New Frontiers in Prostate Cancer Treatment: Are We Ready for Drug Combinations with Novel Agents?
Abstract
:1. Background
2. Article Selection
3. DNA Damage Repair Pathways
4. Single-Agent Therapy and DDR Pathways Correlation
4.1. ARS-Inhibition
4.2. PARP-Inhibition
4.3. ICI-Inhibition
4.4. PSMA-Inhibition
5. Combined Drug Treatments
5.1. Published Clinical Studies
- PARPi plus chemotherapy: A phase 1 dose-escalation study of veliparib combined with paclitaxel and carboplatin was conducted in patients with advanced solid malignancies not responding to previous therapy. Seventy-three patients with documented or probable BRCA mutation were enrolled. The findings demonstrated that the pharmacokinetics of the two chemotherapy drugs was not affected by concomitant veliparib administration. Twenty-two PR and five CR were observed. The toxicity reported was as expected for each drug involved, displaying a good tolerance overall [27].
- PARPi plus ARSi: A phase 2 trial with one-to-one randomization was carried out in 148 mCRPC patients—previously stratified by ETS status (a fusion gene that would enhance PARP-1 inhibition)—to evaluate whether the combination between PARP-1 inhibitor veliparib and ARS inhibitor abiraterone acetate (arm B) is superior to ARS blockade alone (arm A). The findings showed that in patients without DDR pathway alterations (wild-type tumors), there were no significant differences between the two arms in terms of PSA response, tumor response, and PFS, and ETS status did not affect the results. Conversely, 80 patients (33 and 47 for arms A and B, respectively) who harbored DDR gene alterations—detected by NGS of tumor samples—had significantly better results in all the objectives assessed than the counterpart wild-type tumors [28].
- ICI plus PARPi: In a small phase 2 cohort-study, 17 mCRPC patients who were previously treated with ARS inhibitors and not prescreened for gene alterations of DDR pathways received a combination of durvalumab plus olaparib. Nine of 17 patients experienced responses, all of whom showed a response of PSA reduction >50% and four as tumor reduction. A large part of the patient responders had mutations in DDR genes. Anemia and nausea grade 3–4 occurred in 24% and 12% of patients, respectively [29].
- PSMAi plus IL-2: In a phase I dose-escalation study, five mCRPC patients previously receiving chemotherapy conditioning were treated with PSMA-targeted CAR-T cells and continuous infusion of low-dose interleukin 2. The findings successfully showed that two patients engrafted exhibited tumor response as PR along with 50%/70% of PSA reduction; another patient had a minor response. Clinical responses significantly correlated with plasma IL-2 value, and anti-PSMA toxicities did not occur [30].
5.2. Ongoing Trials
- ICI- and PARP-inhibition: An anti-PD-1 inhibitor pembrolizumab and PARPi olaparib combination is being investigated in a phase 1b/2 umbrella study (NCT02861573). Preliminary data in 41 molecularly unselected patients previously treated with docetaxel and one ARS inhibitor have shown a PSA response in 13% of the patients. Grade 3–5 treatment-related adverse events occurred in half of the patients, and the authors stated that the safety profile was in line with the spectrum of toxicity of each drug explored. A phase 2 single-arm open-label study (NCT03565991) is evaluating the combination of anti-PD-L1 avelumab plus PARPi talazoparib in patients with locally advanced or metastatic solid tumors—encompassing also patients with mCRPC—with BRCA1/2 genes or ATM gene defects. Talazoparib is a PARPi with the greatest PARP trapping potency (equal to 1, range 1–5) and the longest half-life (90 h) among the PARPi under development. Two hundred patients are expected to be enrolled into the study.
- ICI and anti-adenosine A2A receptor: It is known that adenosine inhibits the anti-cancer function of T-cells and other immune system cells. According to this, some molecules that block adenosine, hampering the binding to its A2A receptor (also known as ADORA2A) and consequently stimulating the immune system response, are under consideration. It follows that the rationale for combining anti-adenosine agents with ICI is to boost the human immune system. A phase 1 open-label dose escalation trial (NCT02740985) is currently investigating oral dosing of AZD4635 (a selective small agent adenosine A2A receptor antagonist) both as a single agent and in combination with anti-PD-L1 durvalumab or other agents in patients with advanced solid tumors, including those with mCRPC. The estimated enrollment is of 307 patients. Ciforadenant is another oral small molecule anti-adenosine A2A receptor on T-cells and other immune cells. In a phase 1/1b open-label study (NCT02655822), ciforadenant is under development as a monotherapy and plus PD-L1 inhibitor atezolizumab in patients with mCRPC or metastatic renal cell carcinoma. The updated estimated enrollment is 336 patients.
