Repeated Application of Autologous Bone Marrow-Derived Lineage-Negative Stem/Progenitor Cells—Focus on Immunological Pathways in Patients with ALS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Neurological Assessment
2.4. Bone Marrow Collection and Isolation of Lin– Cells
2.5. Lineage-Negative Cells Injection
2.6. Multiplex Luminex Assay
2.7. miRNA Expression Analysis
2.8. Gene Chip Microarray
2.9. Statistical Analysis
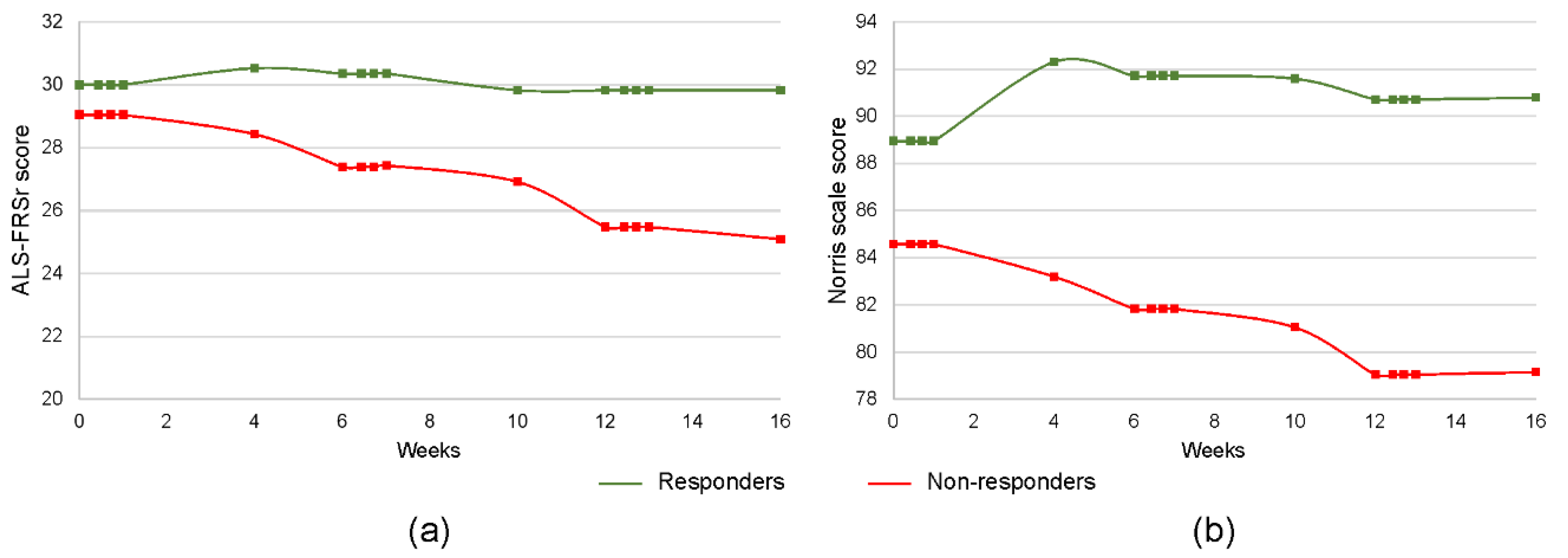
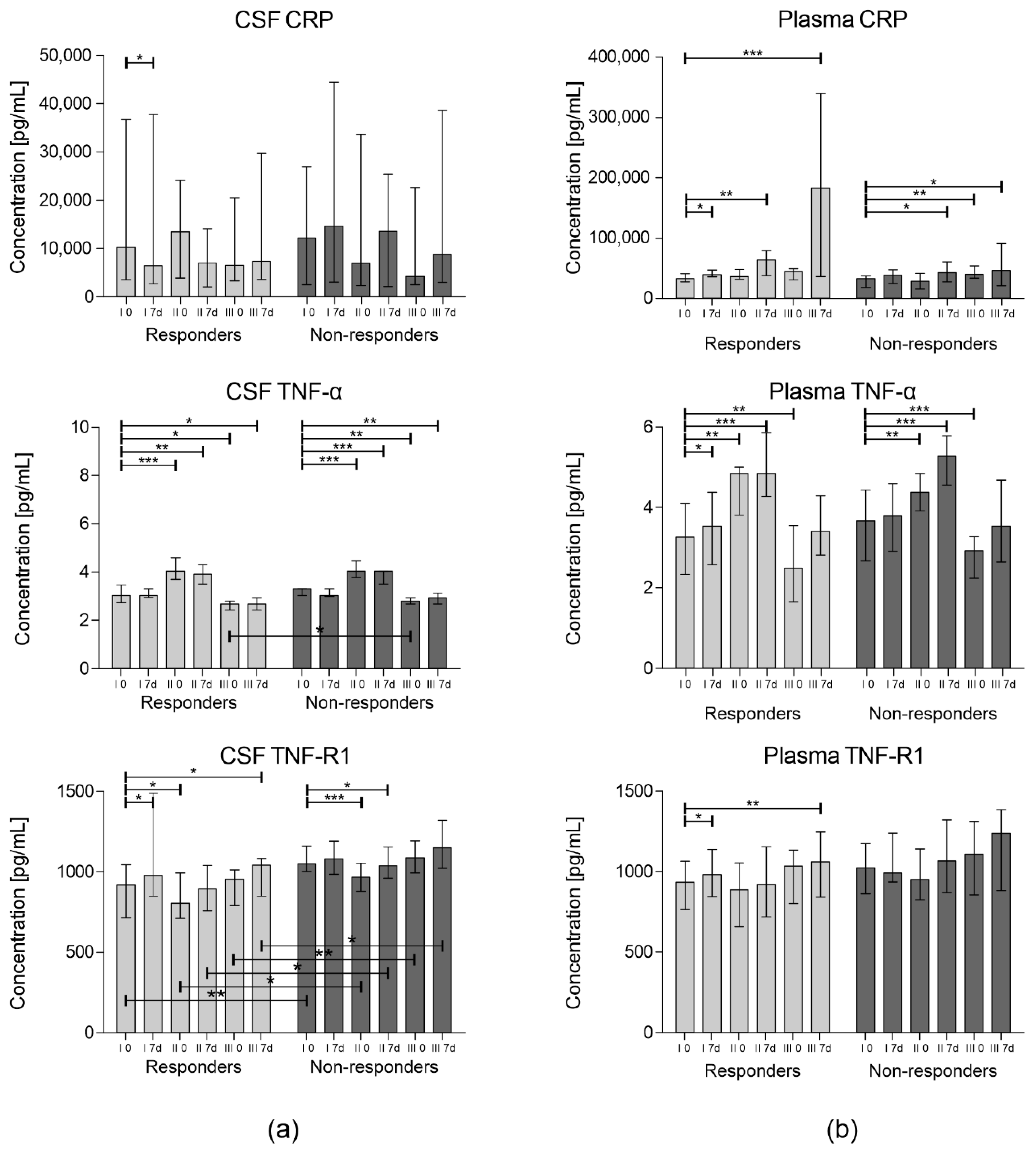
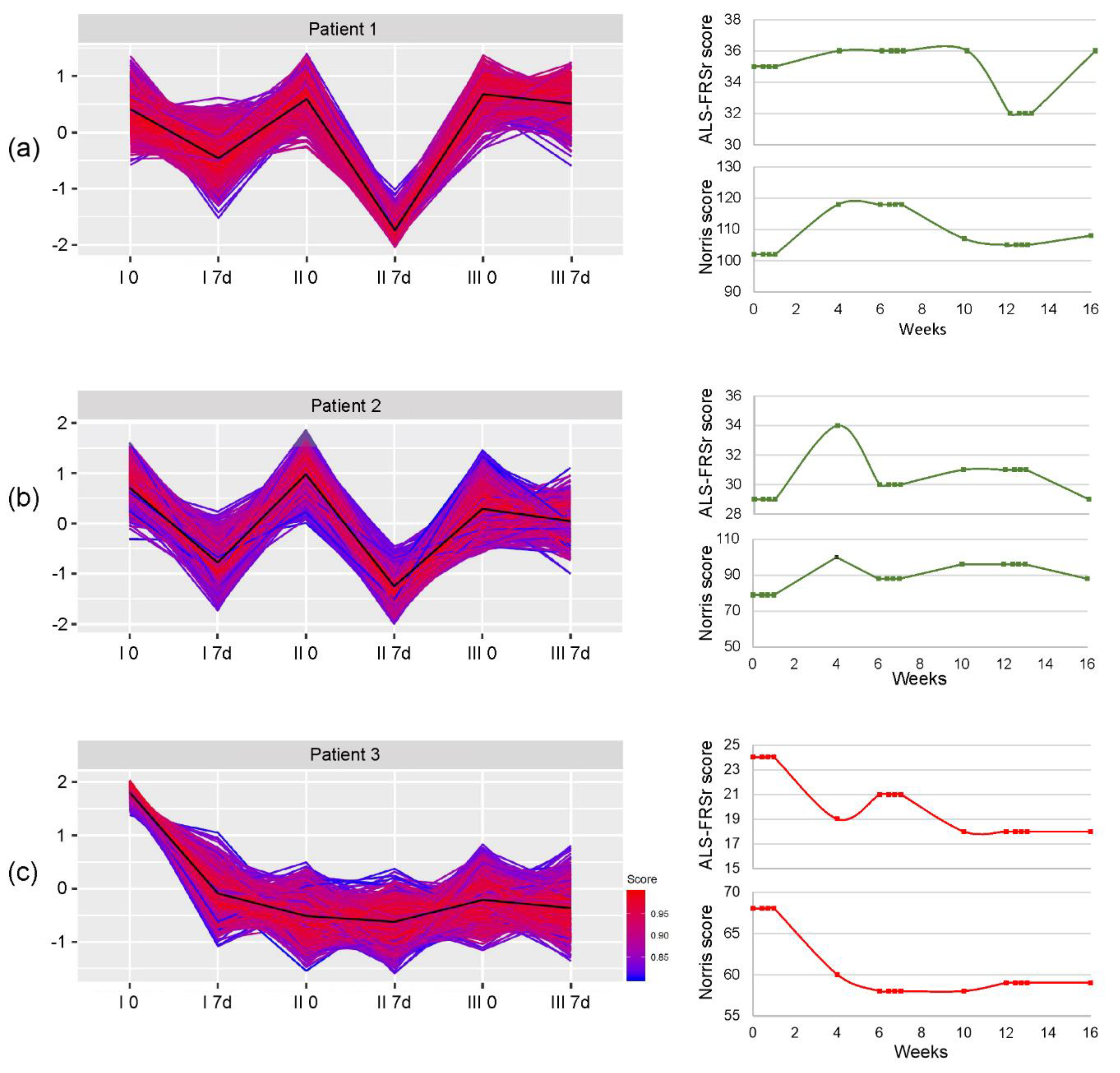
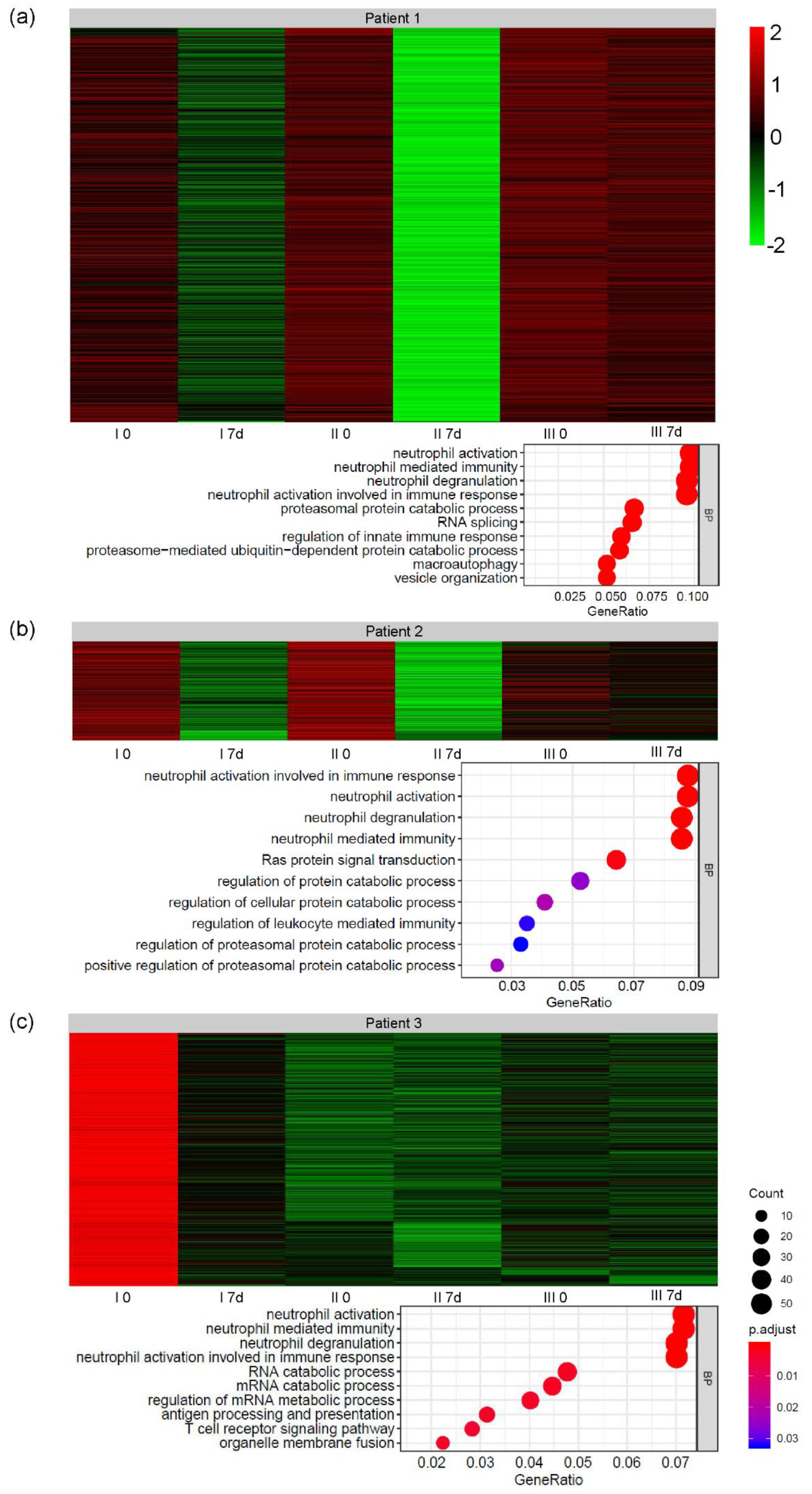
3. Results
3.1. Concentration of Inflammatory Cytokines
3.2. miRNA Expression
3.3. Global Gene Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Amor, S.; Woodroofe, M.N. Innate and adaptive immune responses in neurodegeneration and repair. Immunology 2014, 141, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, O.; Berg, L.H.V.D.; Kiernan, M.C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011, 7, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Wobst, H.J.; Mack, K.L.; Brown, D.G.; Brandon, N.; Shorter, J. The clinical trial landscape in amyotrophic lateral sclerosis—Past, present, and future. Med. Res. Rev. 2020, 40, 1352–1384. [Google Scholar] [CrossRef] [PubMed]
- Bonafede, R.; Mariotti, R. ALS Pathogenesis and Therapeutic Approaches: The Role of Mesenchymal Stem Cells and Extracellular Vesicles. Front. Cell. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, L.; Ferrari, D.; Andjus, P.R.; Buzanska, L.; Cantello, R.; De Marchi, F.; Gelati, M.; Giniatullin, R.; Glover, J.C.; Grilli, M.; et al. Advances in stem cell therapy for amyotrophic lateral sclerosis. Expert Opin. Biol. Ther. 2018, 18, 865–881. [Google Scholar] [CrossRef]
- Hu, Y.; Cao, C.; Qin, X.-Y.; Yu, Y.; Yuan, J.; Zhao, Y.; Cheng, Y. Increased peripheral blood inflammatory cytokine levels in amyotrophic lateral sclerosis: A meta-analysis study. Sci. Rep. 2017, 7, 9094. [Google Scholar] [CrossRef]
- Engelhardt, J.I.; Tajti, J.; Appel, S.H. Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch. Neurol. 1993, 50, 30–36. [Google Scholar] [CrossRef]
