The Effects of PK11195 and Protoporphyrin IX Can Modulate Chronic Alcohol Intoxication in Rat Liver Mitochondria under the Opening of the Mitochondrial Permeability Transition Pore
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Isolation of Rat Liver Mitochondria and the Outer Mitochondrial Membrane
2.3. Evaluation of Mitochondrial Functions
2.4. Sample Preparation
2.5. Electrophoresis and Immunoblotting of the Mitochondrial Proteins
2.6. Statistical Analysis
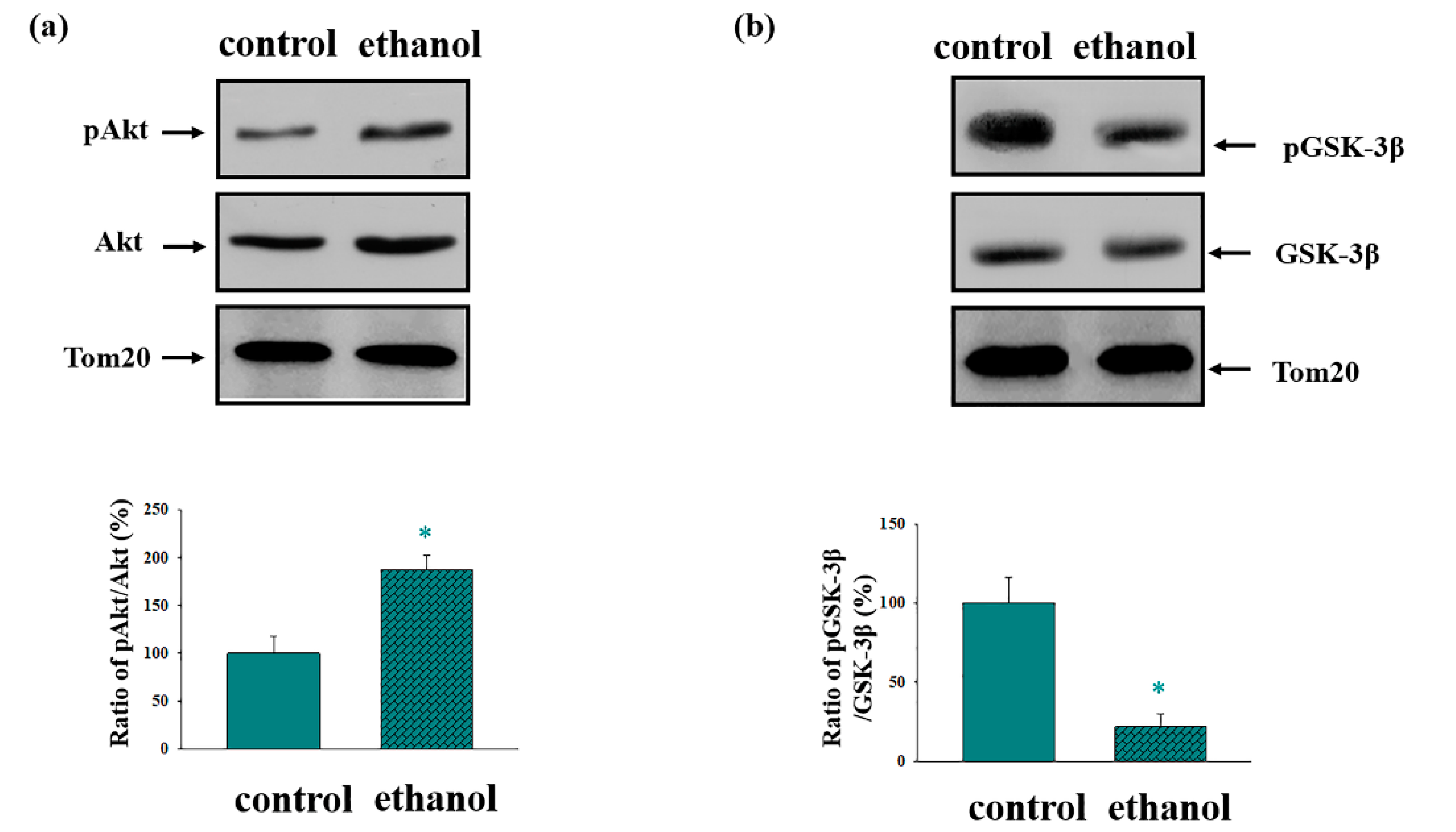
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Mann, R.E.; Smart, R.G.; Govoni, R. The epidemiology of alcoholic liver disease. Alcohol Res. Health 2003, 27, 209–219. [Google Scholar] [PubMed]
- Miranda-Mendez, A.; Lugo-Baruqui, A.; Armendariz-Borunda, J. Molecular basis and current treatment for alcoholic liver disease. Int. J. Environ. Res. Public Health 2010, 7, 1872–1888. [Google Scholar] [CrossRef] [PubMed]
- Bergheim, I.; McClain, C.J.; Arteel, G.E. Treatment of alcoholic liver disease. Dig. Dis. 2005, 23, 275–284. [Google Scholar] [CrossRef]
- Cunningham, C.C.; Coleman, W.B.; Spach, P.I. The effects of chronic ethanol consumption on hepatic mitochondrial energy metabolism. Alcohol Alcohol. 1990, 25, 127–136. [Google Scholar] [CrossRef]
- Cederbaum, A.I.; Lu, Y.; Wu, D. Role of oxidative stress in alcohol-induced liver injury. Arch. Toxicol. 2009, 83, 519–548. [Google Scholar] [CrossRef]
- Mantena, S.K.; King, A.L.; Andringa, K.K.; Eccleston, H.B.; Bailey, S.M. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic. Biol. Med. 2008, 44, 1259–1272. [Google Scholar] [CrossRef]
- Manzo-Avalos, S.; Saavedra-Molina, A. Cellular and mitochondrial effects of alcohol consumption. Int. J. Environ. Res. Public Health 2010, 7, 4281–4304. [Google Scholar] [CrossRef]
- Das, S.; Hajnoczky, N.; Antony, A.N.; Csordas, G.; Gaspers, L.D.; Clemens, D.L.; Hoek, J.B.; Hajnoczky, G. Mitochondrial morphology and dynamics in hepatocytes from normal and ethanol-fed rats. Pflugers Arch. 2012, 464, 101–109. [Google Scholar] [CrossRef]
- Grattagliano, I.; Russmann, S.; Diogo, C.; Bonfrate, L.; Oliveira, P.J.; Wang, D.Q.; Portincasa, P. Mitochondria in chronic liver disease. Curr. Drug Targets 2011, 12, 879–893. [Google Scholar] [CrossRef]
- Auger, C.; Alhasawi, A.; Contavadoo, M.; Appanna, V.D. Dysfunctional mitochondrial bioenergetics and the pathogenesis of hepatic disorders. Front. Cell Dev. Biol. 2015, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhong, W.; Zhang, W.; Zhou, Z. Defect of mitochondrial respiratory chain is a mechanism of ros overproduction in a rat model of alcoholic liver disease: Role of zinc deficiency. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G205–G214. [Google Scholar] [CrossRef] [PubMed]
- Song, B.J.; Abdelmegeed, M.A.; Henderson, L.E.; Yoo, S.H.; Wan, J.; Purohit, V.; Hardwick, J.P.; Moon, K.H. Increased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic fatty liver disease. Oxid. Med. Cell. Longev. 2013, 2013, 781050. [Google Scholar] [CrossRef] [PubMed]