- ICI-based combination: The interleukin-2 receptor (IL-2R) is a protein with three different chains (alpha, beta, and gamma), located on the cell surface of certain immune system cells, e.g., lymphocytes, that interacts with the IL-2 cytokine. CD122 is the beta subunit of the IL-2R and is said to play a role in the T cell-mediated immune response. A phase 1b/2 trial (NCT04052204) is investigating the combination of anti-PD-L1 avelumab plus bempegaldesleukin (NKTR-214, a CD122-biased IL-2 receptor agonist) with PARPi talazoparib or ARSi enzalutamide. The study design includes three treatment arms: avelumab plus bempegaldesleukin (arm A) for locally recurrent/metastatic head and neck cancers; avelumab plus bempegaldesleukin plus talazoparib (arm B); and avelumab plus bempegaldesleukin plus enzalutamide (arm C) for mCRPC patients. In combination B, it is planned to enroll subjects with DDR deficiency for phase 2. The updated estimated enrollment is 127 participants. Immunotherapy with avelumab-based combinations is being explored in an open-label phase I/II study (NCT03217747) in patients with advanced malignancies, including both locally-advanced and metastatic PCa patients. The study design includes six treatment arms, in which avelumab is combined with other monoclonal antibodies (utomilumab and anti-OX40 antibody PF-04518600) and in three arms also with radiotherapy. A sample size of 184 subjects is planned to be reached. The MOVIE study is a phase I/II study (NCT03518606) designed to examine a combination consisting of oral metronomic chemotherapy with vinorelbine and a double immune blockade with durvalumab and an anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) known as tremelimumab in locally advanced/metastatic solid tumors, among which is PCa. It is estimated that 150 participants will be enrolled.
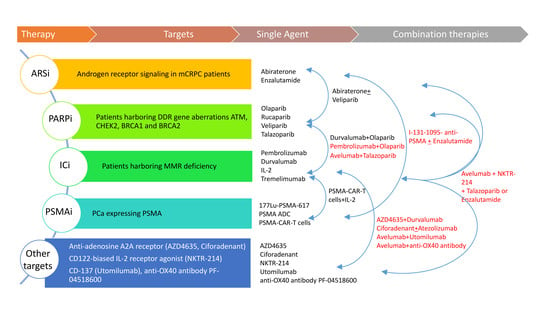
- PSMA-targeting combinations: In a phase I dose-escalation study (NCT04053062), the safety and efficacy of PSMA-specific CAR modified autologous T cells (PSMA-CART cells) is being evaluated in mCRPC patients. Two cohorts of participants have been preplanned, in cohort 1, subjects receive an intravenous single dose of PSMA-CART cells on day 0 after a conditioning regimen (on days -6 to -4) of chemotherapy with cyclophosphamide and fludarabine. Twelve subjects are requested for this study. Another single arm phase I study (NCT03089203) is evaluating the safety and feasibility of dual PSMA-specific/TGFβ-resistant CAR modified autologous T cells (CART-PSMA-TGFβRDN cells) without (cohort 1 and cohort 2) and with (cohort 3) intravenous cyclophosphamide in mCRPC patients. Cohorts 1 and 2 allow the maximum tolerated dose of CART-PSMA-TGFβRDN cells to be identified, while the use of cyclophosphamide in the cohort 3 before CAR-T cells makes use as conditioning chemotherapy. Eighteen patients are estimated to be enrolled into the study. A randomized phase 2 open-label study (NCT03939689) is investigating a radioconjugate radiolabeled with iodine I-131-1095, for delivering iodine cytotoxicity selectively for PSMA-expressing PCa cells, combined with (80 patients) or without (40 patients) enzalutamide in patients with mCRPC. Eligible criteria allow the inclusion of chemotherapy-naïve patients progressing under abiraterone (Figure 1).
