Rab27a Contributes to the Processing of Inflammatory Pain in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Behavior
2.3. Immunohistochemistry
2.4. In Situ Hybridization
2.5. Western Blot
2.6. Real-Time RT-PCR
2.7. Statistics
3. Results
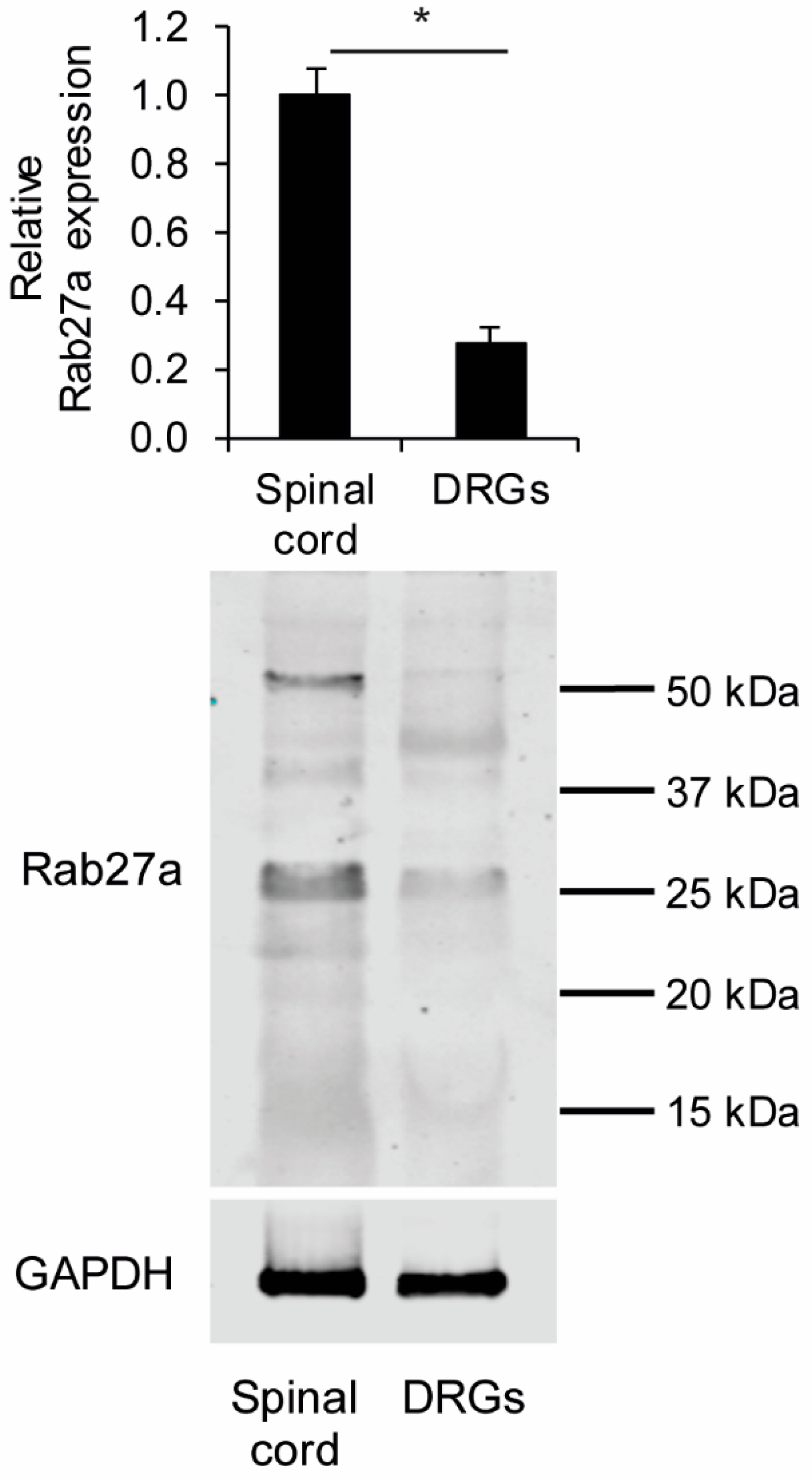
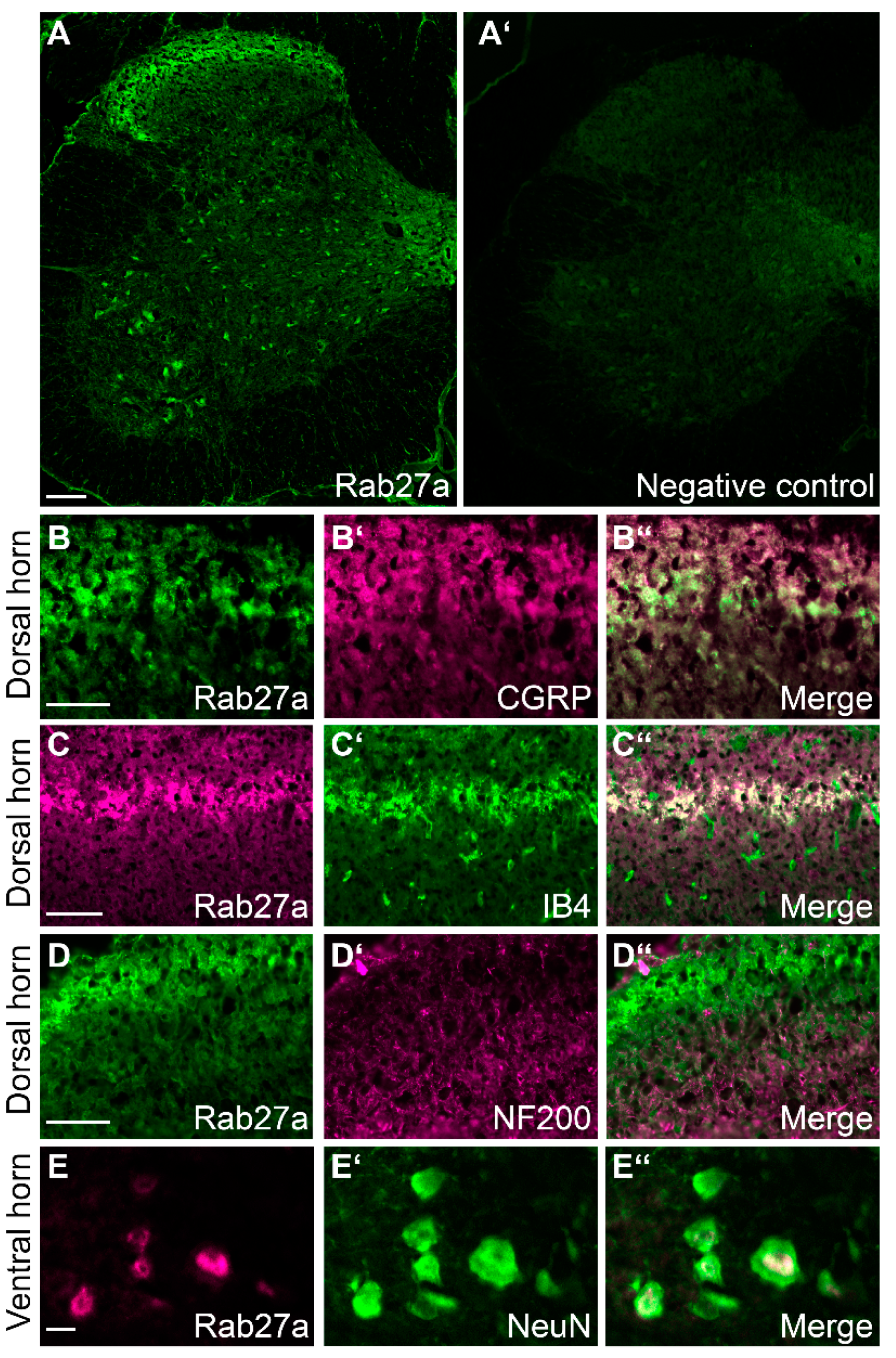
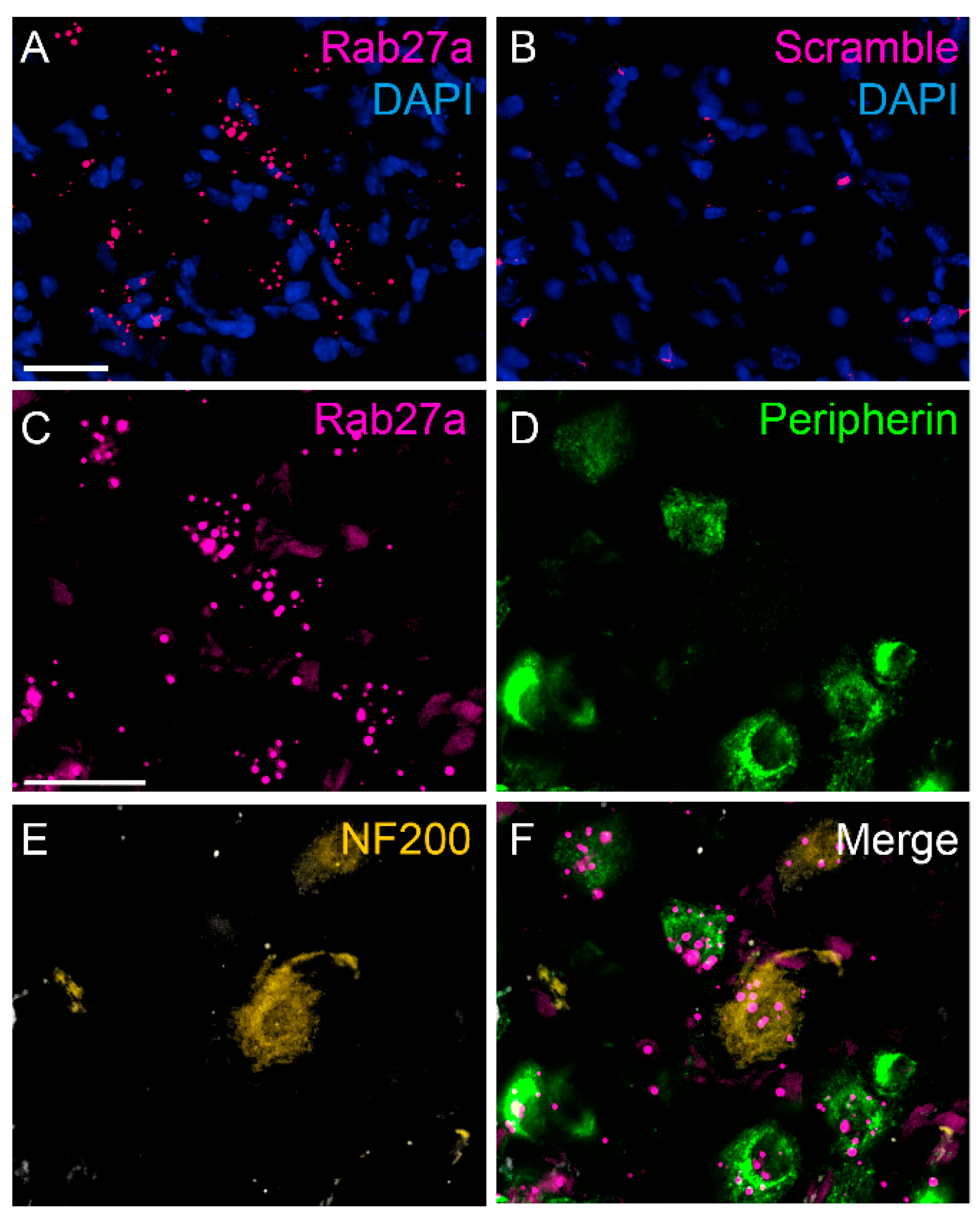
3.1. Rab27a Expression in the Spinal Cord and in Dorsal Root Ganglia
3.2. Rab27aash/ash Mice Display Normal Basal Sensitivity
3.3. Rab27aash/ash Mice Display Reduced Inflammatory Pain Behavior
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease. Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.-R.; Xu, Z.-Z.; Gao, Y.-J. Emerging targets in Neuroinflammation-Driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [Green Version]
- Johannes, C.B.; Le, T.K.; Zhou, X.; Johnston, J.A.; Dworkin, R.H. The prevalence of chronic pain in United States adults: Results of an Internet-Based survey. J. Pain 2010, 11, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Pan, C.; He, S.-M.; Lang, B.; Gao, G.-D.; Wang, X.-L.; Wang, Y. The Ras superfamily of small GTPases in Non-Neoplastic cerebral diseases. Front. Mol. Neurosci. 2019, 12, 121. [Google Scholar] [CrossRef] [Green Version]
- Kallenborn-Gerhardt, W.; Möser, C.V.; Lorenz, J.E.; Steger, M.; Heidler, J.; Scheving, R.; Petersen, J.; Kennel, L.; Flauaus, C.; Lu, R.; et al. Rab7—A novel redox target that modulates inflammatory pain processing. Pain 2017, 158, 1354–1365. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Y.; Xie, K.; Chen, Y.; Wang, H.; Bian, Y.; Wang, Y.; Dong, A.; Yu, Y. Effect of autophagy on allodynia, hyperalgesia and astrocyte activation in a rat model of neuropathic pain. Int. J. Mol. Med. 2018, 42, 2009–2019. [Google Scholar] [CrossRef]
- Mousa, S.A.; Shaqura, B.I.M.; Khalefa, C.; Zollner, L.; Schaad, J.; Schneider, T.S.; Shippenberg, J.F.; Richter, R.; Hellweg, M.; Shakibaei, M. Schafer. Rab7 silencing prevents Mu-Opioid receptor lysosomal targeting and rescues opioid responsiveness to strengthen diabetic neuropathic pain therapy. Diabetes 2013, 62, 1308–1319. [Google Scholar] [CrossRef] [Green Version]