- Graves, M.; Fiala, M.; Dinglasan, L.A.; Liu, N.; Sayre, J.; Chiappelli, F.; Van Kooten, C.; Vinters, H. Inflammation in amyotrophic lateral sclerosis spinal cord and brain is mediated by activated macrophages, mast cells and T cells. Amyotroph. Lateral Scler. 2004, 5, 213–219. [Google Scholar] [CrossRef]
- Donnenfeld, H.; Kascsak, R.; Bartfeld, H. Deposits of IgG and C3 in the spinal cord and motor cortex of ALS patients. J. Neuroimmunol. 1984, 6, 51–57. [Google Scholar] [CrossRef]
- Murdock, B.J.; Bender, D.E.; Kashlan, S.R.; Figueroa-Romero, C.; Backus, C.; Callaghan, B.C.; Goutman, S.A.; Feldman, E. Increased ratio of circulating neutrophils to monocytes in amyotrophic lateral sclerosis. Neurol. Neuroimmunol. Neuroinflammation 2016, 3, e242. [Google Scholar] [CrossRef] [Green Version]
- Murdock, B.J.; Zhou, T.; Kashlan, S.R.; Little, R.J.; Goutman, S.A.; Feldman, E.L. Correlation of Peripheral Immunity with Rapid Amyotrophic Lateral Sclerosis Progression. JAMA Neurol. 2017, 74, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Lunetta, C.; Lizio, A.; Maestri, E.; Sansone, V.A.; Mora, G.; Miller, R.G.; Appel, S.H.; Chiò, A. Serum C-Reactive Protein as a Prognostic Biomarker in Amyotrophic Lateral Sclerosis. JAMA Neurol. 2017, 74, 660–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cereda, C.; Baiocchi, C.; Bongioanni, P.; Cova, E.; Guareschi, S.; Metelli, M.R.; Rossi, B.; Sbalsi, I.; Cuccia, M.C.; Ceroni, M. TNF and sTNFR1/2 plasma levels in ALS patients. J. Neuroimmunol. 2008, 194, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.H.; Moore, D.H.; Miller, R.G.; Florence, J.M.; Verheijde, J.L.; Doorish, C.; Hilton, J.F.; Spitalny, G.M.; MacArthur, R.B.; Mitsumoto, H.; et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: A phase III randomised trial. Lancet Neurol. 2007, 6, 1045–1053. [Google Scholar] [CrossRef]
- Meucci, N.; Orazio, E.N. Intravenous immunoglobulin therapy in amyotrophic lateral sclerosis. J. Neurol. 1996, 243, 117–120. [Google Scholar] [CrossRef]
- Smith, S.A.; Miller, R.G.; Murphy, J.R.; Ringel, S.P. Treatment of ALS with high dose pulse cyclophosphamide. J. Neurol. Sci. 1994, 124, 84–87. [Google Scholar] [CrossRef]