- Andringa, K.K.; King, A.L.; Eccleston, H.B.; Mantena, S.K.; Landar, A.; Jhala, N.C.; Dickinson, D.A.; Squadrito, G.L.; Bailey, S.M. Analysis of the liver mitochondrial proteome in response to ethanol and s-adenosylmethionine treatments: Novel molecular targets of disease and hepatoprotection. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G732–G745. [Google Scholar] [CrossRef]
- Bailey, S.M.; Andringa, K.K.; Landar, A.; Darley-Usmar, V.M. Proteomic approaches to identify and characterize alterations to the mitochondrial proteome in alcoholic liver disease. Methods Mol. Biol. 2008, 447, 369–380. [Google Scholar]
- Fritz, K.S.; Galligan, J.J.; Hirschey, M.D.; Verdin, E.; Petersen, D.R. Mitochondrial acetylome analysis in a mouse model of alcohol-induced liver injury utilizing sirt3 knockout mice. J. Proteome Res. 2012, 11, 1633–1643. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef]
- Rimessi, A.; Giorgi, C.; Pinton, P.; Rizzuto, R. The versatility of mitochondrial calcium signals: From stimulation of cell metabolism to induction of cell death. Biochim. Biophys. Acta 2008, 1777, 808–816. [Google Scholar] [CrossRef]
- Meissner, G. Molecular regulation of cardiac ryanodine receptor ion channel. Cell Calcium 2004, 35, 621–628. [Google Scholar] [CrossRef]
- Kerner, J.; Lee, K.; Tandler, B.; Hoppel, C.L. Vdac proteomics: Post-translation modifications. Biochim. Biophys. Acta 2012, 1818, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Azarashvili, T.; Krestinina, O.; Galvita, A.; Grachev, D.; Baburina, Y.; Stricker, R.; Reiser, G. Identification of phosphorylated form of 2′, 3′-cyclic nucleotide 3′-phosphodiesterase (cnpase) as 46 kda phosphoprotein in brain non-synaptic mitochondria overloaded by calcium. J. Bioenerg. Biomembr. 2014, 46, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Umoh, N.A.; Walker, R.K.; Al-Rubaiee, M.; Jeffress, M.A.; Haddad, G.E. Acute alcohol modulates cardiac function as pi3k/akt regulates oxidative stress. Alcohol. Clin. Exp. Res. 2014, 38, 1847–1864. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Laguesse, S.; Legastelois, R.; Morisot, N.; Ben Hamida, S.; Ron, D. Mtorc1-dependent translation of collapsin response mediator protein-2 drives neuroadaptations underlying excessive alcohol-drinking behaviors. Mol. Psychiatry 2018, 23, 164. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Huang, C.C.Y.; Ma, T.; Wei, X.; Wang, X.; Lu, J.; Wang, J. Distinct synaptic strengthening of the striatal direct and indirect pathways drives alcohol consumption. Biol. Psychiatry 2017, 81, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Azarashvili, T.; Odinokova, I.; Krestinina, O.; Baburina, Y.; Teplova, V.; Jahangir, A.; Holmuhamedov, E. Ethanol exposure increases the activity of mitochondria-associated glycogen synthase kinase-3 beta (gsk-3β): Role in phosphorylation of mitochondrial proteins. Innov. J. Med. Health Sci. 2013, 3, 163–170. [Google Scholar]
