The Role of Cutinsomes in Plant Cuticle Formation
Abstract
:1. Introduction
2. Cutinsomes of Different Plant Species
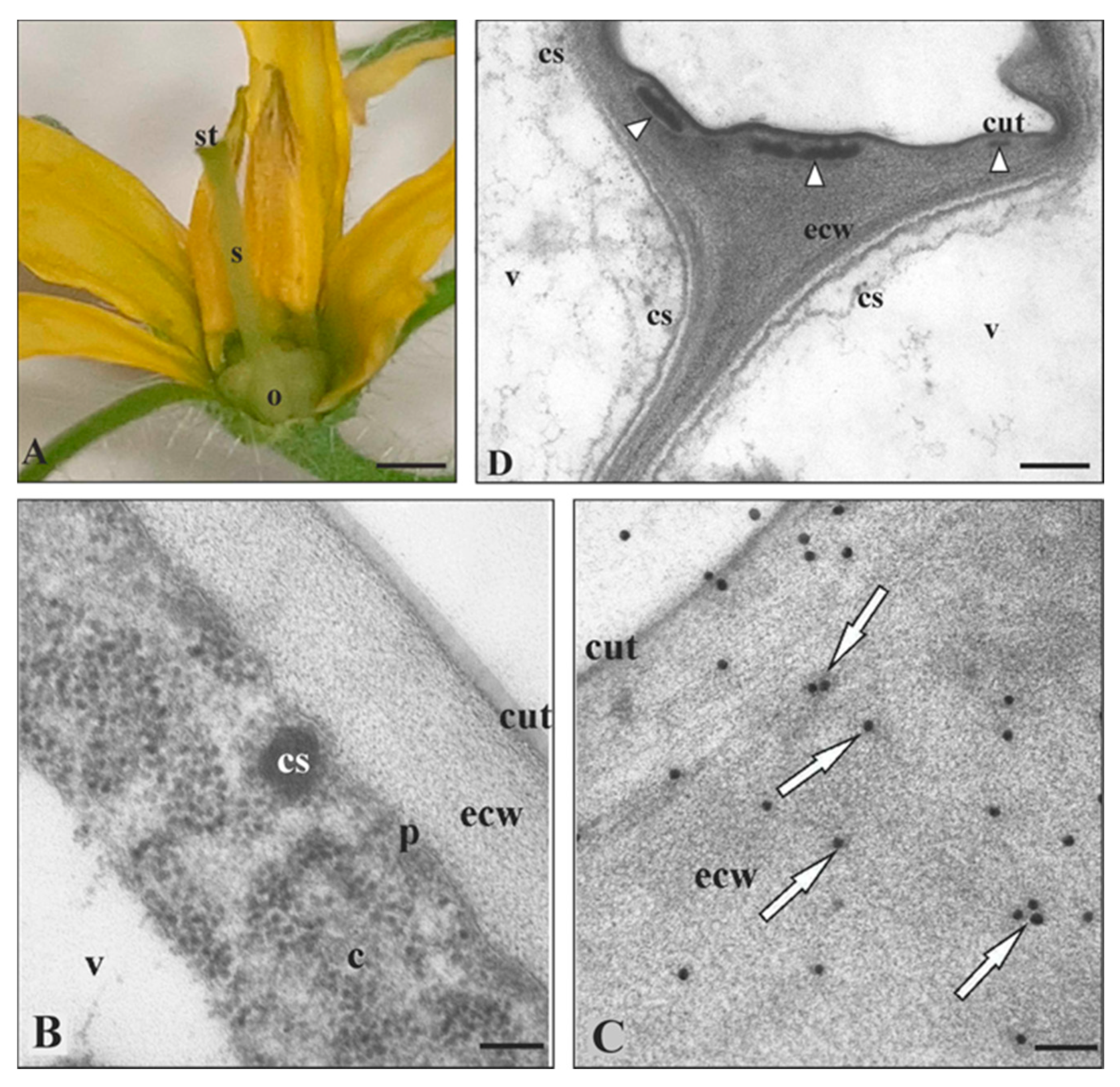
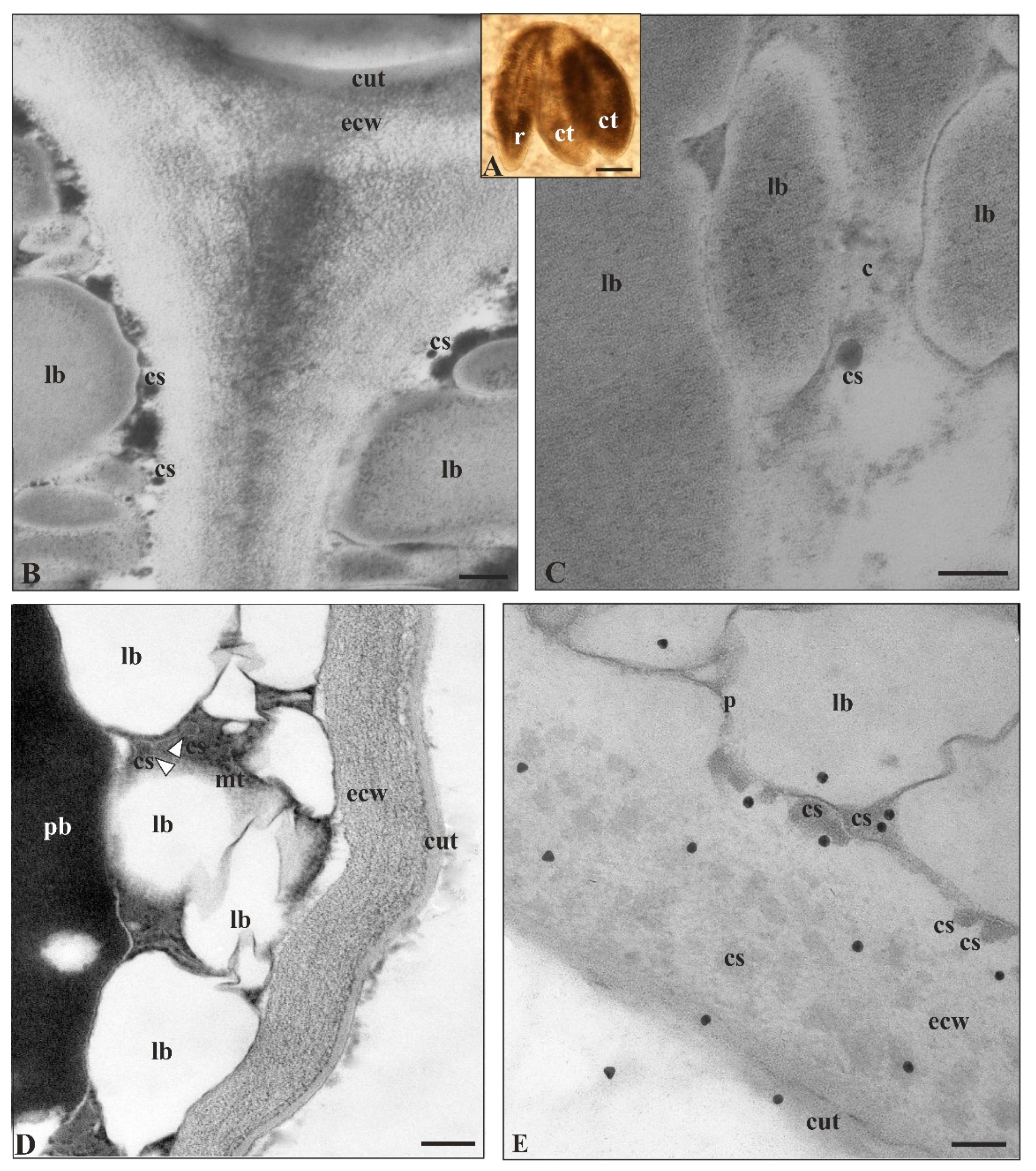
2.1. Solanum Lycopersicum Cutinsomes
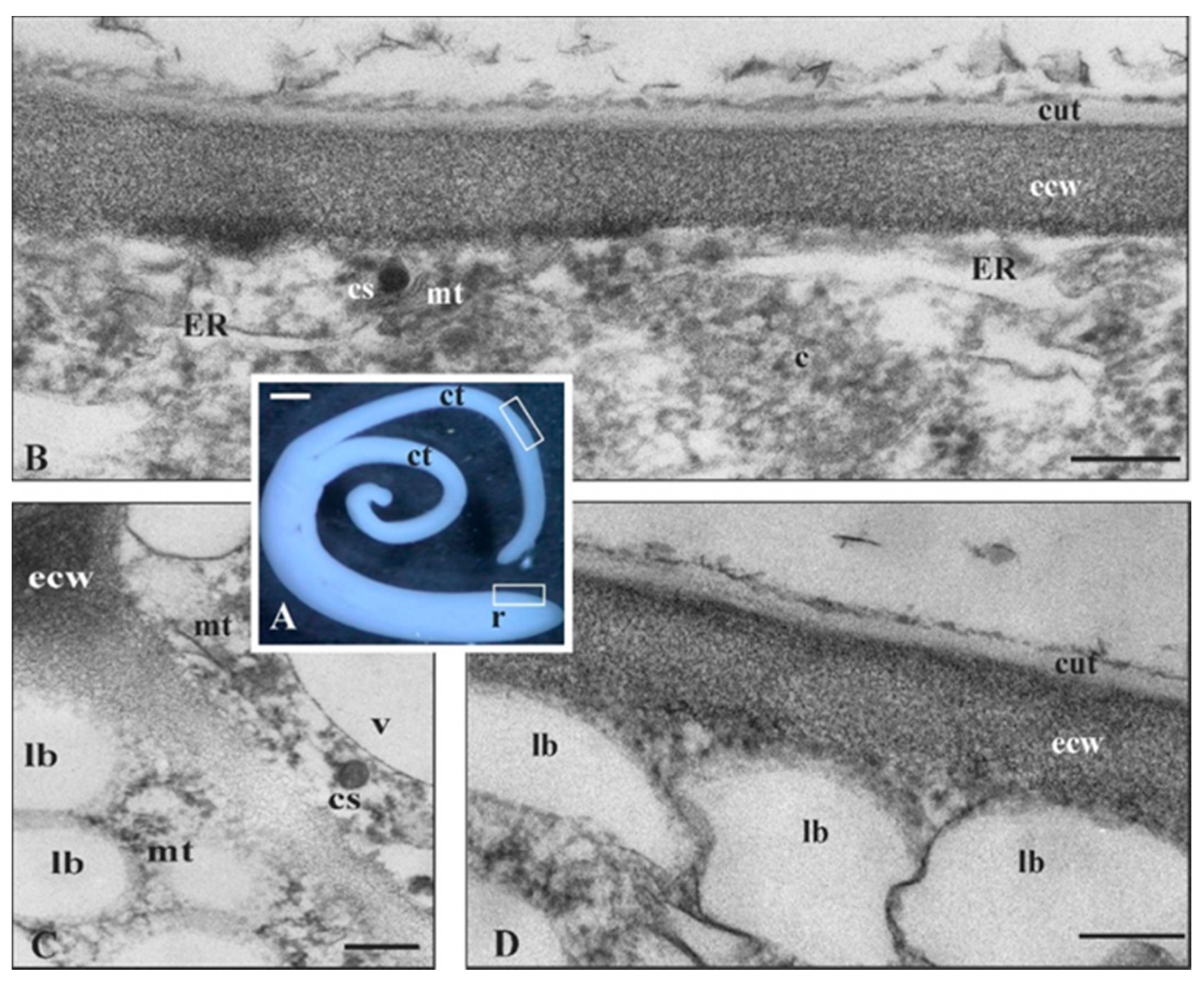
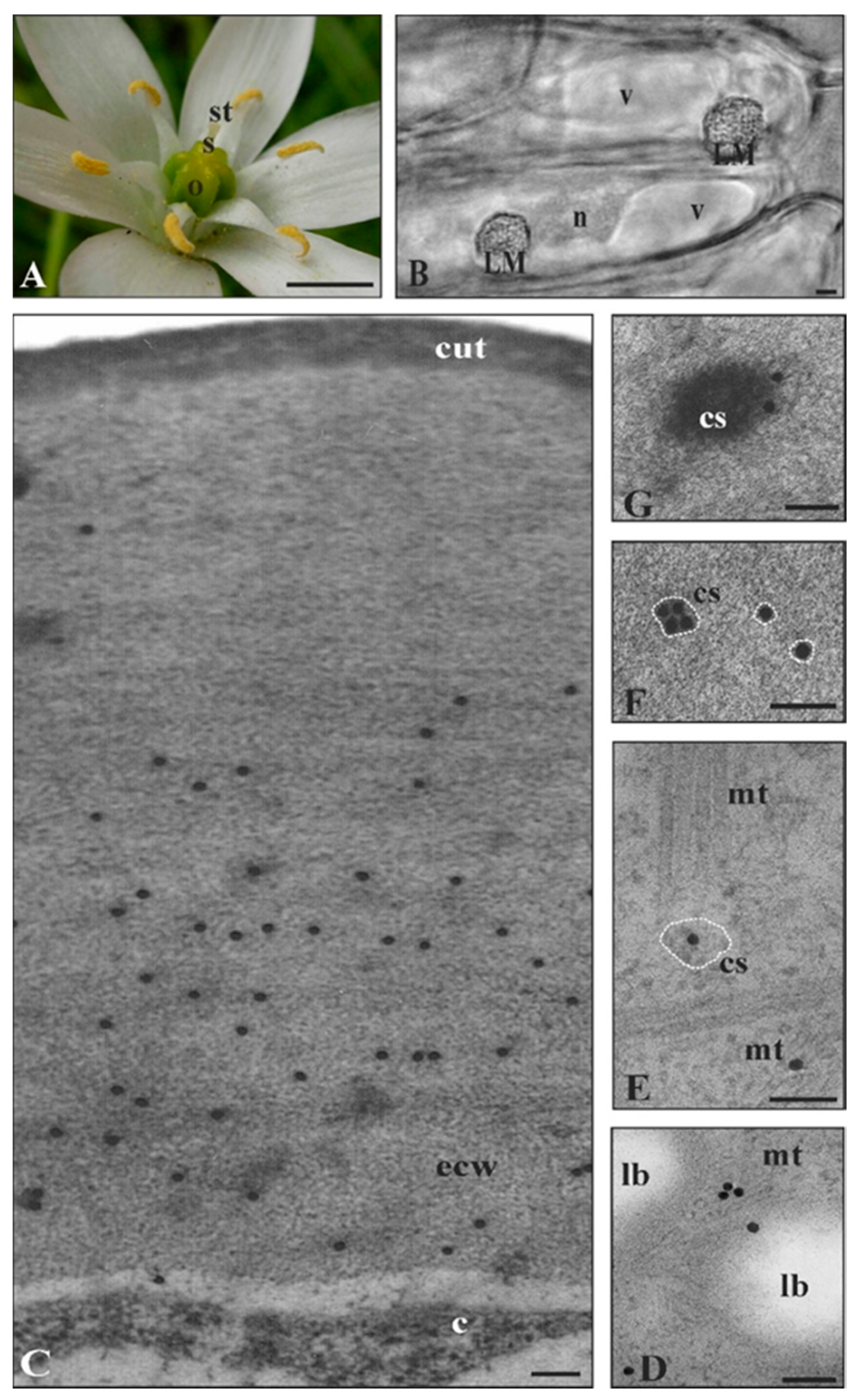
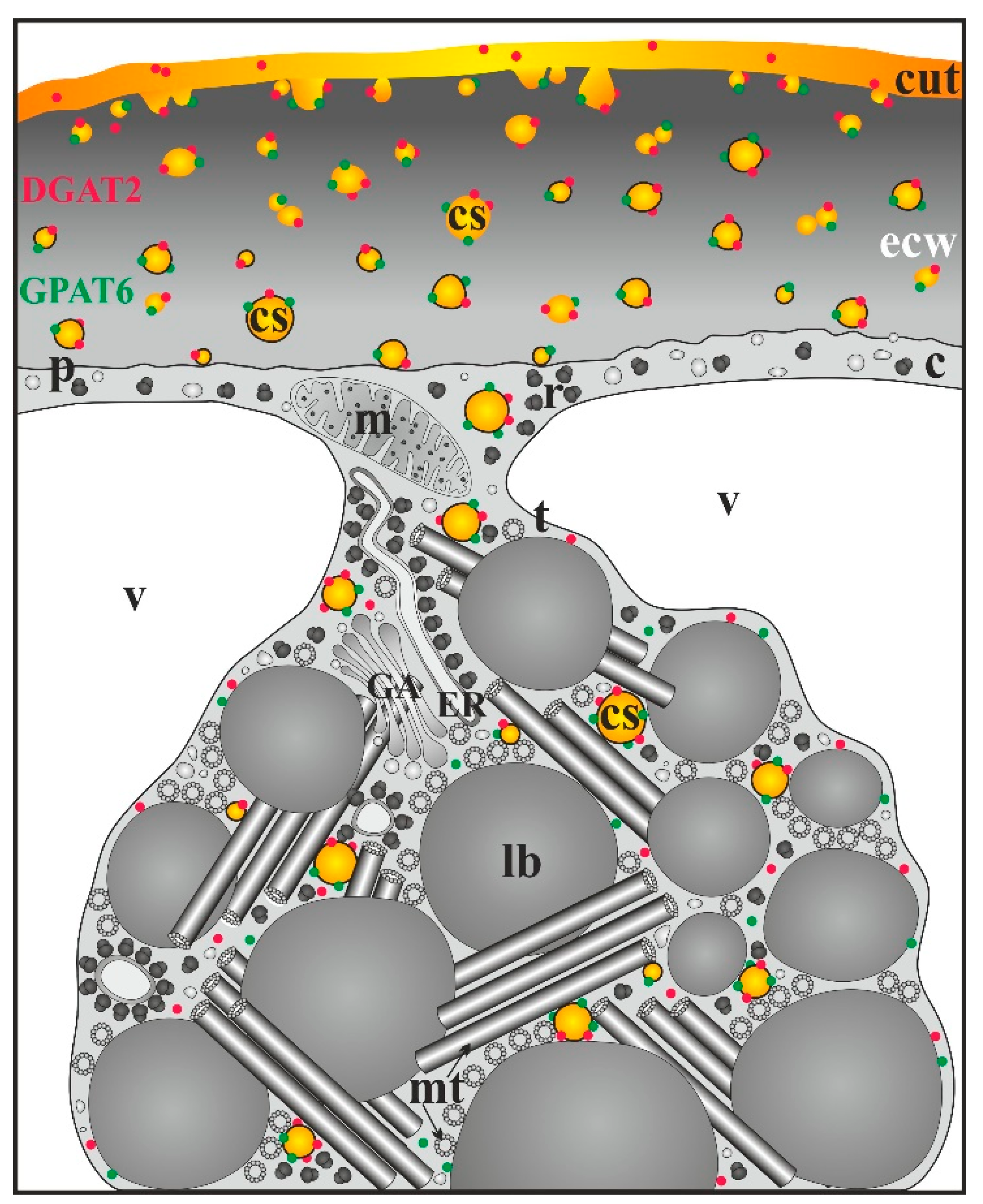
2.2. Ornithogalum Umbellatum Cutinsomes
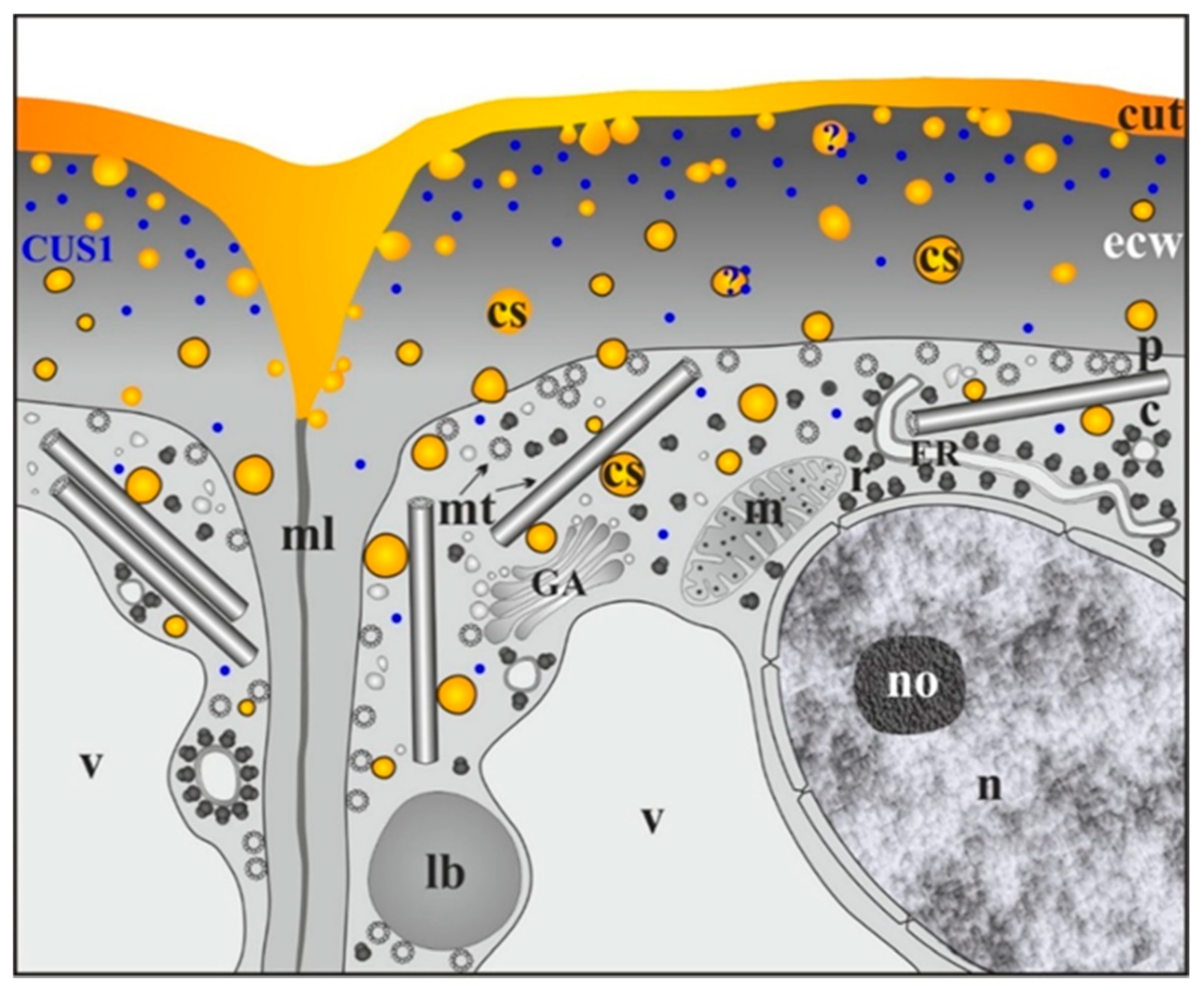
2.3. Arabidopsis Thaliana Cutinsomes
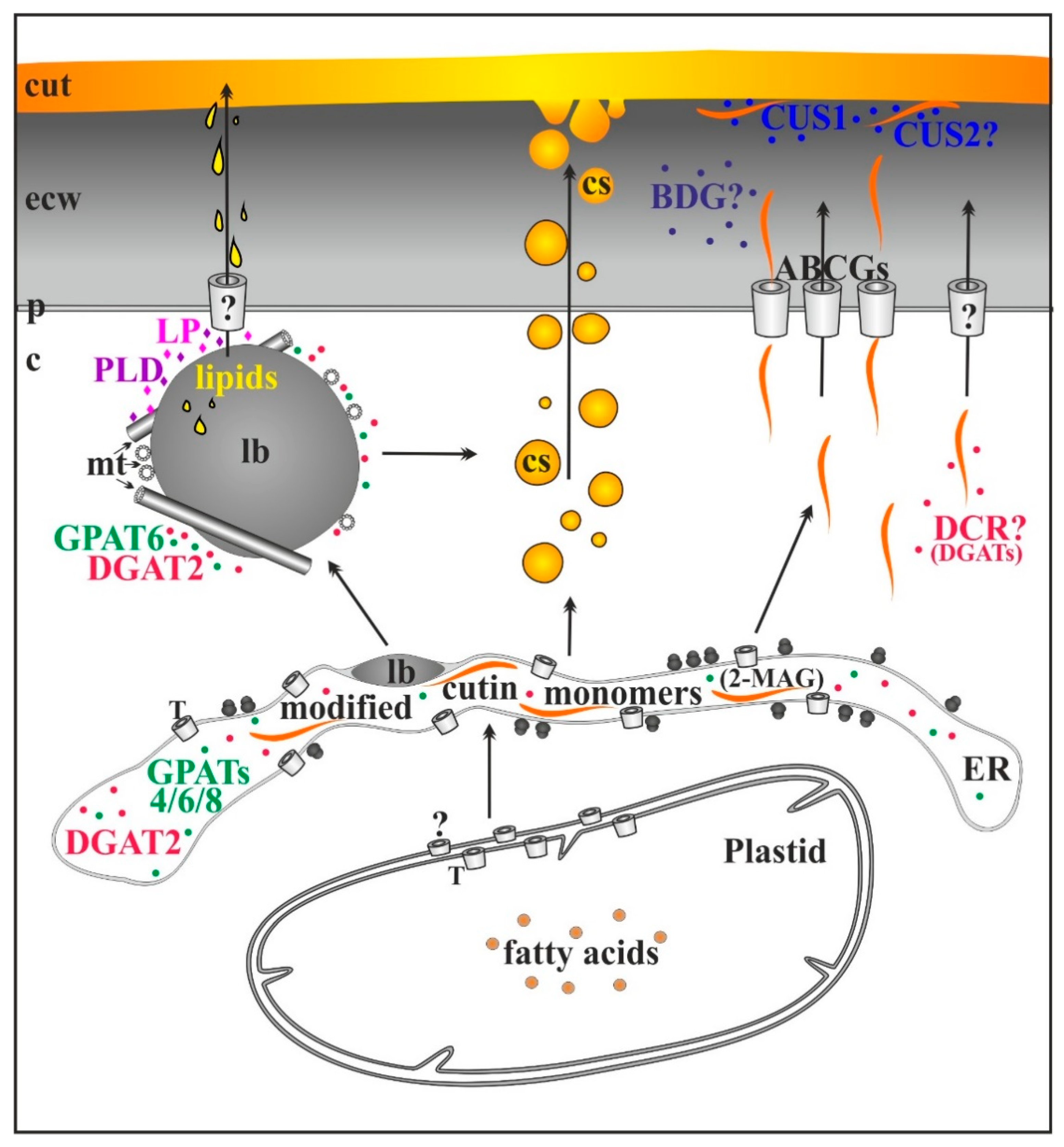
3. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Koch, K.; Barthlott, W. Superhydrophobic and superhydrophilic plant surfaces: An inspiration for biomimetic materials. Philos. Trans. R. Soc. A 2009, 367, 1487–1509. [Google Scholar] [CrossRef]
- Yeats, T.H.; Rose, J.K. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthlott, W.; Mail, M.; Bhushan, B.; Koch, K. Plant surfaces: Structures and functions for biomimetic innovations. Nanomicro Lett. 2017, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domίnguez, E.; Cuartero, J.; Heredia, A. An overview on plant cuticle biomechanics. Plant Sci. 2011, 181, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Khanal, B.P.; Knoche, M. Mechanical properties of cuticles and their primary determinants. J. Exp. Bot. 2017, 68, 5351–5367. [Google Scholar] [CrossRef]
- Lara, I.; Heredia, A.; Domínguez, E. Shelf life potential and the fruit cuticle: The unexpected player. Front. Plant Sci. 2019, 10, 770. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, P.; Akinmoju, T.A.; Nimmakayala, P.; Lopez-Ortiz, C.; Garcia-Lozano, M.; Thompson, B.J.; John Stommel, J.; Reddy, U.K. Integrated metabolomic and transcriptomic analysis to characterize cutin biosynthesis between low- and high-cutin genotypes of Capsicum chinense Jacq. Int. J. Mol. Sci. 2020, 21, 1397. [Google Scholar] [CrossRef] [Green Version]
- Özgen, M.; Palta, J.P.; Smith, J.D. Ripeness stage at harvest influences postharvest life of cranberry fruit: Physiological and anatomical explanations. Postharvest Biol. Technol. 2002, 24, 291–299. [Google Scholar] [CrossRef]
- Ginzberg, I.; Fogelman, E.; Rosenthal, L.; Stern, R.A. Maintenance of high epidermal cell density and reduced calyx-end cracking in developing ‘Pink Lady’ apples treated with a combination of cytokinin 6-benzyladenine and gibberellins A4CA7. Sci. Hortic. 2014, 165, 324–330. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Sweet cherry fruit cracking mechanisms and prevention strategies: A review. Sci. Hortic. 2018, 240, 369–377. [Google Scholar] [CrossRef]
- Jiang, F.; López, A.; Jeon, S.; Tonetto de Freitas, S.; Yu, Q.; Wu, Z.; Labavitch, J.M.; Tian, S.; Powell, A.L.T.; Mitcham, E. Disassembly of the fruit cell wall by the ripening-associated polygalacturonase and expansin influences tomato cracking. Hortic. Res. 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, M.; Coluccia, F.; Torres, M.; L’Haridon, F.; Métraux, J.-P. The cuticle and plant defense to pathogens. Front. Plant Sci. 2014, 5, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gur, L.; Reuveni, M.; Cohen, Y. Occurrence and etiology of Alternaria leaf blotch and fruit spot of apple caused by Alternaria alternata f. sp. mali on cv. Pink lady in Israel. Eur. J. Plant Pathol. 2017, 147, 695–708. [Google Scholar] [CrossRef]
- Ziv, C.; Zhao, Z.; Gao, Y.G.; Xia, Y. Multifunctional roles of plant cuticle during plant-pathogen interactions. Front. Plant Sci. 2018, 9, 1088. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef] [Green Version]
