Annexin A2 in Inflammation and Host Defense
Abstract
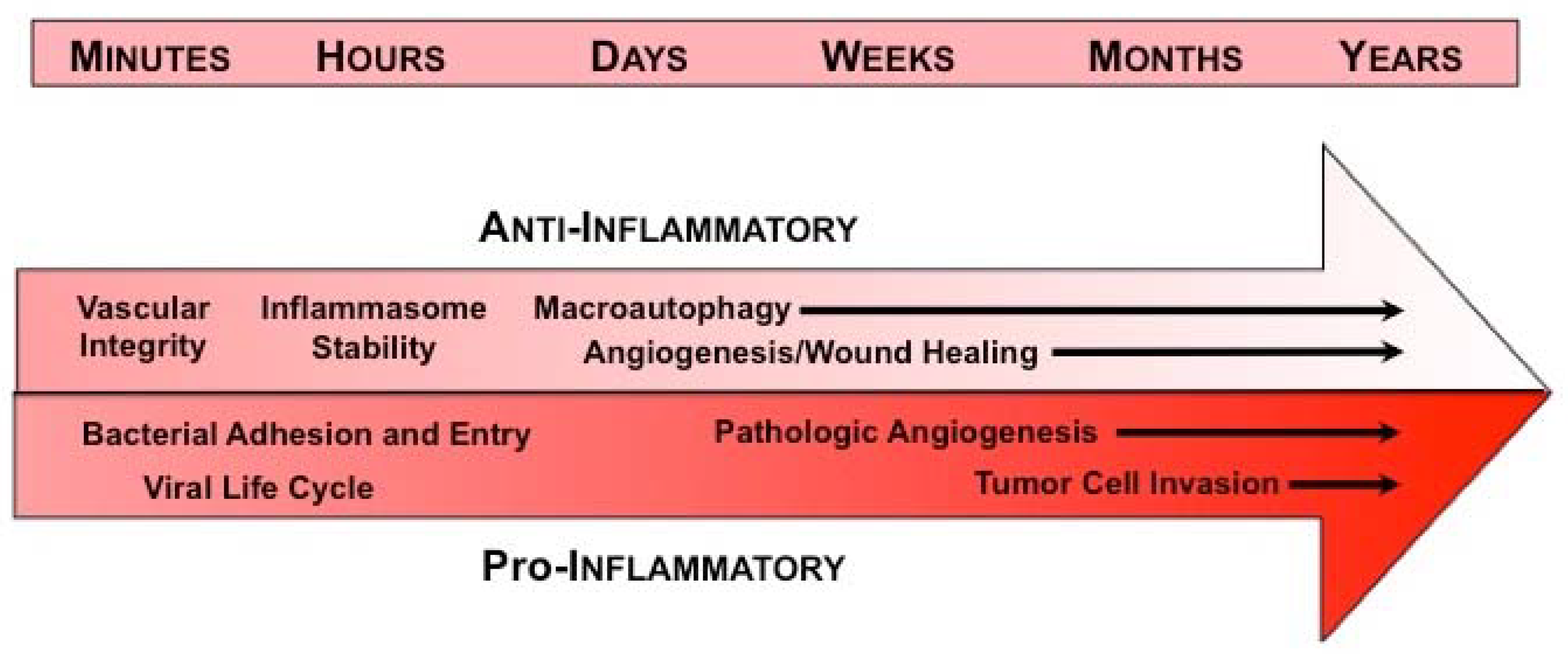
:1. Introduction
2. Annexin A2 and Infection
3. Annexin A2 and Regulation of Vascular Integrity
4. Annexin A2 and Recruitment of Inflammatory Cells
5. Annexin A2 in Inflammasome Dynamics
6. Annexin A2 and Macroautophagy
7. Annexin A2 in Angiogenesis
8. Annexin A2 and Tumor Progression
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerke, V.; Creutz, C.E.; Moss, S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005, 6, 449–461. [Google Scholar] [CrossRef]
- Hajjar, K.A. The Biology of Annexin A2: From Vascular Fibrinolysis to Innate Immunity. Trans. Am. Clin. Climatol. Assoc. 2015, 126, 144–155. [Google Scholar] [PubMed]
- Rescher, U.; Gerke, V. Annexins unique membrane binding proteins with diverse functions. J. Cell Sci. 2004, 117, 2631–2639. [Google Scholar] [CrossRef] [Green Version]
- Cesarman, G.M.; Guevara, C.A.; Hajjar, K.A. An endothelial cell receptor for plasminogen/tissue plasminogen activator (t-PA). II. Annexin II-mediated enhancement of t-PA-dependent plasminogen activation. J. Biol. Chem. 1994, 269, 21198–21203. [Google Scholar]
- Hajjar, K.A.; Jacovina, A.T.; Chacko, J. An endothelial cell receptor for plasminogen/tissue plasminogen activator. I. Identity with annexin II. J. Biol. Chem. 1994, 269, 21191–21197. [Google Scholar]
- Dassah, M.; Deora, A.B.; He, K.; Hajjar, K.A. The Endothelial Cell Annexin A2 System and Vascular Fibrinolysis. Gen. Physiol. Biophys. 2009, 28, F20–F28. [Google Scholar]
- Hajjar, K.A.; Acharya, S.S. Annexin II and Regulation of Cell Surface Fibrinolysis. Ann. N. Y. Acad. Sci. 2000, 902, 265–271. [Google Scholar] [CrossRef]
- Donnelly, S.R.; Moss, S.E. Annexins in the secretory pathway. Cell. Mol. Life Sci. 1997, 53, 533–538. [Google Scholar] [CrossRef]
- Gerke, V.; Moss, S.E. Annexins: From structure to function. Physiol. Rev. 2002, 82, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Hedhli, N.; Falcone, D.J.; Huang, B.; Cesarman-Maus, G.; Kraemer, R.; Zhai, H.; Tsirka, S.E.; Santambrogio, L.; Hajjar, K.A. The annexin A2/S100A10 system in health and disease: Emerging paradigms. J. Biomed. Biotechnol. 2012, 2012, 406273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, A.L.; Ling, Q.; Hajjar, K.A.; Futter, C.E.; Greenwood, J.; Adamson, P.; Wavre-Shapton, S.T.; Moss, S.E.; Hayes, M.J. Annexin A2 regulates phagocytosis of photoreceptor outer segments in the mouse retina. Mol. Biol. Cell 2009, 20, 3896–3904. [Google Scholar] [CrossRef] [Green Version]
- Morozova, K.; Sridhar, S.; Sidhar, S.; Zolla, V.; Clement, C.C.; Scharf, B.; Verzani, Z.; Diaz, A.; Larocca, J.N.; Hajjar, K.A.; et al. Annexin A2 promotes phagophore assembly by enhancing Atg16L+ vesicle biogenesis and homotypic fusion. Nat. Commun. 2015, 6, 5856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, S.E.; Morgan, R.O. The annexins. Genome Biol. 2004, 5, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaiswal, J.K.; Lauritzen, S.P.; Scheffer, L.; Sakaguchi, M.; Bunkenborg, J.; Simon, S.M.; Kallunki, T.; Jäättelä, M.; Nylandsted, J. S100A11 is required for efficient plasma membrane repair and survival of invasive cancer cells. Nat. Commun. 2014, 5, 3795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defour, A.; Medikayala, S.; Van der Meulen, J.H.; Hogarth, M.W.; Holdreith, N.; Malatras, A.; Duddy, W.; Boehler, J.; Nagaraju, K.; Jaiswal, J.K. Annexin A2 links poor myofiber repair with inflammation and adipogenic replacement of the injured muscle. Hum. Mol. Genet. 2017, 26, 1979–1991. [Google Scholar] [CrossRef] [Green Version]
