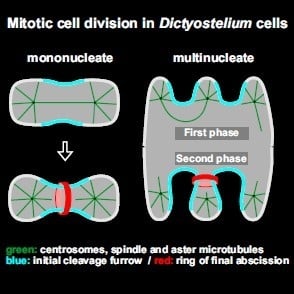
Unilateral Cleavage Furrows in Multinucleate Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Strains and Culture Conditions
2.2. Design of mRFP-Histone 2B Vectors
2.3. Culture Conditions and Sample Preparation for Confocal Microscopy
2.4. Confocal Image Acquisition and Data Processing
3. Results
3.1. Mitosis in Wild-Type and Myosin-II-Null Cells
3.2. Cytokinesis in Mononucleate Wild-Type and Myosin-II-Null Cells
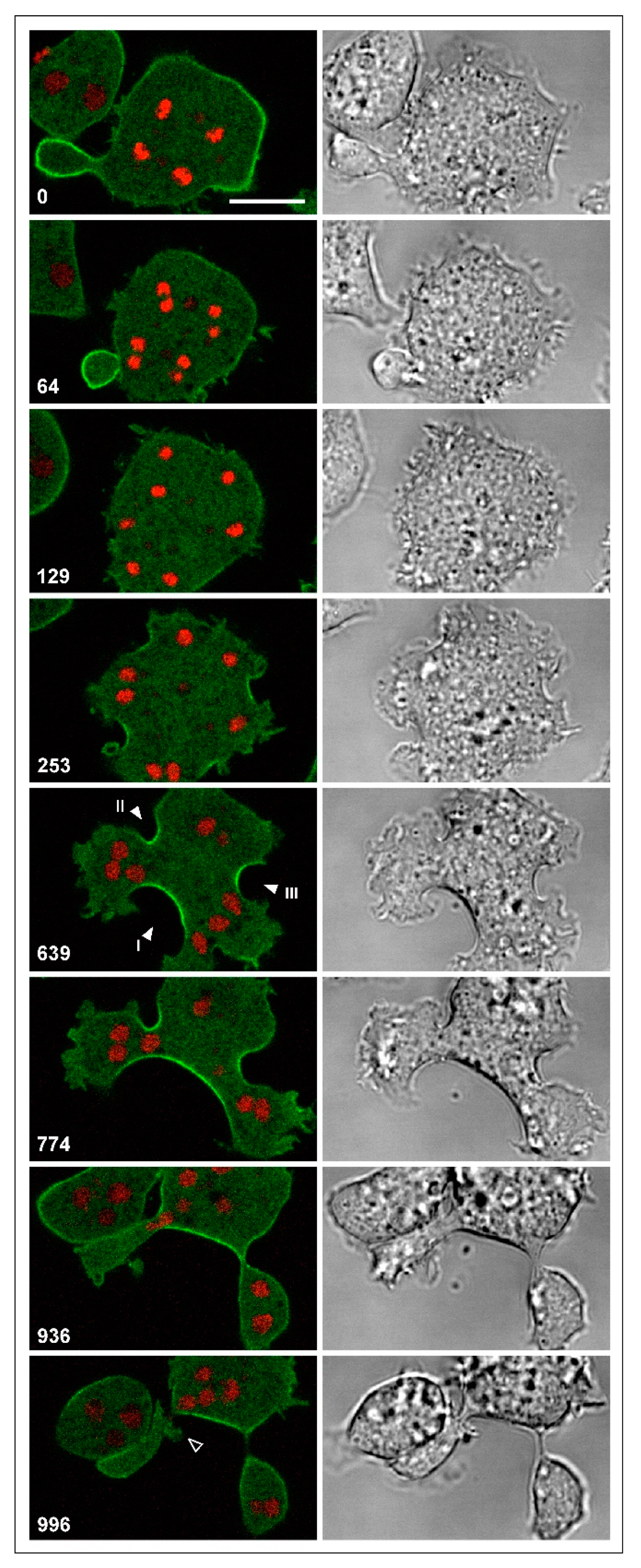
3.3. Unilateral Cleavage Furrows in Multinucleate Wild-Type Cells
3.4. Division of Multinucleate Myosin-II-Null Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henson, J.H.; Ditzler, C.E.; Germain, A.; Irwin, P.M.; Vogt, E.T.; Yang, S.; Wu, X.; Shuster, C.B. The ultrastructural organization of actin and myosin II filaments in the contractile ring: New support for an old model of cytokinesis. Mol. Biol. Cell 2017, 28, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; O’Shaughnessy, B. Molecular mechanism of cytokinesis. Annu. Rev. Biochem. 2019, 88, 661–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swulius, M.T.; Nguyen, L.T.; Ladinsky, M.S.; Ortega, D.R.; Aich, S.; Mishra, M.; Jensen, G.J. Structure of the fission yeast actomyosin ring during constriction. Proc. Natl. Acad. Sci. USA 2018, 115, E1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammarton, T.C. Who needs a contractile actomyosin ring? The plethora of alternative ways to divide a protozoan parasite. Front. Cell. Infect. Microbiol. 2019, 9, 397. [Google Scholar] [CrossRef] [Green Version]
- Solnica-Krezel, L.; Burland, T.G.; Dove, W.F. Variable pathways for developmental changes of mitosis and cytokinesis in Physarum polycephalum. J. Cell Biol. 1991, 113, 591–604. [Google Scholar] [CrossRef]
- Sun, H.S.; Wilde, A.; Harrison, R.E. Chlamydia trachomatis inclusions induce asymmetric cleavage furrow formation and ingression failure in host cells. Mol. Cell Biol. 2011, 31, 5011. [Google Scholar] [CrossRef] [Green Version]
- Kotýnková, K.; Su, K.-C.; West, S.C.; Petronczki, M. Plasma membrane association but not midzone recruitment of RhoGEF ECT2 is essential for cytokinesis. Cell Rep. 2016, 17, 2672–2686. [Google Scholar] [CrossRef] [Green Version]
- Szollosi, D. Cortical cytoplasmic filaments of cleaving eggs: A structural element corresponding to the contractile ring. J. Cell Biol. 1970, 44, 192–209. [Google Scholar] [CrossRef]
- Roberson, M.; Armstrong, J.; Armstrong, P. Adhesive and non-adhesive membrane domains of amphibian embryo cells. J. Cell Sci. 1980, 44, 19. [Google Scholar]
- Rappaport, R.; Rappaport, B.N. Cleavage in Conical Sand Dollar Eggs. Dev. Biol. 1994, 164, 258–266. [Google Scholar] [CrossRef]
- Schejter, E.D.; Wieschaus, E. Functional elements of the cytoskeleton in the early Drosophila embryo. Annu. Rev. Cell Biol. 1993, 9, 67–99. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Sokac, A.M. Back-to-back mechanisms drive actomyosin ring closure during Drosophila embryo cleavage. J. Cell Biol. 2016, 215, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Racowsky, C.; Deng, M. Mechanism of the chromosome-induced polar body extrusion in mouse eggs. Cell Div. 2011, 6, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uraji, J.; Scheffler, K.; Schuh, M. Functions of actin in mouse oocytes at a glance. J. Cell Sci. 2018, 131, jcs218099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savoian, M.S.; Khodjakov, A.; Rieder, C.L. Unilateral and wandering furrows during mitosis in vertebrates: Implications for the mechanism of cytokinesis. Cell Biol. Int. 1999, 23, 805–812. [Google Scholar] [CrossRef]