- Domίnguez, E.; Heredia-Guerrero, J.A.; Heredia, A. Plant cutin genesis: Unanswered question. Trends Plant Sci. 2015, 20, 551–557. [Google Scholar] [CrossRef]
- Fernández, V.; Guzmán-Delgado, P.; Graça, J.; Santos, S.; Gil, L. Cuticle structure in relation to chemical composition: Re-assessing the prevailing model. Front. Plant Sci. 2016, 7, 427. [Google Scholar] [CrossRef] [Green Version]
- Fich, E.A.; Segerson, N.A.; Rose, J.K.C. The plant polyester cutin: Biosynthesis, structure, and biological roles. Annu. Rev. Plant Biol. 2016, 67, 207–233. [Google Scholar] [CrossRef]
- Fernández-Moreno, J.-P.; Levy-Samoha, D.; Malitsky, S.; Monforte, A.J.; Orzaez, D.; Aharoni, A.; Granell, A. Uncovering tomato quantitative trait loci and candidate genes for fruit cuticular lipid composition using the Solanum pennellii introgression line population. J. Exp. Bot. 2017, 68, 2703–2716. [Google Scholar] [CrossRef] [Green Version]
- Heredia-Guerrero, J.A.; Heredia, A.; Domínguez, E.; Cingolani, R.; Bayer, I.S.; Athanassiou, A.; Benítez, J.J. Cutin from agro-waste as a raw material for the production of bioplastics. J. Exp. Bot. 2017, 68, 5401–5410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, A.; Joubès, J. Arabidopsis cuticular waxes: Advances in synthesis, export and regulation. Prog. Lipid Res. 2013, 52, 110–129. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.; Bres, C.; Mauxion, J.-P.; Bakan, B.; Rothan, C. Breeding for cuticle-associated traits in crop species: Traits, targets, and strategies. J. Exp. Bot. 2017, 68, 5369–5387. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Creff, A.; Waters, A.; Tanaka, H.; Goodrich, J.; Ingram, G.C. ZHOUPI controls embryonic cuticle formation via a signalling pathway involving the subtilisin protease ABNORMAL LEAF-SHAPE1 and the receptor kinases GASSHO1 and GASSHO2. Development 2013, 140, 770–779. [Google Scholar] [CrossRef] [Green Version]
- Hen-Avivi, S.; Lashbrooke, J.; Costa, F.; Aharoni, A. Scratching the surface: Genetic regulation of cuticle assembly in fleshy fruit. J. Exp. Bot. 2014, 65, 4653–4664. [Google Scholar] [CrossRef] [Green Version]
- Denay, G.; Creff, A.; Moussu, S.; Wagnon, P.; Thévenin, J.; Gérentes, M.F.; Chambrier, P.; Dubreucq, B.; Ingram, G. Endosperm breakdown in Arabidopsis requires heterodimers of the basic helix–loop–helix proteins ZHOUPI and INDUCER OF CBP EXPRESSION 1. Development 2014, 141, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Nadakuduti, S.S.; Pollard, M.; Kosma, D.K.; Allen, C.; Ohlrogge, J.B.; Barry, C.S. Pleiotropic phenotypes of the sticky peel mutant provide new insight into the role of cutin deficient2 in epidermal cell function in tomato. Plant Physiol. 2012, 159, 945–960. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.; Li, L.; Xie, L.; Chen, M.; Shen, Q.; Pan, Q.; Fu, X.; Shi, P.; Tang, Y.; Huang, H.; et al. A novel HD-ZIP IV/MIXTA complex promotes glandular trichome initiation and cuticle development in Artemisia annua. New Phytol. 2018, 218, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Bessire, M.; Borel, S.; Fabre, G.; Carrac, L.; Efremova, N.; Yephremov, A.; Cao, Y.; Jetter, R.; Jacquat, A.-C.; Me’traux, J.-P.; et al. A member of the PLEIOTROPIC DRUG RESISTANCE family of ATP binding cassette transporters is required for the formation of a functional cuticle in Arabidopsis. Plant Cell 2011, 23, 1958–1970. [Google Scholar] [CrossRef] [Green Version]
- Stępiński, D.; Kwiatkowska, M.; Popłońska, K.; Polit, J.T.; Wojtczak, A.; Domínguez, E.; Heredia, A. Cutinsomes and cuticle enzymes GPAT6 and DGAT2 seem to travel together from lipotubuloid metabolon (LM) to the extracellular matrix of Ornithogalum umbellatum ovary epidermis. Micron 2016, 85, 51–57. [Google Scholar]