- Demonbreun, A.R.; Quattrocelli, M.; Barefield, D.Y.; Allen, M.V.; Swanson, K.E.; McNally, E.M. An actin-dependent annexin complex mediates plasma membrane repair in muscle. J. Cell Biol. 2016, 213, 705–718. [Google Scholar] [CrossRef]
- Koerdt, S.N.; Gerke, V. Annexin A2 is involved in Ca2+-dependent plasma membrane repair in primary human endothelial cells. Biochim. Biophys. Acta 2017, 1864, 1046–1053. [Google Scholar] [CrossRef]
- Jaiswal, J.K.; Nylandsted, J. S100 and annexin proteins identify cell membrane damage as the Achilles heel of metastatic cancer cells. Cell Cycle 2015, 14, 502–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durmus Tekir, S.; Cakir, T.; Ulgen, K. Infection Strategies of Bacterial and Viral Pathogens through Pathogen–Human Protein–Protein Interactions. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signore, A. About inflammation and infection. EJNMMI Res. 2013, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Yu, M.; Guo, Q.; Li, R.; Li, G.; Tan, S.; Li, X.; Wei, Y.; Wu, M. Annexin A2 binds to endosomes and negatively regulates TLR4-triggered inflammatory responses via the TRAM-TRIF pathway. Sci. Rep. 2015, 5, 15859. [Google Scholar] [CrossRef] [Green Version]
- Stukes, S.; Coelho, C.; Rivera, J.; Jedlicka, A.E.; Hajjar, K.A.; Casadevall, A. The Membrane Phospholipid Binding Protein Annexin A2 Promotes Phagocytosis and Nonlytic Exocytosis of Cryptococcus neoformans and Impacts Survival in Fungal Infection. J. Immunol. 2016, 197, 1252–1261. [Google Scholar] [CrossRef] [Green Version]
- Creutz, C.E. The annexins and exocytosis. Science 1992, 258, 924–931. [Google Scholar] [CrossRef]
- Gruenberg, J.; Emans, N. Annexins in membrane traffic. Trends Cell Biol. 1993, 3, 224–227. [Google Scholar] [CrossRef]
- Gabel, M.; Chasserot-Golaz, S. Annexin A2, an essential partner of the exocytotic process in chromaffin cells. J. Neurochem. 2016, 137, 890–896. [Google Scholar] [CrossRef] [Green Version]
- Gerke, V. Annexins A2 and A8 in endothelial cell exocytosis and the control of vascular homeostasis. Biol. Chem. 2016, 397, 995–1003. [Google Scholar] [CrossRef]
- Rentero, C.; Blanco-Muñoz, P.; Meneses-Salas, E.; Grewal, T.; Enrich, C. Annexins—Coordinators of Cholesterol Homeostasis in Endocytic Pathways. Int. J. Mol. Sci. 2018, 19, 1444. [Google Scholar] [CrossRef] [Green Version]
- Enrich, C.; Rentero, C.; Meneses-Salas, E.; Tebar, F.; Grewal, T. Annexins: Ca2+ Effectors Determining Membrane Trafficking in the Late Endocytic Compartment. Adv. Exp. Med. Biol. 2017, 981, 351–385. [Google Scholar] [CrossRef] [PubMed]