- Joilin, G.; Leigh, P.N.; Newbury, S.F.; Hafezparast, M. An Overview of MicroRNAs as Biomarkers of ALS. Front. Neurol. 2019, 10, 186. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Verrilli, M.; Caviedes, A.; Cabrera, A.; Sandoval, S.; Wyneken, U.; Khoury, M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 2016, 320, 129–139. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, H.; Oh, K.-W.; Oh, S.-I.; Koh, S.-H.; Baik, W.; Noh, M.-Y.; Kim, K.S.; Kim, S.H. Biological markers of mesenchymal stromal cells as predictors of response to autologous stem cell transplantation in patients with amyotrophic lateral sclerosis: An investigator-initiated trial and in vivo study. Stem Cells 2014, 32, 2724–2731. [Google Scholar] [CrossRef]
- Kim, S.H.; Oh, K.-W.; Jin, H.K.; Bae, J.-S. Immune inflammatory modulation as a potential therapeutic strategy of stem cell therapy for ALS and neurodegenerative diseases. BMB Rep. 2018, 51, 545–546. [Google Scholar] [CrossRef] [Green Version]
- Forraz, N.; Pettengell, R.; McGuckin, C. Characterization of a lineage-negative stem-progenitor cell population optimized for ex vivo expansion and enriched for LTC-IC. Stem Cells 2004, 22, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Machalińska, A.; Rogińska, D.; Pius-Sadowska, E.; Kawa, M.P.; Paczkowska, E.; Rudnicki, M.; Lejkowska, R.; Baumert, B.; Barcew-Wiszniewska, B.; Machaliński, B. Neuroprotective and antiapoptotic activity of lineage-negative bone marrow cells after intravitreal injection in a mouse model of acute retinal injury. Stem Cells Int. 2015, 2015, 620364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobuś, A.; Baumert, B.; Litwińska, Z.; Golab-Janowska, M.; Stępniewski, J.; Kotowski, M.; Pius-Sadowska, E.; Kawa, M.P.; Gródecka-Szwajkiewicz, D.; Peregud-Pogorzelski, J.; et al. Safety and Feasibility of Lin- Cells Administration to ALS Patients: A Novel View on Humoral Factors and miRNA Profiles. Int. J. Mol. Sci. 2018, 19, 1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumert, B.; Sobuś, A.; Golab-Janowska, M.; Litwińska, Z.; Paczkowska, E.; Łuczkowska, K.; Zawiślak, A.; Milczarek, S.; Osękowska, B.; Meller, A.; et