6. Conclusions and Future Horizons
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PCa | prostate cancer |
| AR | androgen receptor |
| ARS | AR-signaling |
| NGS | next-generation sequencing |
| DDR | DNA damage repair |
| mCRPC | metastatic castration-resistant PCa |
| CSS | cause-specific survival |
| PARP | poly (adenosine diphosphate [ADP]-ribose) polymerase |
| PD | programmed death |
| PSA | prostate-specific antigen |
| ICI | immune checkpoint inhibitors |
| SSB | single-stranded breaks |
| DSB | double-stranded breaks |
| HR | homologous recombination |
| NHEJ | non-homologous end joining |
| MMR | mismatch repair |
| PSMA | prostate-specific membrane antigen |
| CAR-T | chimeric antigen receptor-T |
References
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Armenia, J.; Wankowicz, S.A.M.; Liu, D.; Gao, J.; Kundra, R.; Reznik, E.; Chatila, W.K.; Chakravarty, D.; Han, G.C.; Coleman, I.; et al. The long tail of oncogenic drivers in prostate cancer. Nat. Genet. 2018, 50, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; Mortimer, P.; Swaisland, H.; Lau, A.; O’Connor, M.J.; et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009, 361, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Mateo, J.; Porta, N.; Bianchini, D.; McGovern, U.; Elliott, T.; Jones, R.; Syndikus, I.; Ralph, C.; Jain, S.; Varughese, M.; et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 162–174. [Google Scholar] [CrossRef]
- Schiewer, M.J.; Goodwin, J.F.; Han, S.; Brenner, J.C.; Augello, M.A.; Dean, J.L.; Liu, F.; Planck, J.L.; Ravindranathan, P.; Chinnaiyan, A.M.; et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012, 2, 1134–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asim, M.; Tarish, F.; Zecchini, H.I.; Sanjiv, K.; Gelali, E.; Massie, C.E.; Baridi, A.; Warren, A.Y.; Zhao, W.; Ogris, C.; et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat. Commun. 2017, 8, 374. [Google Scholar] [CrossRef] [Green Version]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Karanika, S.; Karantanos, T.; Li, L.; Corn, P.G.; Thompson, T.C. DNA damage response and prostate cancer: Defects, regulation and therapeutic implications. Oncogene 2015, 34, 2815–2822. [Google Scholar] [CrossRef] [Green Version]
- Wright, W.D.; Shah, S.S.; Heyer, W.D. Homologous recombination and the repair of DNA double-strand breaks. J. Biol. Chem. 2018, 293, 10524–10535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athie, A.; Arce-Gallego, S.; Gonzalez, M.; Morales-Barrera, R.; Suarez, C.; Galobart, T.C.; Viedma, G.H.; Carles, J.; Mateo, J. Targeting DNA repair defects for precision medicine in prostate cancer. Curr. Oncol. Rep. 2019, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Keijzers, G.; Rasmussen, L.J. DNA Mismatch repair and its many roles in eukaryotic cells. Mutat. Res. 2017, 773, 174–187. [Google Scholar] [CrossRef]
- Annala, M.; Struss, W.J.; Warner, E.W.; Beja, K.; Vandekerkhove, G.; Wong, A.; Khalaf, D.; Seppälä, I.L.; So, A.; Lo, G.; et al. Treatment outcomes and tumor loss of heterozygosity in germline DNA repair-deficient prostate cancer. Eur. Urol. 2017, 72, 34–42. [Google Scholar] [CrossRef]