- Roosterman, D.; Cottrell, G.S.; Schmidlin, F.; Steinhoff, M.; Bunnett, N.W. Recycling and resensitization of the neurokinin 1 receptor. J. Biol. Chem. 2004, 279, 30670–30679. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Guo, S.; Wang, S. MicroRNA-16 alleviates inflammatory pain by targeting Ras-Related protein 23 (RAB23) and inhibiting p38 MAPK activation. Med Sci. Monit. 2016, 22, 3894–3901. [Google Scholar] [CrossRef] [Green Version]
- Bolasco, G.; Tracey-White, D.C.; Tolmachova, T.; Thorley, A.J.; Tetley, T.D.; Seabra, M.; Hume, A.N. Loss of Rab27 function results in abnormal lung epithelium structure in mice. Am. J. Physiol. Physiol. 2010, 300, C466–C476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meeths, M.; Bryceson, Y.T.; Rudd, E.; Zheng, C.; Wood, S.M.; Ramme, K.; Beutel, K.; Hasle, H.; Heilmann, C.; Hultenby, K.; et al. Clinical presentation of Griscelli syndrome type 2 and spectrum ofRAB27Amutations. Pediatr. Blood Cancer 2009, 54, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Ménasché, G.; Pastural, E.; Feldmann, J.; Certain, S.; Ersoy, F.; Dupuis, S.; Wulffraat, N.; Bianchi, D.; Fischer, A.; Le Deist, F.; et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat. Genet. 2000, 25, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Ramadass, M.; Catz, S.D. Molecular mechanisms regulating secretory organelles and endosomes in neutrophils and their implications for inflammation. Immunol. Rev. 2016, 273, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Cabrera, A.N.; Matos-Martínez, M.; Sánchez-Villegas, M.C.; Lázaro-Castillo, L.M.; Méndez-León, J.; Martínez-Amigon, J.; Aguilar, M.; García-Escobar, B. The Griscelli-Prunieras syndrome: A case report. Bol. Méd. Hosp. Infant. México 1993, 50, 503–507. [Google Scholar]
- Haddad, E.K.; Wu, X.; Hammer, J.A.; Henkart, P.A. Defective granule exocytosis in Rab27a-Deficient lymphocytes from ashen mice. J. Cell Biol. 2001, 152, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Hume, A.N.; Collinson, L.; Rapak, A.; Gomes, A.; Hopkins, C.R.; Seabra, M. Rab27a regulates the peripheral distribution of melanosomes in Melanocytes. J. Cell Biol. 2001, 152, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Stinchcombe, J.C.; Barral, D.C.; Mules, E.H.; Booth, S.; Hume, A.N.; Machesky, L.M.; Seabra, M.; Griffiths, G.M. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 2001, 152, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.; Yip, R.; Swing, D.A.; O’Sullivan, T.N.; Zhang, Y.; Novak, E.K.; Swank, R.T.; Russell, L.B.; Copeland, N.G.; Jenkins, N.A. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc. Natl. Acad. Sci. USA 2000, 97, 7933–7938. [Google Scholar] [CrossRef] [Green Version]