al. Local and Systemic Humoral Response to Autologous Lineage-Negative Cells Intrathecal Administration in ALS Patients. Int. J. Mol. Sci. 2020, 21, 1070. [Google Scholar] [CrossRef] [Green Version]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Gordon, P.H.; Miller, R.G.; Moore, D. ALSFRS-R. Amyotroph. Lateral Scler. 2004, 5, 90–93. [Google Scholar] [CrossRef]
- Hashizume, A.; Katsuno, M.; Suzuki, K.; Banno, H.; Suga, N.; Mano, T.; Araki, A.; Hijikata, Y.; Grunseich, C.; Kokkinis, A.; et al. A functional scale for spinal and bulbar muscular atrophy: Cross-sectional and longitudinal study. Neuromuscul. Disord. 2015, 25, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Paczkowska, E.; Kaczyńska, K.; Pius-Sadowska, E.; Rogińska, D.; Kawa, M.; Ustianowski, P.; Safranow, K.; Celewicz, Z.; Machaliński, B. Humoral activity of cord blood-derived stem/progenitor cells: Implications for stem cell-based adjuvant therapy of neurodegenerative disorders. PLoS ONE 2013, 8, e83833. [Google Scholar] [CrossRef]
- Paganoni, S.; Macklin, E.A.; Lee, A.; Murphy, A.; Chang, J.; Zipf, A.; Cudkowicz, M.; Atassi, N. Diagnostic timelines and delays in diagnosing amyotrophic lateral sclerosis (ALS). Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 453–456. [Google Scholar] [CrossRef] [Green Version]
- Toivonen, J.M.; Manzano, R.; Oliván, S.; Zaragoza, P.; García-Redondo, A.; Osta, R. MicroRNA-206: A potential circulating biomarker candidate for amyotrophic lateral sclerosis. PLoS ONE 2014, 9, e89065. [Google Scholar] [CrossRef] [Green Version]
- Butovsky, O.; Jedrychowski, M.P.; Cialic, R.; Krasemann, S.; Murugaiyan, G.; Fanek, Z.; Greco, D.J.; Wu, P.M.; Doykan, C.E.; Kiner, O.; et al. Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann. Neurol. 2014, 77, 75–99. [Google Scholar] [CrossRef]