- Stępiński, D.; Kwiatkowska, M.; Wojtczak, A.; Domínguez, E.; Heredia, A.; Popłońska, K. Cutinsomes as building-blocks of Arabidopsis thaliana embryo cuticle. Physiol. Plant. 2017, 161, 560–567. [Google Scholar] [CrossRef] [PubMed]
- DeBono, A.; Yeats, T.H.; Rose, J.K.C.; Bird, D.; Jetter, R.; Kunst, L.; Samuels, L. Arabidopsis LTPG is a glycosylphosphatidylinositol-anchored lipid transfer protein required for export of lipids to the plant surface. Plant Cell 2009, 21, 1230–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.B.; Go, Y.S.; Bae, H.J.; Park, J.H.; Cho, S.H.; Cho, H.J.; Lee, D.S.; Park, O.K.; Hwang, I.; Suh, M.C. Disruption of glycosylphosphatidylinositol-anchored lipid transfer protein gene altered cuticular lipid composition, increased plastoglobules, and enhanced susceptibility to infection by the fungal pathogen Alternaria brassicicola. Plant Physiol. 2009, 150, 42–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Lee, S.B.; Kim, H.J.; Min, M.K.; Hwang, I.; Suh, M.C. Characterization of glycosylphosphatidylinositol-anchored lipid transfer protein 2 (LTPG2) and overlapping function between LTPG/LTPG1 and LTPG2 in cuticular wax export or accumulation in Arabidopsis thaliana. Plant Cell Physiol. 2012, 53, 1391–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffree, C.E. The fine structure of the plant cuticle. In Biology of the Plant Cuticle, 1st ed.; Riederer, M., Müller, C., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 11–125. [Google Scholar]
- Javelle, M.; Vernoud, V.; Rogowsky, P.M.; Ingram, G.C. Epidermis: The formation and functions of a fundamental plant tissue. New Phytol. 2011, 189, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Bakan, B.; Marion, M. Assembly of cutin polyester: From cells to extracellular cell walls. Plants 2017, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Philippe, G.; Sørensen, I.; Jiao, C.; Sun, X.; Fei, Z.; Domozych, D.S.; Rose, J.K. Cutin and suberin: Assembly and origins of specialized lipidic cell wall scaffolds. Curr. Opin. Plant Biol. 2020, 55, 11–20. [Google Scholar] [CrossRef]
- Li, N.; Xu, C.; Li-Beisson, Y.; Philippa, K. Fatty acid and lipid transport in plant cells. Trends Plant Sci. 2016, 21, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Manan, S.; Chen, B.; She, G.; Wan, X.; Zhao, J. Transport and transcriptional regulation of oil production in plants. Crit. Rev. Biotechnol. 2017, 37, 641–655. [Google Scholar] [CrossRef]
- Ohsaki, Y.; Cheng, J.; Suzuki, M.; Shinohara, Y.; Fujita, A.; Fujimoto, T. Biogenesis of cytoplasmic lipid droplets: From the lipid ester globule in the membrane to the visible structure. Biochim. Biophys. Acta 2009, 1791, 399–407. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Popłońska, K.; Wojtczak, A.; Stępiński, D.; Polit, J.T. Lipid body biogenesis and the role of microtubules in lipid synthesis in Ornithogalum umbellatum lipotubuloids. Cell Biol. Int. 2012, 36, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, M.; Popłońska, K.; Stępiński, D.; Wojtczak, A.; Polit, J.T.; Paszak, K. Lipotubuloids-structure and function. In Advances in Selected Plant Physiology Aspects, 1st ed.; Montanaro, G., Dichio, B., Eds.; InTech: Rijeka, Croatia, 2012; pp. 365–388. [Google Scholar]