- Annala, M.; Vandekerkhove, G.; Khalaf, D.; Taavitsainen, S.; Beja, K.; Warner, E.W.; Sunderland, K.; Kollmannsberger, C.; Eigl, B.J.; Finch, D.; et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2018, 8, 444–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateo, J.; Cheng, H.H.; Beltran, H.; Dolling, D.; Xu, W.; Pritchard, C.C.; Mossop, H.; Rescigno, P.; Perez-Lopez, R.; Sailer, V.; et al. Clinical outcome of prostate cancer patients with germline DNA repair mutations: Retrospective analysis from an international study. Eur. Urol. 2018, 73, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Liang, C.; Wang, H.; Chen, Y.; Silberstein, J.L.; Piana, D.; Lai, Z.; Chen, Y.; et al. Germline DNA-repair gene mutations and outcomes in men with metastatic castration-resistant prostate cancer receiving first-line abiraterone and enzalutamide. Eur. Urol. 2018, 74, 218–225. [Google Scholar] [CrossRef]
- Castro, E.; Romero-Laorden, N.; Del Pozo, A.; Lozano, R.; Medina, A.; Puente, J.; Piulats, J.M.; Lorente, D.; Saez, M.I.; Morales-Barrera, R.; et al. PROREPAIR-B: A prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2019, 37, 490–503. [Google Scholar] [CrossRef]
- Mateo, J.; Carreira, S.; Sandhu, S.; Miranda, S.; Mossop, H.; Perez-Lopez, R.; Rodrigues, D.N.; Robinson, D.; Omlin, A.; Tunariu, N.; et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 2015, 373, 1697–1708. [Google Scholar] [CrossRef]
- Abida, W.; Campbell, D.; Patnaik, A.; Shapiro, J.D.; Sautois, B.; Vogelzang, N.J.; Voog, E.G.; Bryce, A.H.; McDermott, R.; Ricci, F.; et al. Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: Analysis from the phase 2 TRITON2 Study. Clin. Cancer Res. 2020, 26, 2487–2496. [Google Scholar] [CrossRef] [Green Version]
- Marshall, C.H.; Sokolova, A.O.; McNatty, A.L.; Cheng, H.H.; Eisenberger, M.A.; Bryce, A.H.; Schweizer, M.T.; Antonarakis, E.S. Differential response to Olaparib treatment among men with metastatic castration-resistant prostate cancer harboring BRCA1 or BRCA2 versus ATM mutations. Eur. Urol. 2019, 76, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Petrylak, D.P.; Vogelzang, N.J.; Chatta, K.; Fleming, M.T.; Smith, D.C.; Appleman, L.J.; Hussain, A.; Modiano, M.; Singh, P.; Tagawa, S.T.; et al. PSMA ADC monotherapy in patients with progressive metastatic castration-resistant prostate cancer following abiraterone and/or enzalutamide: Efficacy and safety in open-label single-arm phase 2 study. Prostate 2020, 80, 99–108. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Dwivedi, S.N.; Bal, C. Radioligand Therapy with 177Lu-PSMA for metastatic castration-resistant prostate cancer: A systematic review and meta-analysis. AJR Am. J. Roentgenol. 2019, 213, 275–285. [Google Scholar] [CrossRef]
- Appleman, L.J.; Beumer, J.H.; Jiang, Y.; Lin, Y.; Ding, F.; Puhalla, S.; Swartz, L.; Owonikoko, T.K.; Donald Harvey, R.; Stoller, R.; et al. Phase 1 study of veliparib (ABT-888), a poly (ADP-ribose) polymerase inhibitor, with carboplatin and paclitaxel in advanced solid malignancies. Cancer Chemother. Pharmacol. 2019, 84, 1289–1301. [Google Scholar] [CrossRef]
- Hussain, M.; Daignault-Newton, S.; Twardowski, P.W.; Albany, C.; Stein, M.N.; Kunju, L.P.; Siddiqui, J.; Wu, Y.M.; Robinson, D.; Lonigro, R.J.; et al. Targeting androgen receptor and DNA repair in metastatic castration-resistant prostate cancer: Results from NCI 9012. J. Clin. Oncol. 2018, 36, 991–999. [Google Scholar] [CrossRef]
- Karzai, F.; VanderWeele, D.; Madan, R.A.; Owens, H.; Cordes, L.M.; Hankin, A.; Couvillon, A.; Nichols, E.; Bilusic, M.; Beshiri, M.L.; et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J. Immunother. Cancer 2018, 6, 141. [Google Scholar] [CrossRef]
