheral nerve regeneration. J. Plast. Reconstr. Aes. 2010, 63, 1544–1552. [Google Scholar] [CrossRef]
- Watanabe, Y.; Sasaki, R.; Matsumine, H.; Yamato, M.; Okano, T. Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. J. Tissue Eng. Regen. Med. 2017, 11, 362–374. [Google Scholar] [CrossRef]
- Faroni, A.; Terenghi, G.; Reid, A.J. Adipose-Derived Stem Cells and Nerve Regeneration: Promises and Pitfalls. In Tissue Engineering of the Peripheral Nerve: Stem Cells and Regeneration Promoting Factors; Geuna, S., Perroteau, I., Tos, P., Battiston, B., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 108, pp. 121–136. [Google Scholar]
- Mantovani, C.; Mahay, D.; Kingham, P.J.; Terenghi, G.; Shawcross, S.G.; Wiberg, M. Bone marrow- and adipose-derived stem cells show expression of myelin mRNAs and proteins. Regen Med. 2010, 5, 403–410. [Google Scholar] [CrossRef]
- Di Summa, P.G.; Kalbermatten, D.F.; Pralong, E.; Raffoul, W.; Kingham, P.J.; Terenghi, G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience 2011, 181, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Di Summa, P.G.; Kingham, P.J.; Campisi, C.C.; Raffoul, W.; Kalbermatten, D.F. Collagen (NeuraGen(R)) nerve conduits and stem cells for peripheral nerve gap repair. Neurosci. Lett. 2014, 572, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Matsumine, H.; Osaki, H.; Ueta, Y.; Tsunoda, S.; Kamei, W.; Hashimoto, K.; Niimi, Y.; Watanabe, Y.; Miyata, M.; et al. Adipose-derived stem cells and the stromal vascular fraction in polyglycolic acid-collagen nerve conduits promote rat facial nerve regeneration. Wound Repair Regen. 2018, 26, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.J.; Wiberg, M.; Terenghi, G.; Kingham, P.J. ECM molecules mediate both Schwann cell proliferation and activation to enhance neurite outgrowth. Tissue Eng. 2007, 13, 2863–2870. [Google Scholar] [CrossRef]
- Di Summa, P.G.; Kalbermatten, D.F.; Raffoul, W.; Terenghi, G.; Kingham, P.J. Extracellular matrix molecules enhance the neurotrophic effect of Schwann cell-like differentiated adipose-derived stem cells and increase cell survival under stress conditions. Tissue Eng. Part A 2013, 19, 368–379. [Google Scholar] [CrossRef]
- Bruckner, G.; Morawski, M.; Arendt, T. Aggrecan-based extracellular matrix is an integral part of the human basal ganglia circuit. Neuroscience 2008, 151, 489–504. [Google Scholar] [CrossRef]
- Gong, H.Y.; Ye, W.; Freddo, T.F.; Hernandez, M.R. Hyaluronic acid in the normal and glaucomatous optic nerve. Exp. Eye Res. 1997, 64, 587–595. [Google Scholar] [CrossRef]
- Hiraga, T.; Ito, S.; Nakamura, H. Cancer Stem-like Cell Marker CD44 Promotes Bone Metastases by Enhancing Tumorigenicity, Cell Motility, and Hyaluronan Production. Cancer Res. 2013, 73, 4112–4122. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wu, Y.P.; Wang, H.F.; Zhang, Y.Q.; Mei, L.; Fang, X.X.; Zhang, X.D.; Zhang, F.; Chen, H.B.; Liu, Y.; et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc. Natl. Acad. Sci. USA 2014, 111, E89–E98. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front. Immunol 2015, 6, 31. [Google Scholar] [CrossRef]