- Das, S.; Wong, R.; Rajapakse, N.; Murphy, E.; Steenbergen, C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ. Res. 2008, 103, 983–991. [Google Scholar] [CrossRef]
- McEnery, M.W.; Snowman, A.M.; Trifiletti, R.R.; Snyder, S.H. Isolation of the mitochondrial benzodiazepine receptor: Association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc. Natl. Acad. Sci. USA 1992, 89, 3170–3174. [Google Scholar] [CrossRef]
- Veenman, L.; Papadopoulos, V.; Gavish, M. Channel-like functions of the 18-kda translocator protein (tspo): Regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr. Pharm. Des. 2007, 13, 2385–2405. [Google Scholar] [CrossRef]
- Corsi, L.; Geminiani, E.; Baraldi, M. Peripheral benzodiazepine receptor (pbr) new insight in cell proliferation and cell differentiation review. Curr. Clin. Pharmacol. 2008, 3, 38–45. [Google Scholar] [CrossRef]
- Lacapere, J.J.; Papadopoulos, V. Peripheral-type benzodiazepine receptor: Structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids 2003, 68, 569–585. [Google Scholar] [CrossRef]
- Dell’osso, B.; Lader, M. Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. Eur. Psychiatry 2013, 28, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.M.; Chen, X.; Boitano, A.; Swenson, L.; Opipari, A.W., Jr.; Glick, G.D. Identification and validation of the mitochondrial f1f0-atpase as the molecular target of the immunomodulatory benzodiazepine bz-423. Chem. Biol. 2005, 12, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Cleary, J.; Johnson, K.M.; Opipari, A.W., Jr.; Glick, G.D. Inhibition of the mitochondrial f1f0-atpase by ligands of the peripheral benzodiazepine receptor. Bioorg. Med. Chem. Lett. 2007, 17, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, V.; von Stockum, S.; Antoniel, M.; Fabbro, A.; Fogolari, F.; Forte, M.; Glick, G.D.; Petronilli, V.; Zoratti, M.; Szabo, I.; et al. Dimers of mitochondrial atp synthase form the permeability transition pore. Proc. Natl. Acad. Sci. USA 2013, 110, 5887–5892. [Google Scholar] [CrossRef]
- Azarashvili, T.; Grachev, D.; Krestinina, O.; Evtodienko, Y.; Yurkov, I.; Papadopoulos, V.; Reiser, G. The peripheral-type benzodiazepine receptor is involved in control of ca2+-induced permeability transition pore opening in rat brain mitochondria. Cell Calcium 2007, 42, 27–39. [Google Scholar] [CrossRef]
- Azarashvili, T.; Krestinina, O.; Yurkov, I.; Evtodienko, Y.; Reiser, G. High-affinity peripheral benzodiazepine receptor ligand, pk11195, regulates protein phosphorylation in rat brain mitochondria under control of ca(2+). J. Neurochem. 2005, 94, 1054–1062. [Google Scholar] [CrossRef]
- Casellas, P.; Galiegue, S.; Basile, A.S. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem. Int. 2002, 40, 475–486. [Google Scholar] [CrossRef]