- Junghans, R.P.; Ma, Q.; Rathore, R.; Gomes, E.M.; Bais, A.J.; Lo, A.S.Y.; Abedi, M.; Davies, R.A.; Cabral, H.J.; Al-Homsi, A.S.; et al. Phase I Trial of Anti-PSMA designer CAR-T cells in prostate cancer: Possible role for interacting Interleukin 2-T Cell pharmacodynamics as a determinant of clinical response. Prostate 2016, 76, 1257–1270. [Google Scholar] [CrossRef]
- Li, L.; Karanika, S.; Yang, G.; Wang, J.; Park, S.; Broom, B.M.; Manyam, G.C.; Wu, W.; Luo, Y.; Basourakos, S.; et al. Androgen Receptor Inhibitor-Induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci. Signal. 2017, 10, 7479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimadamore, A.; Cheng, M.; Santoni, M.; Lopez-Beltran, A.; Battelli, N.; Massari, F.; Galosi, A.B.; Scarpelli, M.; Montironi, R. New prostate cancer targets for diagnosis, imaging, and therapy: Focus on prostate-specific membrane antigen. Front. Oncol. 2018, 8, 653. [Google Scholar] [CrossRef] [Green Version]
- Markowski, M.C.; Shenderov, E.; Eisenberger, M.A.; Kachhap, S.; Pardoll, D.M.; Denmeade, S.R.; Antonarakis, E.S. Extreme responses to immune checkpoint blockade following bipolar androgen therapy and enzalutamide in patients with metastatic castration resistant prostate cancer. Prostate 2020, 80, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Drug | Exploratory analysis | N | Design | Phase | Findings [Ref] |
|---|---|---|---|---|---|
| ARSi Abi./Enza. | Sequencing of 73 PCa genes | 319 | R | NA | 7.5% of pts with germline mutations (> BRCA2) → 3.3 mo mPSA progression [14] |
| ARSi Abi./Enza. | WES of 72 driver PCa genes | 202 | R | NA | BRCA2/ATM defective → shorter TTP [15] |
| ARSi Abi./Enza. | Search of gDDRgm | 390 | R | NA | Carriers vs. non-carriers: no difference as PFS and RR [16] |
| ARSi Abi./Enza. | Search of gDDRm in 50 genes | 172 | R | NA | BRCA/ATM mutations → better survival [17] |
| ARSi Abi./Enza.vs Taxanes | Search of gDDRm in 107 genes | 419 | P | 2 | BRCA2 mutations → better CSS (outcome maintained comparing ARSi vs. Taxanes) [18] |
| PARPi Olaparib | Search of BRCA1/2 mutation | 60 | P | 1 | 22 pts with BRCA1/2 mutation: benefit on Olaparib [5] |
| PARPi Olaparib (TOPARP-A) | WES; transcriptome analysis | 50 | P | 2 | 14 out of 16 responders had DDR defects [19] |
| PARPi Rucaparib (TRITON2) | Mandatory non-BRCA DDR gene defects | 78 | P | 2 | ATM, CDK12, CHEK2: <10% response. PALB2, RAD51B: better and lasting response [20] |
| PARPi Olaparib (TOPARP-B) | Screening for DDR gene defects | 700 | P | 2 | 98 mutated pts received Olaparib 400/300 mg: 54%/39% tumor response [6] |
| PARPi Olaparib | Gene defects and response | 23 | R | NA | BRCA1/2 vs. ATM: 12 vs. 2 mo mPFS [21] |
| Olaparib vs Abi./Enza. (PROfound) | HR DDRgm: A (BRCA1/2 or ATM), B (other genes) | 387 | P | 3 | Cohort A: mr-PFS (pe): 7.4 vs. 3.6 (p < 0.001) Cohorts A and B: mr-PFS: 5.8 vs. 3.5 (p < 0.001) [22] |
| Anti-PD-1 Pembrolizumab | MMR deficiency | 86 | P | 2 | 50% of responders (21 CR) [9] |
| Anti-PD-1/PD-L1 therapy | Molecular tumor profile | 1033 | R | NA | 3.1% of pts had MSI/MMR deficiency→ 6/11 had responses [23] |
| PSMA ADC | PSMA expression on CTC, NE markers | 119 | P | 2 | Chemo-group: 61% SDChemo-naive group: 69% SD, 6% PR 7-mo OS: 92% for both the two groups [24] |
| Drugs | Inclusion Criteria | Objectives | N | Phase | Findings [Ref] |