- Kirschnek, S.; Adams, C.; Gulbins, E. Annexin II is a novel receptor for Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 2005, 327, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, A.; Nakanishi, N.; Ooka, T.; Hayashi, T.; Sugimoto, N.; Tobe, T. Enterohemorrhagic Escherichia coli effector EspL2 induces actin microfilament aggregation through annexin 2 activation. Cell. Microbiol. 2009, 11, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Jolly, C.; Winfree, S.; Hansen, B.; Steele-Mortimer, O. The Annexin A2/p11 complex is required for efficient invasion of Salmonella Typhimurium in epithelial cells. Cell. Microbiol. 2014, 16, 64–77. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Zhang, W.; Chang, Q.; Su, Z.; Gong, D.; Zhou, Y.; Xiao, J.; Drelich, A.; Liu, Y.; Popov, V.; et al. A new role for host annexin A2 in establishing bacterial adhesion to vascular endothelial cells: Lines of evidence from atomic force microscopy and an in vivo study. Lab. Investig. 2019, 99, 1650–1660. [Google Scholar] [CrossRef]
- Hayes, M.J.; Rescher, U.; Gerke, V.; Moss, S.E. Annexin–Actin Interactions. Traffic 2004, 5, 571–576. [Google Scholar] [CrossRef]
- Hayes, M.J.; Shao, D.; Bailly, M.; Moss, S.E. Regulation of actin dynamics by annexin 2. EMBO J. 2006, 25, 1816–1826. [Google Scholar] [CrossRef] [Green Version]
- Tobe, T. Cytoskeleton-modulating effectors of enteropathogenic and enterohemorrhagic Escherichia coli: Role of EspL2 in adherence and an alternative pathway for modulating cytoskeleton through Annexin A2 function. FEBS J. 2010, 277, 2403–2408. [Google Scholar] [CrossRef]
- Taylor, J.R.; Skeate, J.G.; Kast, W.M. Annexin A2 in Virus Infection. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Dziduszko, A.; Ozbun, M.A. Annexin A2 and S100A10 Regulate Human Papillomavirus Type 16 Entry and Intracellular Trafficking in Human Keratinocytes. J. Virol. 2013, 87, 7502–7515. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.R.; Fernandez, D.J.; Thornton, S.M.; Skeate, J.G.; Lühen, K.P.; Da Silva, D.M.; Langen, R.; Kast, W.M. Heterotetrameric annexin A2/S100A10 (A2t) is essential for oncogenic human papillomavirus trafficking and capsid disassembly, and protects virions from lysosomal degradation. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Woodham, A.W.; Da Silva, D.M.; Skeate, J.G.; Raff, A.B.; Ambroso, M.R.; Brand, H.E.; Isas, J.M.; Langen, R.; Kast, W.M. The S100A10 subunit of the annexin A2 heterotetramer facilitates L2-mediated human papillomavirus infection. PLoS ONE 2012, 7, e43519. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Simons, M. Regulation of vascular integrity. J. Mol. Med. 2009, 87, 571–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannotta, M.; Trani, M.; Dejana, E. VE-cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef] [Green Version]
- Heyraud, S.; Jaquinod, M.; Durmort, C.; Dambroise, E.; Concord, E.; Schaal, J.P.; Huber, P.; Gulino-Debrac, D. Contribution of annexin 2 to the architecture of mature endothelial adherens junctions. Mol. Cell. Biol. 2008, 28, 1657–1668. [Google Scholar] [CrossRef] [Green Version]
- Dejana, E.; Orsenigo, F.; Lampugnani, M.G. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 2008, 121, 2115–2122. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Flood, E.C.; Almeida, D.; Yan, L.; Berlin, D.A.; Heerdt, P.M.; Hajjar, K.A. Annexin A2 supports pulmonary microvascular integrity by linking vascular endothelial cadherin and protein tyrosine phosphatases. J. Exp. Med. 2017, 214, 2535–2545. [Google Scholar] [CrossRef]