- De la Rosa, J.M.R.; Pingrajai, P.; Pelliccia, M.; Spadea, A.; Lallana, E.; Gennari, A.; Stratford, I.J.; Rocchia, W.; Tirella, A.; Tirelli, N. Binding and Internalization in Receptor-Targeted Carriers: The Complex Role of CD44 in the Uptake of Hyaluronic Acid-Based Nanoparticles (siRNA Delivery). Adv. Healthc. Mater. 2019, 8, e1901182. [Google Scholar] [CrossRef] [PubMed]
- Spadea, A.; de la Rosa, J.M.R.; Tirella, A.; Ashford, M.B.; Williams, K.J.; Stratford, I.J.; Tirelli, N.; Mehibel, M. Evaluating the Efficiency of Hyaluronic Acid for Tumor Targeting via CD44. Mol. Pharm. 2019, 16, 2481–2493. [Google Scholar] [CrossRef] [PubMed]
- Meszar, Z.; Felszeghy, S.; Veress, G.; Matesz, K.; Szekely, G.; Modis, L. Hyaluronan accumulates around differentiating neurons in spinal cord of chicken embryos. Brain Res. Bull. 2008, 75, 414–418. [Google Scholar] [CrossRef]
- Ikeda, K.; Nakao, J.; Asou, H.; Toya, S.; Shinoda, J.; Uyemura, K. Expression of CD44H in the cells of neural crest origin in peripheral nervous system. Neuroreport 1996, 7, 1713–1716. [Google Scholar]
- Casini, P.; Nardi, I.; Ori, M. RHAMM mRNA expression in proliferating and migrating cells of the developing central nervous system. Gene Expr. Patterns 2010, 10, 93–97. [Google Scholar] [CrossRef]
- Piao, J.H.; Wang, Y.; Duncan, I.D. CD44 is required for the migration of transplanted oligodendrocyte progenitor cells to focal inflammatory demyelinating lesions in the spinal cord. Glia 2013, 61, 361–367. [Google Scholar] [CrossRef]
- Tona, A.; Perides, G.; Rahemtulla, F.; Dahl, D. Extracellular-matrix in regenerating rat sciatic-nerve—A comparative-study on the localization of laminin, hyaluronic-acid, and chondroitin sulfate proteoglycans, including versican. J. Histochem. Cytochem. 1993, 41, 593–599. [Google Scholar] [CrossRef]
- Su, W.P.; Sin, M.; Darrow, A.; Sherman, L.S. Malignant peripheral nerve sheath tumor cell invasion is facilitated by Src and aberrant CD44 expression. Glia 2003, 42, 350–358. [Google Scholar] [CrossRef]
- Mohan, P.; Castellsague, J.; Jiang, J.H.; Allen, K.; Chen, H.L.; Nemirovsky, O.; Spyra, M.; Hu, K.J.; Kluwe, L.; Pujana, M.A.; et al. Genomic imbalance of HMMR/RHAMM regulates the sensitivity and response of malignant peripheral nerve sheath tumour cells to aurora kinase inhibition. Oncotarget 2013, 4, 80–93. [Google Scholar] [CrossRef]
- Merzak, A.; Koocheckpour, S.; Pilkington, G.J. CD44 mediates human glioma cell-adhesion and invasion in-vitro. Cancer Res. 1994, 54, 3988–3992. [Google Scholar]
- Anido, J.; Saez-Borderias, A.; Gonzalez-Junca, A.; Rodon, L.; Folch, G.; Carmona, M.A.; Prieto-Sanchez, R.M.; Barba, I.; Martinez-Saez, E.; Prudkin, L.; et al. TGF-beta Receptor Inhibitors Target the CD44(high)/Id1(high) Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell 2010, 18, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Jung, S.; Salhia, B.; Lee, S.P.; Hubbard, S.; Taylor, M.; Mainprize, T.; Akaishi, K.; van Furth, W.; Rutka, J.T. Hyaluronate receptors mediating glioma cell migration and proliferation. J. Neuro-Oncol. 2001, 53, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Ozgenel, G.Y. Effects of hyaluronic acid on peripheral nerve scarring and regeneration in rats. Microsurgery 2003, 23, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.K.; Nemeth, I.R.; Seckel, B.R.; Chakalis-Haley, D.P.; Swann, D.A.; Kuo, J.W.; Bryan, D.J.; Cetrulo, C.L. Hyaluronic acid enhances peripheral nerve regeneration in vivo. Microsurgery 1998, 18, 270–275. [Google Scholar] [CrossRef]