- Kwiatkowska, M.; Polit, J.T.; Stępiński, D.; Popłońska, K.; Wojtczak, A.; Domίnguez, E.; Heredia, A. Lipotubuloids in ovary epidermis of Ornithogalum umbellatum act as metabolons: Suggestion of the name ‘lipotubuloid metabolon’. J. Exp. Bot. 2015, 66, 1157–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiatkowska, M.; Popłońska, K.; Polit, J.T.; Wojtczak, A.; Stępiński, D.; Paszak, K. Lipid bodies in lipotubuloids of Ornithogalum umbellatum ovary epidermis contain diacylglycerol acyltransferase 2 (DGAT2) and lipase, incorporate 3H-palmitic acid and are connected with cuticle synthesis. Trends Cell Mol. Biol. 2011, 6, 97–108. [Google Scholar]
- Gómez-Patiño, M.B.; Cassani, J.; Jaramillo-Flores, M.E.; Zepeda-Vallejo, L.G.; Sandoval, G.; Jimenez-Estrada, M.; Arrieta-Baez, D. Oligomerization of 10,16-dihydroxyhexadecanoic acid and methyl 10,16-dihydroxyhexadecanoate catalyzed by lipases. Molecules 2013, 18, 9317–9333. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowska, M.; Stępiński, D.; Popłońska, K.; Wojtczak, A.; Polit, J.T. DGAT2 revealed by the immunogold technique in Arabidopsis thaliana lipid bodies associated with microtubules. Folia Histochem. Cytochem. 2012, 50, 427–431. [Google Scholar] [CrossRef]
- Philippe, G.; Gaillard, C.; Petit, J.; Geneix, N.; Dalgalarrondo, M.; Bres, C.; Mauxion, J.-P.; Franke, R.; Rothan, C.; Schreiber, L.; et al. Ester cross-link profiling of the cutin polymer of wild-type and cutin synthase tomato mutants highlights different mechanisms of polymerization. Plant Physiol. 2016, 170, 807–820. [Google Scholar] [CrossRef] [Green Version]
- Panikashvili, D.; Shi, J.X.; Schreiber, L.; Aharoni, A. The Arabidopsis DCR encoding a soluble BAHD acyltransferase is required for cutin polyester formation and seed hydration properties. Plant Physiol. 2009, 151, 1773–1789. [Google Scholar] [CrossRef] [Green Version]
- Rani, S.H.; Krishna, T.H.A.; Saha, S.; Negi, A.S.; Rajasekharan, R. Defective in cuticular ridges (DCR) of Arabidopsis thaliana, a gene associated with surface cutin formation, encodes a soluble diacylglycerol acyltransferase. J. Biol. Chem. 2010, 285, 38337–38347. [Google Scholar] [CrossRef] [Green Version]
- Kurdyukov, S.; Faust, A.; Nawrath, C.; Bär, S.; Voisin, D.; Efremova, N.; Franke, R.; Schreiber, L.; Saedler, H.; Métraux, J.-P.; et al. The epidermis-specific extracellular BODYGUARD controls cuticle development and morphogenesis in Arabidopsis. Plant Cell 2006, 18, 321–339. [Google Scholar] [CrossRef] [Green Version]
- Jakobson, L.; Lindgren, L.O.; Verdier, G.; Laanemets, K.; Brosché, M.; Beisson, F.; Kollist, H. BODYGUARD is required for the biosynthesis of cutin in Arabidopsis. New Phytol. 2016, 211, 614–626. [Google Scholar] [CrossRef] [Green Version]
- Girard, A.-L.; Mounet, F.; Lemaire-Chamley, M.; Gaillard, C.; Elmorjani, K.; Vivancos, J.; Runavot, J.L.; Quemener, B.; Petit, J.; Germain, V.; et al. Tomato GDSL1 is required for cutin deposition in the fruit cuticle. Plant Cell 2012, 24, 3119–3134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeats, T.H.; Martin, L.B.B.; Viart, H.M.-F.; Isaacson, T.; He, Y.; Zhao, L.; Matas, A.J.; Buda, G.J.; Domozych, D.S.; Clausen, M.H.; et al. The identification of cutin synthase: Formation of the plant polyester cutin. Nat. Chem. Biol. 2012, 8, 609–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeats, T.H.; Huang, W.; Chatterjee, S.; Viart, H.M.-F.; Clausen, M.H.; Stark, R.E.; Rose, J.K.C. Tomato Cutin Deficient 1 (CD1) and putative orthologs comprise an ancient family of cutin synthase-like (CUS) proteins that are conserved among land plants. Plant J. 2014, 77, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Segado, P.; Heredia-Guerrero, J.A.; Heredia, A.; Domínguez, E. Cutinsomes and CUTIN SYNTHASE1 function sequentially in tomato fruit cutin deposition in fruits. Plant Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.; Bres, C.; Just, D.; Garcia, V.; Mauxion, J.P.; Marion, D.; Bakan, B.; Joubes, J.; Domergue, F.; Rothan, C. Analyses of tomato fruit brightness mutants uncover both cutin-deficient and cutin-abundant mutants and a new hypomorphic allele of GDSL-lipase. Plant Physiol. 