- Li, W.; Chen, Z.; Yuan, J.; Yu, Z.; Cheng, C.; Zhao, Q.; Huang, L.; Hajjar, K.A.; Chen, Z.; Lo, E.H.; et al. Annexin A2 is a Robo4 ligand that modulates ARF6 activation-associated cerebral trans-endothelial permeability. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2019, 39, 2048–2060. [Google Scholar] [CrossRef]
- McVoy, L.A.; Kew, R.R. CD44 and Annexin A2 Mediate the C5a Chemotactic Cofactor Function of the Vitamin D Binding Protein. J. Immunol. 2005, 175, 4754–4760. [Google Scholar] [CrossRef] [Green Version]
- Brownstein, C.; Deora, A.B.; Jacovina, A.T.; Weintraub, R.; Gertler, M.; Khan, K.M.F.; Falcone, D.J.; Hajjar, K.A. Annexin II mediates plasminogen-dependent matrix invasion by human monocytes: Enhanced expression by macrophages. Blood 2004, 103, 317–324. [Google Scholar] [CrossRef]
- Rankin, C.R.; Hilgarth, R.S.; Leoni, G.; Kwon, M.; Beste, K.A.D.; Parkos, C.A.; Nusrat, A. Annexin A2 Regulates β1 Integrin Internalization and Intestinal Epithelial Cell Migration. J. Biol. Chem. 2013, 288, 15229–15239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Callaway, J.B.; Ting, J.P.Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Scharf, B.; Clement, C.C.; Wu, X.X.; Morozova, K.; Zanolini, D.; Follenzi, A.; Larocca, J.N.; Levon, K.; Sutterwala, F.S.; Rand, J.; et al. Annexin A2 binds to endosomes following organelle destabilization by particulate wear debris. Nat. Commun. 2012, 3, 755. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Shaw, D.K.; Sakhon, O.S.; Snyder, G.A.; Sundberg, E.J.; Santambrogio, L.; Sutterwala, F.S.; Dumler, J.S.; Shirey, K.A.; Perkins, D.J.; et al. The Tick Protein Sialostatin L2 Binds to Annexin A2 and Inhibits NLRC4-Mediated Inflammasome Activation. Infect. Immun. 2016, 84, 1796–1805. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41. [Google Scholar] [CrossRef] [Green Version]
- Ravikumar, B.; Sarkar, S.; Davies, J.E.; Futter, M.; Garcia-Arencibia, M.; Green-Thompson, Z.W.; Jimenez-Sanchez, M.; Korolchuk, V.I.; Lichtenberg, M.; Luo, S.; et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 2010, 90, 1383–1435. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Klionsky, D.J. Autophagosome formation: Core machinery and adaptations. Nat. Cell Biol. 2007, 9, 1102–1109. [Google Scholar] [CrossRef]
- Ravikumar, B.; Futter, M.; Jahreiss, L.; Korolchuk, V.I.; Lichtenberg, M.; Luo, S.; Massey, D.C.O.; Menzies, F.M.; Narayanan, U.; Renna, M.; et al. Mammalian macroautophagy at a glance. J. Cell Sci. 2009, 122, 1707–1711. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Karantza-Wadsworth, V. Role and regulation of autophagy in cancer. Biochim. Biophys. Acta 2009, 1793, 1516–1523. [Google Scholar] [CrossRef] [Green Version]
- Margeta, M. Autophagy Defects in Skeletal Myopathies. Annu. Rev. Pathol. 2020, 15, 261–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, C.D.; Lee, M.S.; Marchetti, P.; Pietropaolo, M.; Towns, R.; Vaccaro, M.I.; Watada, H.; Wiley, J.W. The emerging role of autophagy in the pathophysiology of diabetes mellitus. Autophagy 2011, 7, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 2006, 443, 780–786. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Bento, C.F.; Deretic, V. Therapeutic targeting of autophagy in neurodegenerative and infectious diseases. J. Exp. Med. 2015, 212, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of Cells and Tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, K.; Ghislat, G.; Hochfeld, W.; Renna, M.; Zavodszky, E.; Runwal, G.; Puri, C.; Lee, S.; Siddiqi, F.; Menzies, F.M.; et al. Transcriptional regulation of Annexin A2 promotes starvation-induced autophagy. Nat. Commun. 2015, 6, 8045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Xu, Y. Annexin A2 upregulation protects human retinal endothelial cells from oxygen-glucose deprivation injury by activating autophagy. Exp. Ther. Med. 2019, 18, 2901–2908. [Google Scholar] [CrossRef]
- Li, R.; Tan, S.; Yu, M.; Jundt, M.C.; Zhang, S.; Wu, M. Annexin A2 Regulates Autophagy in Pseudomonas aeruginosa Infection through the Akt1-mTOR-ULK1/2 Signaling Pathway. J. Immunol. 2015, 195, 3901–3911. [Google Scholar] [CrossRef] [Green Version]
- Caramés, B.; Taniguchi, N.; Otsuki, S.; Blanco, F.J.; Lotz, M. Autophagy is a Protective Mechanism in Normal Cartilage and its Aging-related Loss is Linked with Cell Death and Osteoarthritis. Arthritis Rheum. 2010, 62, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Caramés, B.; Olmer, M.; Kiosses, W.B.; Lotz, M.K. The Relationship of Autophagy Defects to Cartilage Damage during Joint Aging in a Mouse Model. Arthritis Rheumatol. 2015, 67, 1568–1576. [Google Scholar] [CrossRef]
- Liu, W.; Hajjar, K.A. The annexin A2 system and angiogenesis. Biol. Chem. 2016, 397, 1005–1016. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D. Inflammation and Angiogenesis. In Inflammation and Angiogenesis; Ribatti, D., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 25–26. ISBN 978-3-319-68448-2. [Google Scholar]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Camarillo, C.; Hernadez de la Cruz, O.N.; Lopez-Gonzalez, J.S. Contribution of Angiogenesis to Inflammation and Cancer. Front. Oncol. 2019, 9, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, Q.; Jacovina, A.T.; Deora, A.; Febbraio, M.; Simantov, R.; Silverstein, R.L.; Hempstead, B.; Mark, W.H.; Hajjar, K.A. Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J. Clin. Investig. 2004, 113, 12. [Google Scholar] [CrossRef] [Green Version]
- Jacovina, A.T.; Deora, A.B.; Ling, Q.; Broekman, M.J.; Almeida, D.; Greenberg, C.B.; Marcus, A.J.; Smith, J.D.; Hajjar, K.A. Homocysteine inhibits neoangiogenesis in mice through blockade of annexin A2-dependent fibrinolysis. J. Clin. Investig. 2009, 119, 3384–3394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Deora, A.B.; He, K.L.; Chen, K.; Sui, G.; Jacovina, A.T.; Almeida, D.; Hong, P.; Burgman, P.; Hajjar, K.A. Hypoxia-inducible factor-1 drives annexin A2 system-mediated perivascular fibrin clearance in oxygen-induced retinopathy in mice. Blood 2011, 118, 2918–2929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Huang, L.; Wu, J.; Zhang, Y.; Pan, D.; Liu, X. Vascular endothelial growth factor upregulates expression of annexin A2 in vitro and in a mouse model of ischemic retinopathy. Mol. Vis. 2009, 15, 1231–1242. [Google Scholar] [PubMed]
- Silva, R.L.E.; Shen, J.; Gong, Y.Y.; Seidel, C.P.; Hackett, S.F.; Kevasan, K.; Jacoby, D.B.; Campochiaro, P.A. Agents That Bind Annexin A2 Suppress Ocular Neovascularization. J. Cell. Physiol. 2010, 225, 855–864. [Google Scholar] [CrossRef] [Green Version]