- Park, Y.D.; Tirelli, N.; Hubbell, J.A. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials 2003, 24, 893–900. [Google Scholar] [CrossRef]
- Ouasti, S.; Donno, R.; Cellesi, F.; Sherratt, M.J.; Terenghi, G.; Tirelli, N. Network connectivity, mechanical properties and cell adhesion for hyaluronic acid/PEG hydrogels. Biomaterials 2011, 32, 6456–6470. [Google Scholar] [CrossRef]
- Vilarino-Feltrer, G.; Martinez-Ramos, C.; Monleon-de-la-Fuente, A.; Valles-Lluch, A.; Moratal, D.; Albacar, J.A.B.; Pradas, M.M. Schwann-cell cylinders grown inside hyaluronic-acid tubular scaffolds with gradient porosity. Acta Biomater. 2016, 30, 199–211. [Google Scholar] [CrossRef]
- Faroni, A.; Mantovani, C.; Shawcross, S.G.; Motta, M.; Terenghi, G.; Magnaghi, V. Schwann-like adult stem cells derived from bone marrow and adipose tissue express gamma-aminobutyric acid type B receptors. J. Neurosci. Res. 2011, 89, 1351–1362. [Google Scholar] [CrossRef]
- Morrissey, T.; Kleitman, N.; Bunge, R. Isolation and functional characterization of Schwann cells derived from adult peripheral nerve. J. Neurosci. 1991, 11, 2433–2442. [Google Scholar] [CrossRef]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Differentiation of adipose-derived stem cells to a Schwann cell phenotype. Tissue Eng. 2007, 13, 1675. [Google Scholar]
- Shinoe, T.; Kuribayashi, H.; Saya, H.; Seiki, M.; Aburatani, H.; Watanabe, S. Identification of CD44 as a cell surface marker for Muller glia precursor cells. J. Neurochem. 2010, 115, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Gorlewicz, A.; Wlodarczyk, J.; Wilczek, E.; Gawlak, M.; Cabaj, A.; Majczynski, H.; Nestorowicz, K.; Herbik, M.A.; Grieb, P.; Slawinska, U.; et al. CD44 is expressed in non-myelinating Schwann cells of the adult rat, and may play a role in neurodegeneration-induced glial plasticity at the neuromuscular junction. Neurobiol. Dis. 2009, 34, 245–258. [Google Scholar] [CrossRef]
- Alfei, L.; Aita, M.; Caronti, B.; De Vita, R.; Margotta, V.; Albani, L.M.; Valente, A.M. Hyaluronate receptor CD44 is expressed by astrocytes in the adult chicken and in astrocyte cell precursors in early development of the chick spinal cord. Eur. J. Histochem. 1999, 43, 29–38. [Google Scholar]
- Liu, Y.; Wu, Y.Y.; Lee, J.C.; Xue, H.P.; Pevny, L.H.; Kaprielian, Z.; Rao, M.S. Oligodendrocyte and astrocyte development in rodents: An in situ and immunohistological analysis during embryonic development. Glia 2002, 40, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Eggli, P.S.; Lucocq, J.; Ott, P.; Graber, W.; Vanderzypen, E. Ultrastructural localization of hyaluronana in myelin sheaths of the rat central and rat and human peripheral nervous systems using hyaluronan-binding protein gold and LINK protein gold. Neuroscience 1992, 48, 737–744. [Google Scholar] [CrossRef]
- Sherman, L.S.; Rizvi, T.A.; Karyala, S.; Ratner, N. CD44 enhances neuregulin signaling by Schwann cells. J. Cell Biol. 2000, 150, 1071–1083. [Google Scholar] [CrossRef]
- Jones, L.L.; Liu, Z.Q.; Shen, J.; Werner, A.; Kreutzberg, G.W.; Raivich, G. Regulation of the cell adhesion molecule CD44 after nerve transection and direct trauma to the mouse brain. J. Comp. Neurol. 2000, 426, 468–492. [Google Scholar] [CrossRef]