- Decaudin, D.; Castedo, M.; Nemati, F.; Beurdeley-Thomas, A.; De Pinieux, G.; Caron, A.; Pouillart, P.; Wijdenes, J.; Rouillard, D.; Kroemer, G.; et al. Peripheral benzodiazepine receptor ligands reverse apoptosis resistance of cancer cells in vitro and in vivo. Cancer Res. 2002, 62, 1388–1393. [Google Scholar]
- Baburina, Y.; Azarashvili, T.; Grachev, D.; Krestinina, O.; Galvita, A.; Stricker, R.; Reiser, G. Mitochondrial 2′, 3′-cyclic nucleotide 3′-phosphodiesterase (cnp) interacts with mptp modulators and functional complexes (i–v) coupled with release of apoptotic factors. Neurochem. Int. 2015, 90, 46–55. [Google Scholar] [CrossRef]
- Azarashvili, T.; Krestinina, O.; Galvita, A.; Grachev, D.; Baburina, Y.; Stricker, R.; Evtodienko, Y.; Reiser, G. Ca2+-dependent permeability transition regulation in rat brain mitochondria by 2′,3′-cyclic nucleotides and 2′,3′-cyclic nucleotide 3′-phosphodiesterase. Am. J. Physiol. Cell Physiol. 2009, 296, C1428–C1439. [Google Scholar] [CrossRef]
- Lieber, C.S.; DeCarli, L.M. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol. 1989, 24, 197–211. [Google Scholar] [PubMed]
- Rice, J.; Lindsay, J. Subcellular fractionation of mitochondria. In Subcellular Fractionation: A Practical Approach; Graham, J., Rickwood, D., Eds.; IRL: Oxford, UK, 1997. [Google Scholar]
- Reiser, G.; Kunzelmann, U.; Steinhilber, G.; Binmoller, F.J. Generation of a monoclonal antibody against the myelin protein cnp (2′,3′-cyclic nucleotide 3′-phosphodiesterase) suitable for biochemical and for immunohistochemical investigations of cnp. Neurochem. Res. 1994, 19, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Leu, H.B.; Chen, Y.; Chen, Y.H.; Epperson, C.M.; Juang, C.; Wang, P.H. Protein kinase b (pkb/akt1) formed signaling complexes with mitochondrial proteins and prevented glycolytic energy dysfunction in cultured cardiomyocytes during ischemia-reperfusion injury. Endocrinology 2014, 155, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, Z.; Gao, J.; Shi, W.; Li, L.; Jiang, S.; Hu, H.; Liu, Z.; Xu, D.; Wu, L. The key roles of gsk-3beta in regulating mitochondrial activity. Cell. Physiol. Biochem. 2017, 44, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Baburina, Y.; Krestinin, R.; Odinokova, I.; Sotnikova, L.; Kruglov, A.; Krestinina, O. Astaxanthin inhibits mitochondrial permeability transition pore opening in rat heart mitochondria. Antioxidants 2019, 8, 576. [Google Scholar] [CrossRef] [PubMed]
- Baburina, Y.; Odinokova, I.; Azarashvili, T.; Akatov, V.; Sotnikova, L.; Krestinina, O. Possible involvement of 2′,3′-cyclic nucleotide-3′-phosphodiesterase in the protein phosphorylation-mediated regulation of the permeability transition pore. Int. J. Mol. Sci. 2018, 19, 3499. [Google Scholar] [CrossRef]
- Bruha, R.; Dvorak, K.; Petrtyl, J. Alcoholic liver disease. World, J. Hepatol. 2012, 4, 81–90. [Google Scholar] [CrossRef]
- Hoshi, M.; Takashima, A.; Noguchi, K.; Murayama, M.; Sato, M.; Kondo, S.; Saitoh, Y.; Ishiguro, K.; Hoshino, T.; Imahori, K. Regulation of mitochondrial pyruvate dehydrogenase activity by tau protein kinase i/glycogen synthase kinase 3beta in brain. Proc. Natl. Acad. Sci. USA 1996, 93, 2719–2723. [Google Scholar] [CrossRef]