- Ginsberg, S.D.; Mufson, E.J.; Alldred, M.J.; Counts, S.E.; Wuu, J.; Nixon, R.A.; Che, S. Upregulation of select rab GTPases in cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. J. Chem. Neuroanat. 2011, 42, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Hui, L.; Geiger, N.; Bloor-Young, D.; Churchill, G.C.; Geiger, J.D.; Chen, X. Release of calcium from endolysosomes increases calcium influx through N-type calcium channels: Evidence for acidic Store-Operated calcium entry in neurons. Cell Calcium 2015, 58, 617–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Tang, S.; Han, X.; Jiang, Z.; Dong, L.; Liu, C.; Liang, X.; Dong, J.; Qiu, C.; Wang, Y.; et al. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat. Commun. 2019, 10, 1639. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Z.; Wei, Z.; Cheng, Q.; Li, X.; Li, W.; Duan, S.; Gu, X. Lysosomal exocytosis in Schwann cells contributes to axon remyelination. Glia 2011, 60, 295–305. [Google Scholar] [CrossRef]
- Chen, G.; Su, W.-F.; Gu, Y.; Wei, Z.-Y.; Shen, Y.-T.; Jin, Z.-H.; Yuan, Y.; Gu, X.-S. Rab27a/Slp2-a complex is involved in Schwann cell myelination. Neural Regen. Res. 2016, 11, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Seabra, M.; Mules, E.H.; Hume, A.N. Rab GTPases, intracellular traffic and disease. Trends Mol. Med. 2002, 8, 23–30. [Google Scholar] [CrossRef]
- Barral, D.C.; Ramalho, J.S.; Anders, R.; Hume, A.N.; Knapton, H.J.; Tolmachova, T.; Collinson, L.M.; Goulding, D.; Authi, K.S.; Seabra, M.C. Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J. Clin. Investig. 2002, 110, 247–257. [Google Scholar] [CrossRef]
- Ranade, S.S.; Woo, S.-H.; Dubin, A.E.; Moshourab, R.; Wetzel, C.; Petrus, M.; Mathur, J.; Bégay, V.; Coste, B.; Mainquist, J.; et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 2014, 516, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Eddy, N.B.; Leimbach, D. Synthetic analgesics. II. Dithienylbutenyl-and dithienylbutylamines. J. Pharm. Exp. 1953, 107, 385–393. [Google Scholar]
- Cortright, D.N.; Krause, J.E.; Broom, D.C. TRP channels and pain. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2007, 1772, 978–988. [Google Scholar] [CrossRef] [Green Version]