|---|---|---|---|---|---|
| PARPi Veliparib + Chemo. (PTX + CBDCA) | advanced solid tumors treated with ≤3 prior regimens, BRCA status not mandated | P. obj.: side effects Recommended phase II dose S. obj.: ORR | 73 | 1 | 22 PR (1 in mCRPC pt) and 5 CR. Overall good tolerability Chemo. PK was not affected by PARPi [27] |
| ARSi Abiraterone + PARPi Veliparib | mCRPC, up to two prior chemotherapy regimens | P. obj.: PSA and RR, ETS response prediction; S. obj.: PFS, biomarkers | 148 | 2 | Arm A (ARSi) vs. Arm B (combo): no difference in wt pts; pts with DDR defects vs. wt: significantly better outcomes [28] |
| Anti-PD-L1 Durvalumab + PARPi Olaparib | Prior 1-2 ARSi; no preplanned DDR prescreening | P. obj.: clinical efficacy; S. obj.: ORR, PSA response, DDR status, biomarkers | 17 | 2 | 9 /17 with PSA response, 4 of whom also disease response (large part of responders had DDRgm) [29] |
| PSMA-targeted CAR-T cells + IL-2 | mCRPC | P. obj.: safety of PSMA-targeting with transduced T cells S. obj.:PSA response | 5 | 1 | 2 PR with PSA response [30] |
| Pathways Involved | Description | (NCT) No. | Phase | N |
|---|---|---|---|---|
| Anti-PD-1 Pembrolizumab + PARPi Olaparib | mCRPC, prior TXT and 1 ARSi therapy | 02861573 | 1b/2 | 41 |
| Anti-PD-L1 Avelumab + PARPi Talazoparib | BRCA1/2 or ATM mutated (also mCRPC) | 03565991 | 2 | 200 |
| AZD4635 + anti-PD-L1 Durvalumab | Advanced solid tumors (also mCRPC) | 02740985 | 1 | 307 |
| Ciforadenant + anti-PD-L1 Atezolizumab | mCRPC and mRCC | 02655822 | 1/1b | 336 |
| Anti-PD-L1 Avelumab + NKTR-214 (Arm A) + PARPi Talazoparib (Arm B) or ARSi Enzalutamide (Arm C) | Arm A: SCCHN; Arms B and C: mCRPC; Arm B enrolls DDR deficiency pts | 04052204 | 1b/2 | 127 |
| Anti-PD-L1 Avelumab-based combinations | Advanced malignancies (also mPCa) | 03217747 | 1/2 | 184 |
| Anti-PD-L1 Durvalumab + anti-CTLA-4 Tremelimumab + oral metronomic Vinorelbine | Solid tumors (also mPCa) | 03518606 | 1/2 | 150 |
| PSMA-CAR-T cells | mCRPC | 04053062 | 1 | 12 |
| CAR-T-PSMA-TGFβRDN cells | mCRPC | 03089203 | 1 | 18 |
| Iodine I-131-1095-radioconjugate anti-PSMA + Enzalutamide | mCRPC | 03939689 | 2 | 120 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aurilio, G.; Cimadamore, A.; Santoni, M.; Nolè, F.; Scarpelli, M.; Massari, F.; Lopez-Beltran, A.; Cheng, L.; Montironi, R. New Frontiers in Prostate Cancer Treatment: Are We Ready for Drug Combinations with Novel Agents? Cells 2020, 9, 1522. https://doi.org/10.3390/cells9061522
Aurilio G, Cimadamore A, Santoni M, Nolè F, Scarpelli M, Massari F, Lopez-Beltran A, Cheng L, Montironi R. New Frontiers in Prostate Cancer Treatment: Are We Ready for Drug Combinations with Novel Agents? Cells. 2020; 9(6):1522. https://doi.org/10.3390/cells9061522
Chicago/Turabian StyleAurilio, Gaetano, Alessia Cimadamore, Matteo Santoni, Franco Nolè, Marina Scarpelli, Francesco Massari, Antonio Lopez-Beltran, Liang Cheng, and Rodolfo Montironi. 2020. "New Frontiers in Prostate Cancer Treatment: Are We Ready for Drug Combinations with Novel Agents?" Cells 9, no. 6: 1522. https://doi.org/10.3390/cells9061522
APA StyleAurilio, G., Cimadamore, A., Santoni, M., Nolè, F., Scarpelli, M., Massari, F., Lopez-Beltran, A., Cheng, L., & Montironi, R. (2020). New Frontiers in Prostate Cancer Treatment: Are We Ready for Drug Combinations with Novel Agents? Cells, 9(6), 1522. https://doi.org/10.3390/cells9061522