- Xi, J.; Wang, H.; Mueller, R.A.; Norfleet, E.A.; Xu, Z. Mechanism for resveratrol-induced cardioprotection against reperfusion injury involves glycogen synthase kinase 3beta and mitochondrial permeability transition pore. Eur. J. Pharmacol. 2009, 604, 111–116. [Google Scholar] [CrossRef]
- Gandy, J.C.; Melendez-Ferro, M.; Bijur, G.N.; Van Leuven, F.; Roche, J.K.; Lechat, B.; Devijver, H.; Demedts, D.; Perez-Costas, E.; Roberts, R.C. Glycogen synthase kinase-3beta (gsk3beta) expression in a mouse model of alzheimer’s disease: A light and electron microscopy study. Synapse 2013, 67, 313–327. [Google Scholar] [CrossRef]
- Miyamoto, S.; Murphy, A.N.; Brown, J.H. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-ii. Cell Death Differ. 2008, 15, 521–529. [Google Scholar] [CrossRef]
- Cai, W.J.; Chen, Y.; Shi, L.X.; Cheng, H.R.; Banda, I.; Ji, Y.H.; Wang, Y.T.; Li, X.M.; Mao, Y.X.; Zhang, D.F.; et al. Akt-gsk3beta signaling pathway regulates mitochondrial dysfunction-associated opa1 cleavage contributing to osteoblast apoptosis: Preventative effects of hydroxytyrosol. Oxid. Med. Cell. Longev. 2019, 2019, 4101738. [Google Scholar] [CrossRef] [PubMed]
- Doble, B.W.; Woodgett, J.R. Gsk-3: Tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003, 116, 1175–1186. [Google Scholar] [CrossRef]
- Jensen, J.; Brennesvik, E.O.; Lai, Y.C.; Shepherd, P.R. Gsk-3beta regulation in skeletal muscles by adrenaline and insulin: Evidence that pka and pkb regulate different pools of gsk-3. Cell. Signal. 2007, 19, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. Akt/pkb signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fu, H.; Zhang, H.; Xu, F.; Zou, Z.; Liu, M.; Wang, Q.; Miao, M.; Shi, X. Hydrogen sulfide preconditioning protects rat liver against ischemia/reperfusion injury by activating akt-gsk-3beta signaling and inhibiting mitochondrial permeability transition. PLoS ONE 2013, 8, e74422. [Google Scholar]
- Pastorino, J.G.; Hoek, J.B. Ethanol potentiates tumor necrosis factor-alpha cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology 2000, 31, 1141–1152. [Google Scholar] [CrossRef]
- Pastorino, J.G.; Marcineviciute, A.; Cahill, A.; Hoek, J.B. Potentiation by chronic ethanol treatment of the mitochondrial permeability transition. Biochem. Biophys. Res. Commun. 1999, 265, 405–409. [Google Scholar] [CrossRef]
- King, A.L.; Swain, T.M.; Mao, Z.; Udoh, U.S.; Oliva, C.R.; Betancourt, A.M.; Griguer, C.E.; Crowe, D.R.; Lesort, M.; Bailey, S.M. Involvement of the mitochondrial permeability transition pore in chronic ethanol-mediated liver injury in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G265–G277. [Google Scholar] [CrossRef]
- Baines, C.P.; Kaiser, R.A.; Sheiko, T.; Craigen, W.J.; Molkentin, J.D. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 2007, 9, 550–555. [Google Scholar] [CrossRef]