- Mogil, J.S.; Wilson, S.G.; Bon, K.; Lee, S.E.; Chung, K.; Raber, P.; Pieper, J.O.; Hain, H.S.; Belknap, J.K.; Hubert, L.; et al. Heritability of nociception I: Responses of 11 inbred mouse strains on 12 measures of nociception. Pain 1999, 80, 67–82. [Google Scholar] [CrossRef]
- Kallenborn-Gerhardt, W.; Schröder, K.; Del Turco, D.; Lu, R.; Kynast, K.; Kosowski, J.; Niederberger, E.; Shah, A.M.; Brandes, R.P.; Geisslinger, G.; et al. NADPH Oxidase-4 maintains neuropathic pain after peripheral nerve injury. J. Neurosci. 2012, 32, 10136–10145. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Bausch, A.E.; Kallenborn-Gerhardt, W.; Stoetzer, C.; De Bruin, N.; Ruth, P.; Geisslinger, G.; Leffler, A.; Lukowski, R.; Schmidtko, A. Slack channels expressed in sensory neurons control neuropathic pain in mice. J. Neurosci. 2015, 35, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Schmidtko, A.; Luo, C.; Gao, W.; Geisslinger, G.; Kuner, R.; Tegeder, I. Genetic deletion of synapsin II reduces neuropathic pain due to reduced glutamate but increased GABA in the spinal cord dorsal horn. Pain 2008, 139, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Hunskaar, S.; Fasmer, O.B.; Hole, K. Formalin test in mice, a useful technique for evaluating mild analgesics. J. Neurosci. Methods 1985, 14, 69–76. [Google Scholar] [CrossRef]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M. TRPA1 mediates Formalin-Induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, R.; Flauaus, C.; Kennel, L.; Petersen, J.; Drees, O.; Kallenborn-Gerhardt, W.; Ruth, P.; Lukowski, R.; Schmidtko, A. KCa 3.1 channels modulate the processing of noxious chemical stimuli in mice. Neuropharmacology 2017, 125, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Meller, S.; Gebhart, G. Intraplantar zymosan as a reliable, quantifiable model of thermal and mechanical hyperalgesia in the rat. Eur. J. Pain 1997, 1, 43–52. [Google Scholar] [CrossRef]
- Pitzer, C.; Kuner, R.; Tappe-Theodor, A. Voluntary and evoked behavioral correlates in inflammatory pain conditions under different social housing conditions. Pain Rep. 2016, 1, e564. [Google Scholar] [CrossRef]
- Schmidtko, A.; Gao, W.; König, P.; Heine, S.; Motterlini, R.; Ruth, P.; Schlossmann, J.; Koesling, R.; Niederberger, E.; Tegeder, I.; et al. cGMP Produced by No-Sensitive guanylyl cyclase essentially contributes to inflammatory and neuropathic pain by using targets different from cGMP-Dependent protein kinase I. J. Neurosci. 2008, 28, 8568–8576. [Google Scholar] [CrossRef]