- Ricci, C.; Marzocchi, C.; Battistini, S. MicroRNAs as Biomarkers in Amyotrophic Lateral Sclerosis. Cells 2018, 7, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumert, B.; Przybycień, K.; Paczkowska, E.; Kotowski, M.; Pius-Sadowska, E.; Safranow, K.; Peregud-Pogorzelski, J.; Kornacewicz-Jach, Z.; Peregud-Pogorzelska, M.; Machaliński, B. Novel Evidence of the Increase in Angiogenic Factor Plasma Levels after Lineage-Negative Stem/Progenitor Cell Intracoronary Infusion in Patients with Acute Myocardial Infarction. Int. J. Mol. Sci. 2019, 20, 3330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sproston, N.R.; Ashworth, J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Falvo, J.V.; Tsytsykova, A.V.; Goldfeld, A.E. Transcriptional control of the TNF gene. Curr. Dir. Autoimmun. 2010, 11, 27–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botchkina, G.I.; Meistrell, M.E.; Botchkina, I.L.; Tracey, K.J. Expression of TNF and TNF Receptors (p55 and p75) in the Rat Brain after Focal Cerebral Ischemia. Mol. Med. 1997, 3, 765–781. [Google Scholar] [CrossRef] [Green Version]
- Chen, G. TNF-R1 Signaling: A Beautiful Pathway. Science 2002, 296, 1634–1635. [Google Scholar] [CrossRef] [Green Version]
- Sedger, L.; McDermott, M.F. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants – past, present and future. Cytokine Growth Factor Rev. 2014, 25, 453–472. [Google Scholar] [CrossRef] [Green Version]
- Probert, L.; Akassoglou, K.; Pasparakis, M.; Kontogeorgos, G.; Kollias, G. Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factor alpha. Proc. Natl. Acad. Sci. USA 1995, 92, 11294–11298. [Google Scholar] [CrossRef] [Green Version]
- Lv, S.; Song, H.-L.; Zhou, Y.; Li, L.-X.; Cui, W.; Wang, W.; Liu, P. Tumour necrosis factor-α affects blood-brain barrier permeability and tight junction-associated occludin in acute liver failure. Liver Int. 2010, 30, 1198–1210. [Google Scholar] [CrossRef]
- Patel, H.J.; Patel, B.M. TNF-α and cancer cachexia: Molecular insights and clinical implications. Life Sci. 2017, 170, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Frankola, K.A.; Greig, N.H.; Luo, W.; Tweedie, D. Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol. Disord. Drug Targets 2011, 10, 391–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taoufik, E.; Petit, E.; Divoux, D.; Tseveleki, V.; Mengozzi, M.; Roberts, M.L.; Valable, S.; Ghezzi, P.; Quackenbush, J.; Brines, M.L.; et al. TNF receptor I sensitizes neurons to erythropoietin- and VEGF-mediated neuroprotection after ischemic and excitotoxic injury. Proc. Natl. Acad. Sci. USA 2008, 105, 6185–6190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brambilla, L.; Guidotti, G.; Martorana, F.; Iyer, A.; Aronica, E.; Valori, C.F.; Rossi, D. Disruption of the astrocytic TNFR1-GDNF axis accelerates motor neuron degeneration and disease progression in amyotrophic lateral sclerosis. Hum. Mol. Genet. 2016, 25, 3080–3095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Chen, J.; Sen, S. MicroRNA as Biomarkers and Diagnostics. J. Cell. Physiol. 2015, 231, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Zilahi, E.; Adamecz, Z.; Bodoki, L.; Griger, Z.; Póliska, S.; Nagy-Vincze, M.; Dankó, K. Dysregulated expression profile of myomiRs in the skeletal muscle of patients with polymyositis. EJIFCC 2019, 30, 237–245. [Google Scholar] [PubMed]
- O’Connell, R.M.; Chaudhuri, A.A.; Rao, D.S.; Baltimore, D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc. Natl. Acad. Sci. USA 2009, 106, 7113–7118. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhao, Q.; Matta, R.; Meng, X.; Liu, X.; Liu, C.-G.; Nelin, L.D.; Liu, Y. Inducible Nitric-oxide Synthase Expression Is Regulated by Mitogen-activated Protein Kinase Phosphatase-1. J. Biol. Chem. 2009, 284, 27123–27134. [Google Scholar] [CrossRef] [Green Version]
- Henry, R.J.; Doran, S.J.; Barrett, J.P.; Meadows, V.E.; Sabirzhanov, B.; Stoica, B.; Loane, D.J.; Faden, A. Inhibition of miR-155 Limits Neuroinflammation and Improves Functional Recovery After Experimental Traumatic Brain Injury in Mice. Neurotherapeutics 2018, 16, 216–230. [Google Scholar] [CrossRef] [Green Version]
- Koval, E.D.; Shaner, C.; Zhang, P.; Du Maine, X.; Fischer, K.; Tay, J.; Chau, B.N.; Wu, G.F.; Miller, T.M. Method for widespread microRNA-155 inhibition prolongs survival in ALS-model mice. Hum. Mol. Genet. 2013, 22, 4127–4135. [Google Scholar] [CrossRef] [Green Version]