- McEnery, M.W. The mitochondrial benzodiazepine receptor: Evidence for association with the voltage-dependent anion channel (vdac). J. Bioenerg. Biomembr. 1992, 24, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Sileikyte, J.; Blachly-Dyson, E.; Sewell, R.; Carpi, A.; Menabo, R.; Di Lisa, F.; Ricchelli, F.; Bernardi, P.; Forte, M. Regulation of the mitochondrial permeability transition pore by the outer membrane does not involve the peripheral benzodiazepine receptor (translocator protein of 18 kda (tspo)). J. Biol. Chem. 2014, 289, 13769–13781. [Google Scholar] [CrossRef] [PubMed]
- Morin, D.; Musman, J.; Pons, S.; Berdeaux, A.; Ghaleh, B. Mitochondrial translocator protein (tspo): From physiology to cardioprotection. Biochem. Pharmacol. 2016, 105, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Tian, M.; Yin, S.; Lai, S.; Zhou, Y.; Chen, J.; He, M.; Liao, Z. Downregulation of tspo expression inhibits oxidative stress and maintains mitochondrial homeostasis in cardiomyocytes subjected to anoxia/reoxygenation injury. Biomed. Pharmacother. 2020, 121, 109588. [Google Scholar] [CrossRef] [PubMed]
- Zeineh, N.; Nagler, R.; Gabay, M.; Weizman, A.; Gavish, M. Effects of cigarette smoke on tspo-related mitochondrial processes. Cells 2019, 8, 694. [Google Scholar] [CrossRef] [PubMed]
- Bader, S.; Wolf, L.; Milenkovic, V.M.; Gruber, M.; Nothdurfter, C.; Rupprecht, R.; Wetzel, C.H. Differential effects of tspo ligands on mitochondrial function in mouse microglia cells. Psychoneuroendocrinology 2019, 106, 65–76. [Google Scholar] [CrossRef]
- Krestinina, O.; Azarashvili, T.; Baburina, Y.; Galvita, A.; Grachev, D.; Stricker, R.; Reiser, G. In aging, the vulnerability of rat brain mitochondria is enhanced due to reduced level of 2′,3′-cyclic nucleotide-3′-phosphodiesterase (cnp) and subsequently increased permeability transition in brain mitochondria in old animals. Neurochem. Int. 2015, 80, 41–50. [Google Scholar] [CrossRef]
- Krestinina, O.; Baburina, Y.; Krestinin, R.; Odinokova, I.; Fadeeva, I.; Sotnikova, L. Astaxanthin prevents mitochondrial impairment induced by isoproterenol in isolated rat heart mitochondria. Antioxidants 2020, 9, 262. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Nahon-Crystal, E.; Shteinfer-Kuzmine, A.; Gupta, R. Vdac1, mitochondrial dysfunction, and alzheimer’s disease. Pharmacol. Res. 2018, 131, 87–101. [Google Scholar] [CrossRef]
- Reina, S.; Guarino, F.; Magri, A.; De Pinto, V. Vdac3 as a potential marker of mitochondrial status is involved in cancer and pathology. Front. Oncol. 2016, 6, 264. [Google Scholar] [CrossRef]
- Reina, S.; Checchetto, V.; Saletti, R.; Gupta, A.; Chaturvedi, D.; Guardiani, C.; Guarino, F.; Scorciapino, M.A.; Magri, A.; Foti, S.; et al. Vdac3 as a sensor of oxidative state of the intermembrane space of mitochondria: The putative role of cysteine residue modifications. Oncotarget 2016, 7, 2249–2268. [Google Scholar] [CrossRef] [PubMed]