- Andreasen, P.A.; Egelund, R.; Petersen, H.H. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell. Mol. Life Sci. 2000, 57, 25–40. [Google Scholar] [CrossRef]
- Sharma, M.; Ownbey, R.T.; Sharma, M.C. Breast cancer cell surface annexin II induces cell migration and neoangiogenesis via tPA dependent plasmin generation. Exp. Mol. Pathol. 2010, 88, 278–286. [Google Scholar] [CrossRef]
- Sharma, M.R.; Koltowski, L.; Ownbey, R.T.; Tuszynski, G.P.; Sharma, M.C. Angiogenesis-associated protein annexin II in breast cancer: Selective expression in invasive breast cancer and contribution to tumor invasion and progression. Exp. Mol. Pathol. 2006, 81, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Blackman, M.R.; Sharma, M.C. Antibody-directed neutralization of annexin II (ANX II) inhibits neoangiogenesis and human breast tumor growth in a xenograft model. Exp. Mol. Pathol. 2012, 92, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.C.; Tuszynski, G.P.; Blackman, M.R.; Sharma, M. Long-term efficacy and downstream mechanism of anti-annexinA2 monoclonal antibody (anti-ANX A2 mAb) in a pre-clinical model of aggressive human breast cancer. Cancer Lett. 2016, 373, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nygaard, S.J.; Haugland, H.K.; Kristoffersen, E.K.; Lund-Johansen, M.; Laerum, O.D.; Tysnes, O.B. Expression of annexin II in glioma cell lines and in brain tumor biopsies. J. Neurooncol. 1998, 38, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Reeves, S.A.; Chavez-Kappel, C.; Davis, R.; Rosenblum, M.; Israel, M.A. Developmental regulation of annexin II (Lipocortin 2) in human brain and expression in high grade glioma. Cancer Res. 1992, 52, 6871–6876. [Google Scholar]
- Onishi, M.; Ichikawa, T.; Kurozumi, K.; Inoue, S.; Maruo, T.; Otani, Y.; Fujii, K.; Ishida, J.; Shimazu, Y.; Yoshida, K.; et al. Annexin A2 regulates angiogenesis and invasion phenotypes of malignant glioma. Brain Tumor Pathol. 2015, 32, 184–194. [Google Scholar] [CrossRef]
- Zhai, H.; Acharya, S.; Gravanis, I.; Mehmood, S.; Seidman, R.J.; Shroyer, K.R.; Hajjar, K.A.; Tsirka, S.E. Annexin A2 Promotes Glioma Cell Invasion and Tumor Progression. J. Neurosci. 2011, 31, 14346–14360. [Google Scholar] [CrossRef] [Green Version]
- Ohno, Y.; Izumi, M.; Kawamura, T.; Nishimura, T.; Mukai, K.; Tachibana, M. Annexin II represents metastatic potential in clear-cell renal cell carcinoma. Br. J. Cancer 2009, 101, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, U.; Woenckhaus, C.; Pietschmann, S.; Junker, H.; Maile, S.; Schultz, K.; Protzel, C.; Giebel, J. Expression of annexin II in conventional renal cell carcinoma is correlated with Fuhrman grade and clinical outcome. Virchows Arch. Int. J. Pathol. 2004, 445, 368–374. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, G.; Cai, J.; Wang, Y.; Qu, X.; He, L.; Liu, F.; Zhang, Y.; Lin, K.; Ma, S.; et al. Annexin A2 is a discriminative serological candidate in early hepatocellular carcinoma. Carcinogenesis 2013, 34, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Rocha, M.R.; Barcellos-de-Souza, P.; Sousa-Squiavinato, A.C.M.; Fernandes, P.V.; de Oliveira, I.M.; Boroni, M.; Morgado-Diaz, J.A. Annexin A2 overexpression associates with colorectal cancer invasiveness