- Schnell, S.A.; Staines, W.; Wessendorf, M.W. Reduction of Lipofuscin-Like autofluorescence in fluorescently labeled tissue. J. Histochem. Cytochem. 1999, 47, 719–730. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, A.; Okada, R.; Nagao, K.; Kawamata, Y.; Hanyu, A.; Yoshimoto, S.; Takasugi, M.; Watanabe, S.; Kanemaki, M.T.; Obuse, C.; et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017, 8, 15287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisel, A.; Hochgerner, H.; Lönnerberg, P.; Johnsson, A.; Memic, F.; Van Der Zwan, J.; Häring, M.; Braun, E.; Borm, L.E.; La Manno, G.; et al. Molecular architecture of the mouse nervous system. Cell 2018, 174, 999–1014.e22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catz, S.D. The role of Rab27a in the regulation of neutrophil function. Cell. Microbiol. 2014, 16, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic 2013, 14, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Krzewski, K.; Cullinane, A.R. Evidence for defective Rab GTPase-dependent cargo traffic in immune disorders. Exp. Cell Res. 2013, 319, 2360–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.-H.; Fang, R.; Fang, J.; He, S.; Liu, T. Functional implications of Rab27 GTPases in Cancer. Cell Commun. Signal. 2018, 16, 44. [Google Scholar] [CrossRef] [Green Version]
- Tolmachova, T.; Anders, R.; Stinchcombe, J.; Bossi, G.; Griffiths, G.M.; Huxley, C.; Seabra, M. A general role for Rab27a in secretory cells. Mol. Biol. Cell 2004, 15, 332–344. [Google Scholar] [CrossRef]
- Yamaoka, M.; Ishizaki, T.; Kimura, T. GTP- and GDP-Dependent Rab27a effectors in pancreatic Beta-Cells. Biol. Pharm. Bull. 2015, 38, 663–668. [Google Scholar] [CrossRef] [Green Version]
- LaCroix-Fralish, M.L.; Austin, J.-S.; Zheng, F.Y.; Levitin, D.J.; Mogil, J.S. Patterns of pain: Meta-Analysis of microarray studies of pain. Pain 2011, 152, 1888–1898. [Google Scholar] [CrossRef]
- Caterina, M.J. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A Heat-Activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Vandewauw, I.; De Clercq, K.; Mulier, M.; Held, K.; Pinto, S.; Van Ranst, N.; Segal, A.; Voet, T.; Vennekens, R.; Zimmermann, K.; et al. A TRP channel trio mediates acute noxious heat sensing. Nature 2018, 555, 662–666. [Google Scholar] [CrossRef]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A Capsaicin-Receptor homologue with a high threshold for noxious heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef]