2014, 164, 888–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, L.; Brown, J.; Segerson, N.A.; Rose, J.K.C.; Roeder, A.H.K. CUTIN SYNTHASE 2 maintains progressively developing cuticular ridges in Arabidopsis sepals. Mol. Plant. 2017, 10, 560–574. [Google Scholar] [CrossRef] [Green Version]
- Riederer, M.; Schönherr, J. Development of plant cuticles fine structure and cutin composition of Clivia miniata Reg. leaves. Planta 1988, 174, 127–138. [Google Scholar] [CrossRef]
- Heide-Jørgensen, H.S. Cuticle development and ultrastructure—evidence for a procuticle of high osmium affinity. Planta 1991, 183, 511–519. [Google Scholar] [CrossRef]
- Cook, M.E.; Graham, L.E. Structural similarities between surface layers of selected Charophycean algae and bryophytes and the cuticles of vascular plants. Int. J Plant Sci. 1998, 159, 780–787. [Google Scholar] [CrossRef]
- Benίtez, J.J.; Garcίa-Seguraq, R.; Heredia, A. Plant biopolyester cutin: A tough way to its chemical synthesis. Biochim. Biophys. Acta 2004, 1674, 1–3. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benίtez, J.J.; Heredia, A. Self-assembled polyhydroxy fatty acids: A mechanism for plant cutin synthesis. BioEssays 2008, 30, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Guerrero, J.A.; Domίnguez, E.; Luna, M.; Benίtez, J.J.; Heredia, A. Structural characterization of polyhydroxy fatty acid nanoparticles related to plant lipid biopolyesters. Chem. Phys. Lipids 2010, 163, 329–333. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; San-Miguel, M.A.; Luna, M.; Domίnguez, E.; Heredia, A.; Benίtez, J.J. Structure and support induced structure disruption of soft nanoparticles obtained from hydroxylated fatty acids. Soft Matter 2011, 7, 4357–4363. [Google Scholar] [CrossRef] [Green Version]
- Domίnguez, E.; Heredia-Guerrero, J.A.; Benίtez, J.J.; Heredia, A. Self-assembly of supramolecular lipid nanoparticles in the formation of plant biopolyester cutin. Mol. BioSyst. 2010, 6, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Domίnguez, E.; Heredia-Guerrero, J.A.; Heredia, A. The biophysical design of plant cuticles: An overview. New Phytol. 2011, 189, 938–984. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Puyol, S.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A.; Heredia-Guerrero, J.A. Pectin-lipid self-assembly: Influence on the formation of polyhydroxy fatty acids nanoparticles. PLoS ONE 2015, 10, e0124639. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Wojtczak, A.; Popłońska, K.; Polit, J.T.; Stępiński, D.; Domίnguez, E.; Heredia, A. Cutinsomes and lipotubuloids appear to participate in cuticle formation in Ornithogalum umbellatum ovary epidermis: EM-immunogold research. Protoplasma 2014, 251, 1151–1161. [Google Scholar] [CrossRef] [Green Version]
- Segado, P.; Domínguez, E.; Heredia, A. Ultrastructure of the epidermal cell wall and cuticle of tomato fruit (Solanum lycopersicum L.) during development. Plant Physiol. 2016, 70, 935–946. [Google Scholar] [CrossRef] [Green Version]
- Pollard, M.; Beisson, F.; Li, Y.H.; Ohlrogge, J.B. Building lipid barriers: Biosynthesis of cutin and suberin. Trends Plant Sci. 2008, 13, 236–246. [Google Scholar] [CrossRef]