- Fukui, Y.; Inoue, S. Cell division in Dictyostelium with special emphasis on actomyosin organization in cytokinesis. Cell Motil. Cytoskel. 1991, 18, 41–54. [Google Scholar] [CrossRef]
- De Lozanne, A.; Spudich, J.A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science 1987, 236, 1086–1091. [Google Scholar] [CrossRef]
- Knecht, D.A.; Loomis, W.F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science 1987, 236, 1081–1085. [Google Scholar] [CrossRef]
- Manstein, D.J.; Titus, M.A.; De Lozanne, A.; Spudich, J.A. Gene replacement in Dictyostelium: Generation of myosin null mutants. EMBO J. 1989, 8, 923–932. [Google Scholar] [CrossRef]
- Neujahr, R.; Heizer, C.; Gerisch, G. Myosin II-independent processes in mitotic cells of Dictyostelium discoideum: Redistribution of the nuclei, re-arrangement of the actin system and formation of the cleavage furrow. J. Cell Sci. 1997, 110, 123. [Google Scholar]
- Neujahr, R.; Albrecht, R.; Köhler, J.; Matzner, M.; Schwartz, J.M.; Westphal, M.; Gerisch, G. Microtubule-mediated centrosome motility and the positioning of cleavage furrows in multinucleate myosin II-null cells. J. Cell Sci. 1998, 111, 1227. [Google Scholar] [PubMed]
- Faix, J.; Steinmetz, M.; Boves, H.; Kammerer, R.A.; Lottspeich, F.; Mintert, U.; Murphy, J.; Stock, A.; Aebi, U.; Gerisch, G. Cortexillins, major determinants of cell shape and size, are actin-bundling proteins with a parallel coiled-coil tail. Cell 1996, 86, 631–642. [Google Scholar] [CrossRef]
- Faix, J.; Weber, I.; Mintert, U.; Köhler, J.; Lottspeich, F.; Marriott, G. Recruitment of cortexillin into the cleavage furrow is controlled by Rac1 and IQGAP-related proteins. EMBO J. 2001, 20, 3705–3715. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shu, S.; Yu, S.; Lee, D.-Y.; Piszczek, G.; Gucek, M.; Wang, G.; Korn, E.D. Biochemical and biological properties of cortexillin III, a component of Dictyostelium DGAP1–cortexillin complexes. Mol. Biol. Cell 2014, 25, 2026–2038. [Google Scholar] [CrossRef] [PubMed]
- Gerisch, G.; Weber, I. Cytokinesis without myosin II. Curr. Opin. Cell Biol. 2000, 12, 126–132. [Google Scholar] [CrossRef]
- Weber, I.; Neujahr, R.; Du, A.; Köhler, J.; Faix, J.; Gerisch, G. Two-step positioning of a cleavage furrow by cortexillin and myosin II. Curr. Biol. 2000, 10, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, V.; Iglesias, P.A.; Robinson, D.N. Cytokinesis: Robust cell shape regulation. Semin. Cell Dev. Biol. 2016, 53, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Weber, I.; Gerisch, G.; Heizer, C.; Murphy, J.; Badelt, K.; Stock, A.; Schwartz, J.M.; Faix, J. Cytokinesis mediated through the recruitment of cortexillins into the cleavage furrow. EMBO J. 1999, 18, 586–594. [Google Scholar] [CrossRef]
- Gerisch, G.; Ecke, M.; Neujahr, R.; Prassler, J.; Stengl, A.; Hoffmann, M.; Schwarz, U.S.; Neumann, E. Membrane and actin reorganization in electropulse-induced cell fusion. J. Cell Sci. 2013, 126 Pt 9, 2069–2078. [Google Scholar] [CrossRef] [Green Version]