- Wu, S.C.; Chen, C.H.; Chang, J.K.; Fu, Y.C.; Wang, C.K.; Eswaramoorthy, R.; Lin, Y.S.; Wang, Y.H.; Lin, S.Y.; Wang, G.J.; et al. Hyaluronan initiates chondrogenesis mainly via CD44 in human adipose-derived stem cells. J. Appl. Physiol. 2013, 114, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.A.; Idrus, R.B.; Saim, A.B.; Sathappan, S.; Chua, K.H. Characterization of human adipose-derived stem cells and expression of chondrogenic genes during induction of cartilage differentiation. Clinics (Sao Paulo) 2012, 67, 99–106. [Google Scholar] [CrossRef]
- Folgiero, V.; Migliano, E.; Tedesco, M.; Iacovelli, S.; Bon, G.; Torre, M.L.; Sacchi, A.; Marazzi, M.; Bucher, S.; Falcioni, R. Purification and characterization of adipose-derived stem cells from patients with lipoaspirate transplant. Cell Transplant. 2010, 19, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Turley, E.A.; Hossain, M.Z.; Sorokan, T.; Jordan, L.M.; Nagy, J.I. Astrocyte and microglial motility in-vitro is functionally dependent on the hyaluronan receptor RHAMM. Glia 1994, 12, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Lynn, B.D.; Li, X.B.; Cattini, P.A.; Turley, E.A.; Nagy, J.I. Identification of sequence, protein isoforms, and distribution of the hyaluronan-binding protein RHAMM in adult and developing rat brain. J. Comp. Neurol. 2001, 439, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Lynn, B.D.; Turley, E.A.; Nagy, J.I. Subcellular distribution, calmodulin interaction, and mitochondrial association of the hyaluronan-binding protein RHAMM in rat brain. J. Neurosci. Res. 2001, 65, 6–16. [Google Scholar] [CrossRef]
- Savani, R.C.; Wang, C.; Yang, B.H.; Zhang, S.W.; Kinsella, M.G.; Wight, T.N.; Stern, R.; Nance, D.M.; Turley, E.A. Migration of bovine aortic smooth-muscle cells after wounding injuty—The role of hyaluronan and RHAMM. J. Clin. Investig. 1995, 95, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Savani, R.C.; Cao, G.Y.; Pooler, P.M.; Zaman, A.; Zhou, Z.; DeLisser, H.M. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J. Biol. Chem. 2001, 276, 36770–36778. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Byers, H.R.; Vink, J.; Stamenkovic, I. CD44H regulated tumor-cell migration on hyaluronate-coated substrate. J. Cell Biol. 1992, 118, 971–977. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouasti, S.; Faroni, A.; Kingham, P.J.; Ghibaudi, M.; Reid, A.J.; Tirelli, N. Hyaluronic Acid (HA) Receptors and the Motility of Schwann Cell(-Like) Phenotypes. Cells 2020, 9, 1477. https://doi.org/10.3390/cells9061477
Ouasti S, Faroni A, Kingham PJ, Ghibaudi M, Reid AJ, Tirelli N. Hyaluronic Acid (HA) Receptors and the Motility of Schwann Cell(-Like) Phenotypes. Cells. 2020; 9(6):1477. https://doi.org/10.3390/cells9061477
Chicago/Turabian StyleOuasti, Sihem, Alessandro Faroni, Paul J. Kingham, Matilde Ghibaudi, Adam J. Reid, and Nicola Tirelli. 2020. "Hyaluronic Acid (HA) Receptors and the Motility of Schwann Cell(-Like) Phenotypes" Cells 9, no. 6: 1477. https://doi.org/10.3390/cells9061477
APA StyleOuasti, S., Faroni, A., Kingham, P. J., Ghibaudi, M., Reid, A. J., & Tirelli, N. (2020). Hyaluronic Acid (HA) Receptors and the Motility of Schwann Cell(-Like) Phenotypes. Cells, 9(6), 1477. https://doi.org/10.3390/cells9061477