- Gerard, H.C.; Osman, S.F.; Fett, W.F.; Moreau, R.A. Separation, identification and quantification of monomers from cutin polymers by high-performance liquid chromatography and evaporative light-scattering detection. Phytochem. Anal. 1992, 3, 139–144. [Google Scholar] [CrossRef]
- Berhin, A.; de Bellis, D.; Franke, R.B.; Buono, R.A.; Nowack, M.K.; Nawrath, C. The root cap cuticle: A cell wall structure for seedling establishment and lateral root formation. Cell 2019, 176, 1367–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiatkowska, M.; Polit, J.T.; Popłońska, K.; Stępiński, D.; Wojtczak, A. Immunogold metod evidences that kinesin and myosin bind to and coupe microtubules and actin filaments in lipotubuloids of Ornithogalum umbellatum ovary epidermis. Acta Physiol. Plant. 2013, 35, 1967–1977. [Google Scholar] [CrossRef] [Green Version]
- Yeats, T.H.; Rose, J.K.C. The biochemistry and biology of extracellular plant lipid-transfer proteins (LTPs). Protein Sci. 2008, 17, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shumborski, S.J.; Samuels, A.L.; Bird, D.A. Fine structure of the Arabidopsis stem cuticle: Effects of fixation and changes over development. Planta 2016, 244, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, S.; Garroum, I.; Daraspe, J.; De Bellis, D.; Olsson, V.; Mucciolo, A.; Butenko, M.A.; Humbel, B.M.; Nawrath, C. Connecting the molecular structure of cutin to ultrastructure and physical properties of the cuticle in petals of Arabidopsis. Plant Physiol. 2017, 173, 1146–1163. [Google Scholar] [CrossRef] [Green Version]
- Franke, R.; Briesen, I.; Wojciechowski, T.; Faust, A.; Yephremov, A.; Nawrath, C.; Schreiber, L. Apoplastic polyesters in Arabidopsis surface tissues—A typical suberin and particular cutin. Phytochemistry 2005, 66, 2643–2658. [Google Scholar] [CrossRef] [Green Version]
- Shao, S.; Meyer, C.J.; Ma, F.; Peterson, C.A.; Bernards, M.A. The outermost cuticle of soybean seeds: Chemical composition and function during imbibition. J. Exp. Bot. 2007, 58, 1071–1082. [Google Scholar] [CrossRef] [Green Version]
- Ingram, G.; Nawrath, C. The roles of the cuticle in plant development: Organ adhesions and beyond. J. Exp. Bot. 2017, 68, 5307–5321. [Google Scholar] [CrossRef]
- Szczuka, E.; Szczuka, A. Cuticle fluorescence during embryogenesis of Arabidopsis thaliana (L.) Heynh. Acta Biol. Cracov. Ser. Bot. 2003, 45, 63–67. [Google Scholar]
- De Giorgi, J.; Piskurewicz, U.; Loubery, S.; Utz-Pugin, A.; Bailly, C.; Mène-Saffrané, L.; Lopez-Molina, L. An endosperm-associated cuticle is required for Arabidopsis seed viability, dormancy and early control of germination. PLoS Genet. 2015, 11, e1005708. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Johnston, N.; Talideh, E.; Mitchell, S.; Jeffree, C.; Goodrich, J.; Ingram, G. The endosperm-specific ZHOUPI gene of Arabidopsis thaliana regulates endosperm breakdown and embryonic epidermal development. Development 2008, 135, 3501–3509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, S.E.; Verhertbruggen, Y.; Hervé, C.; Ordaz-Ortiz, J.J.; Farkas, V.; Pedersen, H.L.; Willats, W.G.; Knox, J.P. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 2008, 8, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán, P.; Fernández, V.; García, M.L.; Khayet, M.; Fernández, A.; Gil, L. Localization of polysaccharides in isolated and intact cuticles of eucalypt, poplar and pear leaves by enzyme-gold labelling. Plant Physiol. Biochem. 