- Gerin, I.; Bommer, G.T.; McCoin, C.S.; Sousa, K.M.; Krishnan, V.; MacDougald, O.A. Roles for miRNA-378/378* in adipocyte gene expression and lipogenesis. Am. J. Physiol. Metab. 2010, 299, E198–E206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulyté, A.; Lorente-Cebrián, S.; Gao, H.; Mejhert, N.; Agustsson, T.; Arner, P.; Ryden, M.; Dahlman, I. MicroRNA profiling links miR-378 to enhanced adipocyte lipolysis in human cancer cachexia. Am. J. Physiol. Metab. 2014, 306, E267–E274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.H.; Valdez, G.; Moresi, V.; Qi, X.; McAnally, J.; Elliott, J.L.; Bassel-Duby, R.; Sanes, J.R.; Olson, E.N. MicroRNA-206 Delays ALS Progression and Promotes Regeneration of Neuromuscular Synapses in Mice. Science 2009, 326, 1549–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horak, M.; Novak, J.; Bienertová-Vašků, J. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 2016, 410, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nakasa, T.; Ishikawa, M.; Shi, M.; Shibuya, H.; Adachi, N.; Ochi, M. Acceleration of muscle regeneration by local injection of muscle-specific microRNAs in rat skeletal muscle injury model. J. Cell. Mol. Med. 2009, 14, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Trias, E.; King, P.H.; Si, Y.; Kwon, Y.; Varela, V.; Ibarburu, S.; Kovacs, M.; Moura, I.C.; Beckman, J.S.; Hermine, O.; et al. Mast cells and neutrophils mediate peripheral motor pathway degeneration in ALS. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Guo, W.; Naujock, M.; Fumagalli, L.; Vandoorne, T.; Baatsen, P.; Boon, R.; Ordovás, L.; Patel, A.; Welters, M.; Vanwelden, T.; et al. HDAC6 inhibition reverses axonal transport defects in motor neurons derived from FUS-ALS patients. Nat. Commun. 2017, 8, 861. [Google Scholar] [CrossRef]
- Zhang, Z.; Almeida, S.; Lu, Y.; Nishimura, A.L.; Peng, L.; Sun, D.; Wu, B.; Karydas, A.M.; Tartaglia, M.C.; Fong, J.C.; et al. Downregulation of MicroRNA-9 in iPSC-Derived Neurons of FTD/ALS Patients with TDP-43 Mutations. PLoS ONE 2013, 8, e76055. [Google Scholar] [CrossRef] [Green Version]

| Responders (n = 17) | Non-Responders (n = 23) | p-Value | ||
|---|---|---|---|---|
| Age (mean ± SD, years) | 53.1 ± 9.5 | 54.4 ± 8.8 | 0.76 | |
| Gender (Male/Female) | 8/9 | 14/9 | 0.52 | |