- Cho, H.; Yang, Y.D.; Lee, J.; Lee, B.; Kim, T.; Jang, Y.; Back, S.K.; Na, H.S.; Harfe, B.D.; Wang, F.; et al. The Calcium-Activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat. Neurosci. 2012, 15, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; Voets, T. Heat pain and cold pain. In The Oxford Handbook of the Neurobiology of Pain; Oxford University Press (OUP): Oxford, UK, 2019. [Google Scholar]
- Lee, H.; Iida, T.; Mizuno, A.; Suzuki, M.; Caterina, M.J. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J. Neurosci. 2005, 25, 1304–1310. [Google Scholar] [CrossRef] [Green Version]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.; Murray, A.N.; Spencer, K.S.R.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef]
- Vriens, J.; Owsianik, G.; Hofmann, T.; Philipp, S.E.; Stab, J.; Chen, X.; Benoit, M.; Xue, F.; Janssens, A.; Kerselaers, S.; et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011, 70, 482–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, S.K.; Horiuchi, H.; Fukuda, M. Rab27a regulates epithelial sodium channel (ENaC) activity through Synaptotagmin-Like protein (SLP-5) and Munc13-4 effector mechanism. Biochem. Biophys. Res. Commun. 2006, 344, 651–657. [Google Scholar] [CrossRef]
- Saxena, S.K.; Kaur, S. Rab27a negatively regulates CFTR chloride channel function in colonic epithelia: Involvement of the effector proteins in the regulatory mechanism. Biochem. Biophys. Res. Commun. 2006, 346, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Reichhart, N.; Markowski, M.; Ishiyama, S.; Wagner, A.; Crespo-Garcia, S.; Schorb, T.; Ramalho, J.; Milenkovic, V.M.; Föckler, R.; Seabra, M.; et al. Rab27a GTPase modulates L-Type Ca2+ channel function via interaction with the II–III linker of CaV1.3 subunit. Cell. Signal. 2015, 27, 2231–2240. [Google Scholar] [CrossRef]