- Krestinina, O.V.; Grachev, D.E.; Odinokova, I.V.; Reiser, G.; Evtodienko, Y.V.; Azarashvili, T.S. Effect of peripheral benzodiazepine receptor (pbr/tspo) ligands on opening of ca2+-induced pore and phosphorylation of 3.5-kda polypeptide in rat brain mitochondria. Biochemistry 2009, 74, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Azarashvili, T.; Krestinina, O.; Baburina, Y.; Odinokova, I.; Akatov, V.; Beletsky, I.; Lemasters, J.; Papadopoulos, V. Effect of the crac peptide, vlnyyvw, on mptp opening in rat brain and liver mitochondria. Int. J. Mol. Sci. 2016, 17, 2096. [Google Scholar] [CrossRef]
- Johnson, K.M.; Cleary, J.; Fierke, C.A.; Opipari, A.W., Jr.; Glick, G.D. Mechanistic basis for therapeutic targeting of the mitochondrial f1f0-atpase. ACS Chem. Biol. 2006, 1, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Baburina, Y.; Odinokova, I.; Azarashvili, T.; Akatov, V.; Lemasters, J.J.; Krestinina, O. 2′,3′-cyclic nucleotide 3′-phosphodiesterase as a messenger of protection of the mitochondrial function during melatonin treatment in aging. Bba-Biomembranes 2017, 1859, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Krestinina, O.; Fadeev, R.; Lomovsky, A.; Baburina, Y.; Kobyakova, M.; Akatov, V. Melatonin can strengthen the effect of retinoic acid in hl-60 cells. Int. J. Mol. Sci. 2018, 19, 2873. [Google Scholar] [CrossRef]
- Black, K.L.; Ikezaki, K.; Santori, E.; Becker, D.P.; Vinters, H.V. Specific high-affinity binding of peripheral benzodiazepine receptor ligands to brain tumors in rat and man. Cancer 1990, 65, 93–97. [Google Scholar] [CrossRef]
- Ferrarese, C.; Appollonio, I.; Frigo, M.; Gaini, S.M.; Piolti, R.; Frattola, L. Benzodiazepine receptors and diazepam-binding inhibitor in human cerebral tumors. Ann. Neurol. 1989, 26, 564–568. [Google Scholar] [CrossRef]
- Ikezaki, K.; Black, K.L.; Santori, E.M.; Smith, M.L.; Becker, D.P.; Payne, B.A.; Toga, A.W. Three-dimensional comparison of peripheral benzodiazepine binding and histological findings in rat brain tumor. Neurosurgery 1990, 27, 78–82. [Google Scholar] [CrossRef]
- Veenman, L.; Gavish, M. Peripheral-type benzodiazepine receptors: Their implication in brain disease. Drug Dev. Res. 2000, 50, 355–370. [Google Scholar] [CrossRef]
- Miyazawa, N.; Diksic, M.; Yamamoto, Y. Chronological study of peripheral benzodiazepine binding sites in the rat brain stab wounds using [3h] pk-11195 as a marker for gliosis. Acta Neurochir. 1995, 137, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Pubill, D.; Verdaguer, E.; Canudas, A.M.; Sureda, F.X.; Escubedo, E.; Camarasa, J.; Pallas, M.; Camins, A. Orphenadrine prevents 3-nitropropionic acid-induced neurotoxicity in vitro and in vivo. Br. J. Pharmacol. 2001, 132, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Leschiner, S.; Spanier, I.; Weisinger, G.; Weizman, A.; Gavish, M. Pk 11195 attenuates kainic acid-induced seizures and alterations in peripheral-type benzodiazepine receptor (pbr) protein components in the rat brain. J. Neurochem. 2002, 80, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Carmel, I.; Fares, F.A.; Leschiner, S.; Scherubl, H.; Weisinger, G.; Gavish, M. Peripheral-type benzodiazepine receptors in the regulation of proliferation of mcf-7 human breast carcinoma cell line. Biochem. Pharmacol. 1999, 58, 273–278. [Google Scholar] [CrossRef]