2014, 76, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán, P.; Fernández, V.; Graça, J.; Cabral, V.; Kayali, N.; Khayet, M.; Gil, L. Chemical and structural analysis of Eucalyptus globulus and E. camaldulensis leaf cuticles: A lipidized cell wall region. Front. Plant Sci. 2014, 5, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loubéry, S.; De Giorgi, J.; Utz-Pugin, A.; Demonsais, L.; Lopez-Molina, L. A maternally deposited endosperm cuticle contributes to the physiological defects of transparent testa seeds. Plant Physiol. 2018, 177, 1218–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mérida, T.; Schönherr, J.; Schmidt, H.W. Fine structure of plant cuticles in relation to water permeability: The fine structure of the cuticle of Clivia miniata Reg. leaves. Planta 1981, 152, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Stpiczyńska, M.; Kamińska, M.; Davies, K.L.; Pansarin, E.R. Nectar-secreting and nectarless Epidendrum: Structure of the inner floral spur. Front. Plant Sci. 2018, 9, 840. [Google Scholar] [CrossRef]
- Konarska, A.; Masierowska, M. Structure of floral nectaries and female-biased nectar production in protandrous species Geranium macrorrhizum and Geranium phaeum. Protoplasma 2020, 257, 501–523. [Google Scholar] [CrossRef]
- Chwil, M.; Kostryco, M.; Matraszek-Gawron, R. Comparative studies on structure of the floral nectaries and the abundance of nectar production of Prunus laurocerasus L. Protoplasma 2019, 256, 1705–1726. [Google Scholar] [CrossRef] [Green Version]
- D’Angeli, S.; Falasca, G.; Matteucci, M.; Altamura, M.M. Cold perception and gene expression differ in Olea europaea seed coat and embryo during drupe cold acclimation. New Phytol. 2013, 197, 123–138. [Google Scholar] [CrossRef]
- Correia, V.G.; Bento, A.; Pais, J.; Rodrigues, R.; Haliński, Ł.P.; Frydrych, M.; Greenhalgh, A.; Stepnowski, P.; Vollrath, F.; King, A.W.T.; et al. The molecular structure and multifunctionality of the cryptic plant polymer suberin. Mater. Today Bio 2020, 5, 100039. [Google Scholar] [CrossRef] [PubMed]
- Hemsley, A.R.; Jenkins, P.D.; Collinson, M.E.; Vincent, B. Experimental modelling of exine self-assembly. Bot. J. Linn. Soc. 1996, 121, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Hemsley, A.R.; Vincent, B.; Collinson, M.E.; Griffiths, P.C. Simulated self-assembly of spore exines. Ann. Bot. 1998, 82, 105–109. [Google Scholar] [CrossRef]
- Hemsley, A.R.; Griffiths, P.C.; Mathias, R.; Moore, S.E.M. A model for the role of surfactants in the assembly of exine sculpture. Grana 2003, 42, 38–42. [Google Scholar] [CrossRef]
- Gabarayeva, N.I.; Hemsley, A.R. Merging concepts: The role of self-assembly in the development of pollen wall structure. Rev. Palaeobot. Palyno. 2006, 138, 121–139. [Google Scholar] [CrossRef]
- Hemsley, A.R.; Gabarayeva, N.I. Exine development: The importance of looking through a colloid chemistry “window”. Plant Syst. Evol. 2007, 263, 25–49. [Google Scholar] [CrossRef]
- Gabarayeva, N.I.; Grigorjeva, V.V. Experimental modelling of exine-like structures. Grana 2013, 52, 241–257. [Google Scholar] [CrossRef]
- Gabarayeva, N.; Grigorjeva, V. Simulation of exine patterns by self-assembly. Plant Syst. Evol. 2016, 302, 1135–1156. [Google Scholar] [CrossRef]
- Lavrentovich, M.O.; Horsley, E.M.; Radja, A.; Sweeney, A.M.; Kamien, R.D. First-order patterning transitions on a sphere as a route to cell morphology. Proc. Nat. Acad. Sci. USA 2016, 113, 5189–5194. [Google Scholar] [CrossRef] [Green Version]
- Gabarayeva, N.I.; Grigorjeva, V.V. Self-assembly as the underlying mechanism for exine development in Larix decidua DC. Planta 2017, 246, 471–493. [Google Scholar] [CrossRef]