- Chen, D.; Guo, J.; Miki, T.; Tachibana, M.; Gahl, W.A. Molecular cloning and characterization of Rab27a and Rab27b, novel human rab proteins shared by melanocytes and platelets. Biochem. Mol. Med. 1997, 60, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomi, H.; Mori, K.; Itohara, S.; Izumi, T. Rab27b is expressed in a wide range of exocytic cells and involved in the delivery of secretory granules near the plasma membrane. Mol. Biol. Cell 2007, 18, 4377–4386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, L.I.; Dalghi, M.G.; Clayton, D.R.; Ruiz, W.G.; Khandelwal, P.; Apodaca, G. RAB27B requirement for Stretch-Induced exocytosis in bladder umbrella cells. Am. J. Physiol. Physiol. 2018, 314, C349–C365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.L.; Brzezinska, A.A.; Tolmachova, T.; Munafo, D.B.; Ellis, B.A.; Seabra, M.; Hong, H.; Catz, S.D. Rab27a and Rab27b regulate neutrophil azurophilic granule exocytosis and NADPH oxidase activity by independent mechanisms. Traffic 2010, 11, 533–547. [Google Scholar] [CrossRef] [Green Version]
- Ostrowski, M.; Carmo, N.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nature 2009, 12, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Mizuno, K.; Wasmeier, C.; Wavre-Shapton, S.T.; Recchi, C.; Catz, S.D.; Futter, C.; Tolmachova, T.; Hume, A.N.; Seabra, M. Distinct and opposing roles for Rab27a/Mlph/MyoVa and Rab27b/Munc13-4 in mast cell secretion. FEBS J. 2012, 280, 892–903. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gross, T.; Wack, G.; Syhr, K.M.J.; Tolmachova, T.; Seabra, M.C.; Geisslinger, G.; Niederberger, E.; Schmidtko, A.; Kallenborn-Gerhardt, W. Rab27a Contributes to the Processing of Inflammatory Pain in Mice. Cells 2020, 9, 1488. https://doi.org/10.3390/cells9061488
Gross T, Wack G, Syhr KMJ, Tolmachova T, Seabra MC, Geisslinger G, Niederberger E, Schmidtko A, Kallenborn-Gerhardt W. Rab27a Contributes to the Processing of Inflammatory Pain in Mice. Cells. 2020; 9(6):1488. https://doi.org/10.3390/cells9061488
Chicago/Turabian StyleGross, Tilman, Gesine Wack, Katharina M. J. Syhr, Tanya Tolmachova, Miguel C. Seabra, Gerd Geisslinger, Ellen Niederberger, Achim Schmidtko, and Wiebke Kallenborn-Gerhardt. 2020. "Rab27a Contributes to the Processing of Inflammatory Pain in Mice" Cells 9, no. 6: 1488. https://doi.org/10.3390/cells9061488
APA StyleGross, T., Wack, G., Syhr, K. M. J., Tolmachova, T., Seabra, M. C., Geisslinger, G., Niederberger, E., Schmidtko, A., & Kallenborn-Gerhardt, W. (2020). Rab27a Contributes to the Processing of Inflammatory Pain in Mice. Cells, 9(6), 1488. https://doi.org/10.3390/cells9061488