- Gavish, M.; Bachman, I.; Shoukrun, R.; Katz, Y.; Veenman, L.; Weisinger, G.; Weizman, A. Enigma of the peripheral benzodiazepine receptor. Pharmacol. Rev. 1999, 51, 629–650. [Google Scholar]
- Kelly-Hershkovitz, E.; Weizman, R.; Spanier, I.; Leschiner, S.; Lahav, M.; Weisinger, G.; Gavish, M. Effects of peripheral-type benzodiazepine receptor antisense knockout on ma-10 leydig cell proliferation and steroidogenesis. J. Biol. Chem. 1998, 273, 5478–5483. [Google Scholar] [CrossRef]
- Landau, M.; Weizman, A.; Zoref-Shani, E.; Beery, E.; Wasseman, L.; Landau, O.; Gavish, M.; Brenner, S.; Nordenberg, J. Antiproliferative and differentiating effects of benzodiazepine receptor ligands on b16 melanoma cells. Biochem. Pharmacol. 1998, 56, 1029–1034. [Google Scholar] [CrossRef]







| Control | Ethanol | Control | Ethanol | Control | Ethanol | |
|---|---|---|---|---|---|---|
| Pairs No | Body weight before treatment, g | Body weight before treatment, g | Body weight after treatment, g | Body weight after treatment, g | Liver weight, g | Liver weight, g |
| Pair 1 | 165.0 | 165.0 | 346.0 | 316.0 | 10.3 | 11.7 |
| Pair 2 | 173.0 | 178.0 | 334.0 | 322.0 | 10.6 | 11.4 |
| Pair 3 | 169.0 | 170.0 | 338.0 | 326.0 | 10.9 | 11.8 |
| Pair 4 | 163.0 | 160.0 | 343.0 | 306.0 | 10.4 | 11.4 |
| mean | 167.5 ± 4.4 | 168.2 ± 7.7 | 340.2 ± 5.3 | 317.5 ± 8.7 | 10.5 ± 0.3 | 11.6 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baburina, Y.; Odinokova, I.; Krestinina, O. The Effects of PK11195 and Protoporphyrin IX Can Modulate Chronic Alcohol Intoxication in Rat Liver Mitochondria under the Opening of the Mitochondrial Permeability Transition Pore. Cells 2020, 9, 1774. https://doi.org/10.3390/cells9081774
Baburina Y, Odinokova I, Krestinina O. The Effects of PK11195 and Protoporphyrin IX Can Modulate Chronic Alcohol Intoxication in Rat Liver Mitochondria under the Opening of the Mitochondrial Permeability Transition Pore. Cells. 2020; 9(8):1774. https://doi.org/10.3390/cells9081774
Chicago/Turabian StyleBaburina, Yulia, Irina Odinokova, and Olga Krestinina. 2020. "The Effects of PK11195 and Protoporphyrin IX Can Modulate Chronic Alcohol Intoxication in Rat Liver Mitochondria under the Opening of the Mitochondrial Permeability Transition Pore" Cells 9, no. 8: 1774. https://doi.org/10.3390/cells9081774
APA StyleBaburina, Y., Odinokova, I., & Krestinina, O. (2020). The Effects of PK11195 and Protoporphyrin IX Can Modulate Chronic Alcohol Intoxication in Rat Liver Mitochondria under the Opening of the Mitochondrial Permeability Transition Pore. Cells, 9(8), 1774. https://doi.org/10.3390/cells9081774