- Radja, A.; Horsley, E.M.; Lavrentovich, M.O.; Sweeney, A.M. Pollen cell wall patterns form from modulated phases. Cell 2019, 176, 856–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabarayeva, N.I.; Grigorjeva, V.V.; Shavarda, A.L. Mimicking pollen and spore walls: Self-assembly in action. Ann. Bot. 2019, 123, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Gabarayeva, N.I.; Grigorjeva, V.V.; Lavrentovich, M.O. Artificial pollen walls simulated by the tandem processes of phase separation and self-assembly in vitro. New Phytol. 2020, 225, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Tsugama, D.; Fujino, K.; Liu, S.; Takano, T. A GDSL-type esterase/lipase gene, GELP77, is necessary for pollen dissociation and fertility in Arabidopsis. Biochem. Biophys. Res. Commun. 2020, 526, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Davison, T.S.; Henz, S.R.; Pape, U.J.; Demar, M.; Vingron, M.; Schölkopf, B.; Weigel, D.; Lohmann, J.U. A gene expression map of Arabidopsis thaliana development. Nat. Genet. 2005, 37, 501–506. [Google Scholar] [CrossRef]
- Jacq, A.; Pernot, C.; Martinez, Y.; Domergue, F.; Payré, B.; Jamet, E.; Burlat, V.; Pacquit, V.B. The Arabidopsis lipid transfer protein 2 (AtLTP2) is involved in cuticle–cell wall interface integrity and in etiolated hypocotyl permeability. Front. Plant Sci. 2017, 8, 263. [Google Scholar] [CrossRef] [Green Version]
- McCourt, R.M.; Delwiche, C.F.; Karol, K.G. Charophyte algae and land plant origins. Trends Ecol. Evol. 2004, 19, 661–666. [Google Scholar] [CrossRef]
- Delwiche, C.F.; Cooper, E.D. The evolutionary origin of terrestrial flora. Curr. Biol. 2015, 25, R899–R910. [Google Scholar] [CrossRef] [Green Version]
- Kondo, S.; Hori, K.; Sasaki-Sekimoto, Y.; Kobayashi, A.; Kato, T.; Yuno-Ohta, N.; Nobusawa, T.; Ohtaka, K.; Shimojima, M.; Ohta, H. Primitive extracellular lipid components on the surface of the Charophytic alga Klebsormidium flaccidum and their possible biosynthetic pathways as deduced from the genome sequence. Front. Plant Sci. 2016, 7, 952. [Google Scholar] [CrossRef] [Green Version]
- Niklas, K.J.; Cobb, E.D.; Matas, A.J. The evolution of hydrophobic cell wall biopolymers: From algae to angiosperms. J. Exp. Bot. 2017, 68, 5261–52691. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, T.; Sakayama, H.; de Vries, J.; Buschmann, H.; Saint-Marcoux, D.; Ullrich, K.K.; Haas, F.B.; Vanderstraeten, L.; Becker, D.; Lang, D.; et al. The Chara genome: Secondary complexity and implications for plant terrestrialization. Cell 2018, 174, 448–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, C.; Sørensen, I.; Sun, X.; Sun, H.; Behar, H.; Alseekh, S.; Philippe, G.; Lopez, K.P.; Sun, L.; Reed, R.; et al. The Penium margaritaceum genome: Hallmarks of the origins of land plants. Cell 2020, 181, 1097–1111. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępiński, D.; Kwiatkowska, M.; Wojtczak, A.; Polit, J.T.; Domínguez, E.; Heredia, A.; Popłońska, K. The Role of Cutinsomes in Plant Cuticle Formation. Cells 2020, 9, 1778. https://doi.org/10.3390/cells9081778
Stępiński D, Kwiatkowska M, Wojtczak A, Polit JT, Domínguez E, Heredia A, Popłońska K. The Role of Cutinsomes in Plant Cuticle Formation. Cells. 2020; 9(8):1778. https://doi.org/10.3390/cells9081778
Chicago/Turabian StyleStępiński, Dariusz, Maria Kwiatkowska, Agnieszka Wojtczak, Justyna Teresa Polit, Eva Domínguez, Antonio Heredia, and Katarzyna Popłońska. 2020. "The Role of Cutinsomes in Plant Cuticle Formation" Cells 9, no. 8: 1778. https://doi.org/10.3390/cells9081778
APA StyleStępiński, D., Kwiatkowska, M., Wojtczak, A., Polit, J. T., Domínguez, E., Heredia, A., & Popłońska, K. (2020). The Role of Cutinsomes in Plant Cuticle Formation. Cells, 9(8), 1778. https://doi.org/10.3390/cells9081778