- Rossier, C.; Gerisch, G.; Malchow, D. Action of a slowly hydrolysable cyclic AMP analogue on developing cells of Dictyostelium discoideum. J. Cell Sci. 1979, 35, 321. [Google Scholar]
- Robinson, D.N.; Cavet, G.; Warrick, H.M.; Spudich, J.A. Quantitation of the distribution and flux of myosin-II during cytokinesis. BMC Cell Biol. 2002, 3, 4. [Google Scholar] [CrossRef]
- Fischer, M.; Haase, I.; Simmeth, E.; Gerisch, G.; Müller-Taubenberger, A. A brilliant monomeric red fluorescent protein to visualize cytoskeleton dynamics in Dictyostelium. FEBS Lett. 2004, 577, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Campbell, R.E.; Tour, O.; Palmer, A.E.; Steinbach, P.A.; Baird, G.S.; Zacharias, D.A.; Tsien, R.Y. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 2002, 99, 7877. [Google Scholar] [CrossRef] [Green Version]
- Müller-Taubenberger, A. Application of fluorescent protein tags as reporters in live-cell imaging studies. In Dictyostelium Discoideum Protocols; Eichinger, L., Rivero, F., Eds.; Humana Press: Totowa, NJ, USA, 2006; Volume 346, pp. 229–246. [Google Scholar]
- Gritz, L.; Davies, J. Plasmid-encoded hygromycin B resistance: The sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene 1983, 25, 179–188. [Google Scholar] [CrossRef]
- Malchow, D.; Nägele, B.; Schwarz, H.; Gerisch, G. Membrane-bound cyclic AMP phosphodiesterase in chemotactically responding cells of Dictyostelium discoideum. Eur. J. Biochem. 1972, 28, 136–142. [Google Scholar] [CrossRef]
- Fukui, Y.; Yumura, S.; Yumura, T.K. Agar-overlay immunofluorescence: High-resolution studies of cytoskeletal components and their changes during chemotaxis. Methods Cell Biol. 1987, 28, 347–356. [Google Scholar]
- Samereier, M.; Meyer, I.; Koonce, M.P.; Gräf, R. Live cell-Imaging techniques for analyses of microtubules in Dictyostelium. In Methods in Cell Biology; Cassimeris, L., Tran, P., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 97, pp. 341–357. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leo, M.; Santino, D.; Tikhonenko, I.; Magidson, V.; Khodjakov, A.; Koonce, M.P. Rules of engagement: Centrosome–nuclear connections in a closed mitotic system. Biol. Open 2012, 1, 1111. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, J.R.; Roos, U.P.; Neighbors, B.; McDonald, K.L. Architecture of the microtubule component of mitotic spindles from Dictyostelium discoideum. J. Cell Sci. 1985, 75, 93. [Google Scholar]
- Wienke, D.C.; Knetsch, M.L.W.; Neuhaus, E.M.; Reedy, M.C.; Manstein, D.J. Disruption of a dynamin homologue affects endocytosis, organelle morphology, and cytokinesis in Dictyostelium discoideum. Mol. Biol. Cell 1999, 10, 225–243. [Google Scholar] [CrossRef] [Green Version]
- Neujahr, R.; Heizer, C.; Albrecht, R.; Ecke, M.; Schwartz, J.-M.; Weber, I.; Gerisch, G. Three-dimensional patterns and redistribution of myosin II and actin in mitotic Dictyostelium cells. J. Cell Biol. 1997, 139, 1793. [Google Scholar] [CrossRef] [Green Version]
- Rappaport, R. Experiments concerning the cleavage stimulus in sand dollar eggs. J. Exp. Zool. 1961, 148, 81–89. [Google Scholar] [CrossRef]
- Rappaport, R. Cytokinesis in Animal Cells. In Biomechanics of Active Movement and Deformation of Cells; Akkaş, N., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; Volume H42, pp. 1–34. [Google Scholar]