| Disease duration (mean ± SD, months) | 27.9 ± 20.2 | 28.9 ± 29.7 | 0.29 | |
| Disease onset (bulbar/limb) | 5/12 | 5/18 | 0.72 | |
| Number of administered Lin– cells (mean ± SD) | I | 8.1 × 106 ± 6.3 | 5.5 × 106 ± 6.0 | 0.17 |
| II | 7.9 × 106 ± 6.0 | 6.7 × 106 ± 6.8 | 0.24 | |
| III | 7.5 × 106 ± 9.8 | 7.0 × 106 ± 5.0 | 0.48 | |
| Baseline ALS-FRSr (mean ± SD, points) | 30.1 ± 4.3 | 29 ± 5.6 | 0.39 | |
| Baseline Norris scale (mean ± SD, points) | 90.2 ± 16.2 | 79.4 ± 14.3 | 0.25 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumert, B.; Sobuś, A.; Gołąb-Janowska, M.; Paczkowska, E.; Łuczkowska, K.; Rogińska, D.; Zawiślak, A.; Milczarek, S.; Osękowska, B.; Pawlukowska, W.; et al. Repeated Application of Autologous Bone Marrow-Derived Lineage-Negative Stem/Progenitor Cells—Focus on Immunological Pathways in Patients with ALS. Cells 2020, 9, 1822. https://doi.org/10.3390/cells9081822
Baumert B, Sobuś A, Gołąb-Janowska M, Paczkowska E, Łuczkowska K, Rogińska D, Zawiślak A, Milczarek S, Osękowska B, Pawlukowska W, et al. Repeated Application of Autologous Bone Marrow-Derived Lineage-Negative Stem/Progenitor Cells—Focus on Immunological Pathways in Patients with ALS. Cells. 2020; 9(8):1822. https://doi.org/10.3390/cells9081822
Chicago/Turabian StyleBaumert, Bartłomiej, Anna Sobuś, Monika Gołąb-Janowska, Edyta Paczkowska, Karolina Łuczkowska, Dorota Rogińska, Alicja Zawiślak, Sławomir Milczarek, Bogumiła Osękowska, Wioletta Pawlukowska, and et al. 2020. "Repeated Application of Autologous Bone Marrow-Derived Lineage-Negative Stem/Progenitor Cells—Focus on Immunological Pathways in Patients with ALS" Cells 9, no. 8: 1822. https://doi.org/10.3390/cells9081822
APA StyleBaumert, B., Sobuś, A., Gołąb-Janowska, M., Paczkowska, E., Łuczkowska, K., Rogińska, D., Zawiślak, A., Milczarek, S., Osękowska, B., Pawlukowska, W., Meller, A., Machowska-Sempruch, K., Wełnicka, A., Safranow, K., Nowacki, P., & Machaliński, B. (2020). Repeated Application of Autologous Bone Marrow-Derived Lineage-Negative Stem/Progenitor Cells—Focus on Immunological Pathways in Patients with ALS. Cells, 9(8), 1822. https://doi.org/10.3390/cells9081822