and TGF-ß induced epithelial mesenchymal transition via Src/ANXA2/STAT3. Sci. Rep. 2018, 8, 11285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andey, T.; Marepally, S.; Patel, A.; Jackson, T.; Sarkar, S.; O’Connell, M.; Reddy, R.C.; Chellappan, S.; Singh, P.; Singh, M. Cationic lipid guided short-hairpin RNA interference of annexin A2 attenuates tumor growth and metastasis in a mouse lung cancer stem cell model. J. Control. Release Off. J. Control. Release Soc. 2014, 184, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Pathogen | AnxA2 role | References |
|---|---|---|
| Bacteria | ||
| Anti-Inflammatory Actions | ||
| Klebsiella pneumoniae | Reduced cytokine response | [24] |
| Pro-Inflammatory Actions | ||
| Pseudomonas aeruginosa | Plasma membrane adhesion | [32] |
| Escherichia coli | Plasma membrane adhesion | [33,38] |
| Salmonella typhimurium | Host cell invasion | [34] |
| Rickettsia australis | Plasma membrane adhesion | [35] |
| Fungus | ||
| Anti-Inflammatory Actions | ||
| Cryptococcus neoformans | Phagocytosis and exocytosis | [25] |
| Pro-Inflammatory Actions | ||
| none reported | --- | --- |
| Virus | ||
| Anti-Inflammatory Actions | ||
| Human Papillomavirus | Attachment and intracellular trafficking | [40,41,42] |
| Pro-Inflammatory Actions | ||
| none reported | --- | --- |
| Action | Model System | Result in Anxa2-/- | References |
|---|---|---|---|
| Infection | Klebsiella pneumonia | Increased mortality | [24] |
| Cryptococcal pneumonia | Increased mortality and enhanced inflammatory response | [25] | |
| Rickettsia australis | Increased bacteria in blood and reduced adhesion to vascular endothelial cells | [35] | |
| Vascular integrity | Hypoxia | Increased vascular leak in lungs and skin | [47] |
| Tracer injection in embryonic mice | Increased leakage of 10-kDA-dextran | [48] | |
| Inflammasome Dynamics | Anaplasmaphagocytophilum peritonitis | Increased bacterial load, splenomegaly, and cytopenias | [55] |
| Macroautophagy | Starvation-induced autophagy | Abrogation of autophagosome biogenesis | [66] |
| Pseudomonasaeruginosa pneumonia | Reduced survival, increased bacterial dissemination, and blunted autophagy | [68] | |
| Angiogenesis | Cornea pocket assay | Reduced growth factor induced neoangiogenesis | [75] |
| Matrigel implant assay | Reduced growth factor induced neoangiogenesis | [78] | |
| Oxygen-induced retinopathy | Reduced angiogenesis and fibrinolysis with fibrin accumulation | [77] | |
| Tumor progression | Intracerebral glioma cell implantation | Increased tumor size and reduced vascularity | [88] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dallacasagrande, V.; Hajjar, K.A. Annexin A2 in Inflammation and Host Defense. Cells 2020, 9, 1499. https://doi.org/10.3390/cells9061499
Dallacasagrande V, Hajjar KA. Annexin A2 in Inflammation and Host Defense. Cells. 2020; 9(6):1499. https://doi.org/10.3390/cells9061499
Chicago/Turabian StyleDallacasagrande, Valentina, and Katherine A. Hajjar. 2020. "Annexin A2 in Inflammation and Host Defense" Cells 9, no. 6: 1499. https://doi.org/10.3390/cells9061499
APA StyleDallacasagrande, V., & Hajjar, K. A. (2020). Annexin A2 in Inflammation and Host Defense. Cells, 9(6), 1499. https://doi.org/10.3390/cells9061499