- Field, C.M.; Alberts, B.M. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J. Cell Biol. 1995, 131, 165–178. [Google Scholar] [CrossRef]
- Jananji, S.; Risi, C.; Lindamulage, I.K.S.; Picard, L.-P.; Van Sciver, R.; Laflamme, G.; Albaghjati, A.; Hickson, G.R.X.; Kwok, B.H.; Galkin, V.E. Multimodal and polymorphic interactions between anillin and actin: Their implications for cytokinesis. J. Mol. Biol. 2017, 429, 715–731. [Google Scholar] [CrossRef]
- Piekny, A.J.; Glotzer, M. Anillin is a scaffold protein that links RhoA, Actin, and Myosin during cytokinesis. Curr. Biol. 2008, 18, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Piekny, A.J.; Maddox, A.S. The myriad roles of Anillin during cytokinesis. Semin. Cell Dev. Biol. 2010, 21, 881–891. [Google Scholar] [CrossRef] [Green Version]
- Kučera, O.; Janda, D.; Siahaan, V.; Dijkstra, S.H.; Pilátová, E.; Zatecka, E.; Diez, S.; Braun, M.; Lansky, Z. Anillin propels myosin-independent constriction of actin rings. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Taira, R.; Yumura, S. A novel mode of cytokinesis without cell-substratum adhesion. Sci. Rep. 2017, 7, 17694. [Google Scholar] [CrossRef]
- Fey, P.; Dodson, R.J.; Basu, S.; Chisholm, R.L. One stop shop for everything Dictyostelium: DictyBase and the Dicty Stock Center in 2012. In Methods Mol. Biol.; Eichinger, L., Rivero, F., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 59–92. [Google Scholar]







| Mononucleate Cells | Wild-Type | Myosin-II-Null |
|---|---|---|
| Velocity of spindle elongation | 44 ± 15 s.d. nm/s | 56 nm/s |
| Maximal spindle length | 11.8 ± 1.3 s.d. µm | 14.0 µm |
| Number of measured cells | 7 | 1 for velocity, 3 for spindle length |
| Multinucleate Cells | Wild-Type | Myosin-II-Null |
| Velocity of spindle elongation | 46 ± 11 s.d. nm/s | 51 ± 14 s.d. nm/s |
| Maximal spindle length | 14.9 ± 2.1 s.d. µm | 13.9 ± 1.4 s.d. µm |
| Number of measured spindles | 17 in 5 cells | 18 in 6 cells |
| Pre-Culture | Dividing Nuclei | Completed | Failed |
|---|---|---|---|
| (1) On substrate | Mono-nucleate | 3 | 4 [1] |
| Multi-nucleate | 2 [1] | 1 [1] | |
| (2) Shaken culture | Mono-nucleate | 2 | 1 |
| Multi-nucleate | 20 | 9 | |
| (3) Electric pulse-fused | Mono-nucleate | 2 | 0 |
| Multi-nucleate | 8 | 3 | |
| Total | 37 | 18 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bindl, J.; Molnar, E.S.; Ecke, M.; Prassler, J.; Müller-Taubenberger, A.; Gerisch, G. Unilateral Cleavage Furrows in Multinucleate Cells. Cells 2020, 9, 1493. https://doi.org/10.3390/cells9061493
Bindl J, Molnar ES, Ecke M, Prassler J, Müller-Taubenberger A, Gerisch G. Unilateral Cleavage Furrows in Multinucleate Cells. Cells. 2020; 9(6):1493. https://doi.org/10.3390/cells9061493
Chicago/Turabian StyleBindl, Julia, Eszter Sarolta Molnar, Mary Ecke, Jana Prassler, Annette Müller-Taubenberger, and Günther Gerisch. 2020. "Unilateral Cleavage Furrows in Multinucleate Cells" Cells 9, no. 6: 1493. https://doi.org/10.3390/cells9061493
APA StyleBindl, J., Molnar, E. S., Ecke, M., Prassler, J., Müller-Taubenberger, A., & Gerisch, G. (2020). Unilateral Cleavage Furrows in Multinucleate Cells. Cells, 9(6), 1493. https://doi.org/10.3390/cells9061493