Tat-Cannabinoid Receptor Interacting Protein Reduces Ischemia-Induced Neuronal Damage and Its Possible Relationship with 14-3-3η
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Effects of Tat-CRIP1a in HT22 Cells
2.1.1. Cell Preparation
2.1.2. Construction of Expression Vectors
2.1.3. Penetration Efficacy of Tat-CRIP1a Proteins in the HT22 Cells
2.1.4. Effects of Tat-CRIP1a Proteins on Cell Death and DNA Damage Exposed to H2O2 in the HT22 Cells
2.1.5. Effects of Tat-CRIP1a Proteins on ROS Levels Exposed to H2O2 in the HT22 Cells
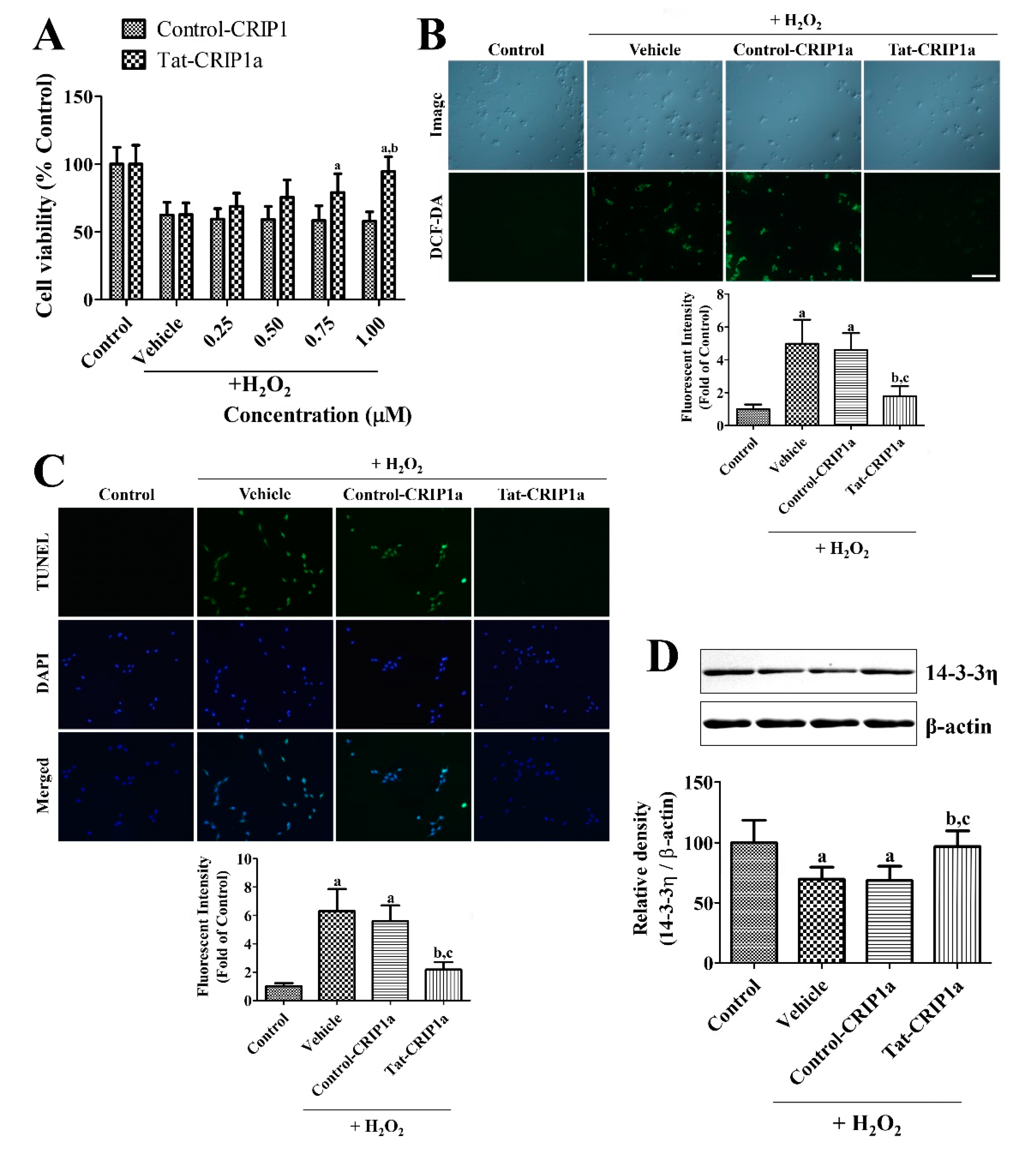
2.1.6. Effects of Tat-CRIP1a Proteins on 14-3-3η Levels in the HT22 Cells
2.2. Changes of CRIP1a after Ischemia and In Vivo Effects of Tat-CRIP1a against Ischemic Damage in Gerbils
2.2.1. Experimental Animals
2.2.2. Induction of Transient Forebrain Ischemia
2.2.3. Experimental Design
2.2.4. Open Field Test
2.2.5. Tissue Processing and Immunohistochemistry
2.2.6. Semi-Quantification of Data
2.2.7. Statistical Analysis
3. Results
3.1. In Vitro Effects of Tat-CRIP1a in HT22 Cells
3.1.1. Expression and Purification of Tat-CRIP1a Protein
3.1.2. In Vitro Efficacy of Intracellular Delivery of Tat-CRIP1a Protein in HT22 Cells
3.1.3. Effects of Tat-CRIP1a Proteins on Cell Death, DNA Damage, ROS, and 14-3-3η Levels Following Exposure to H2O2
3.2. Changes of CRIP1a after Ischemia and the In Vivo Effects of Tat-CRIP1a Following Ischemic Damage in Gerbils
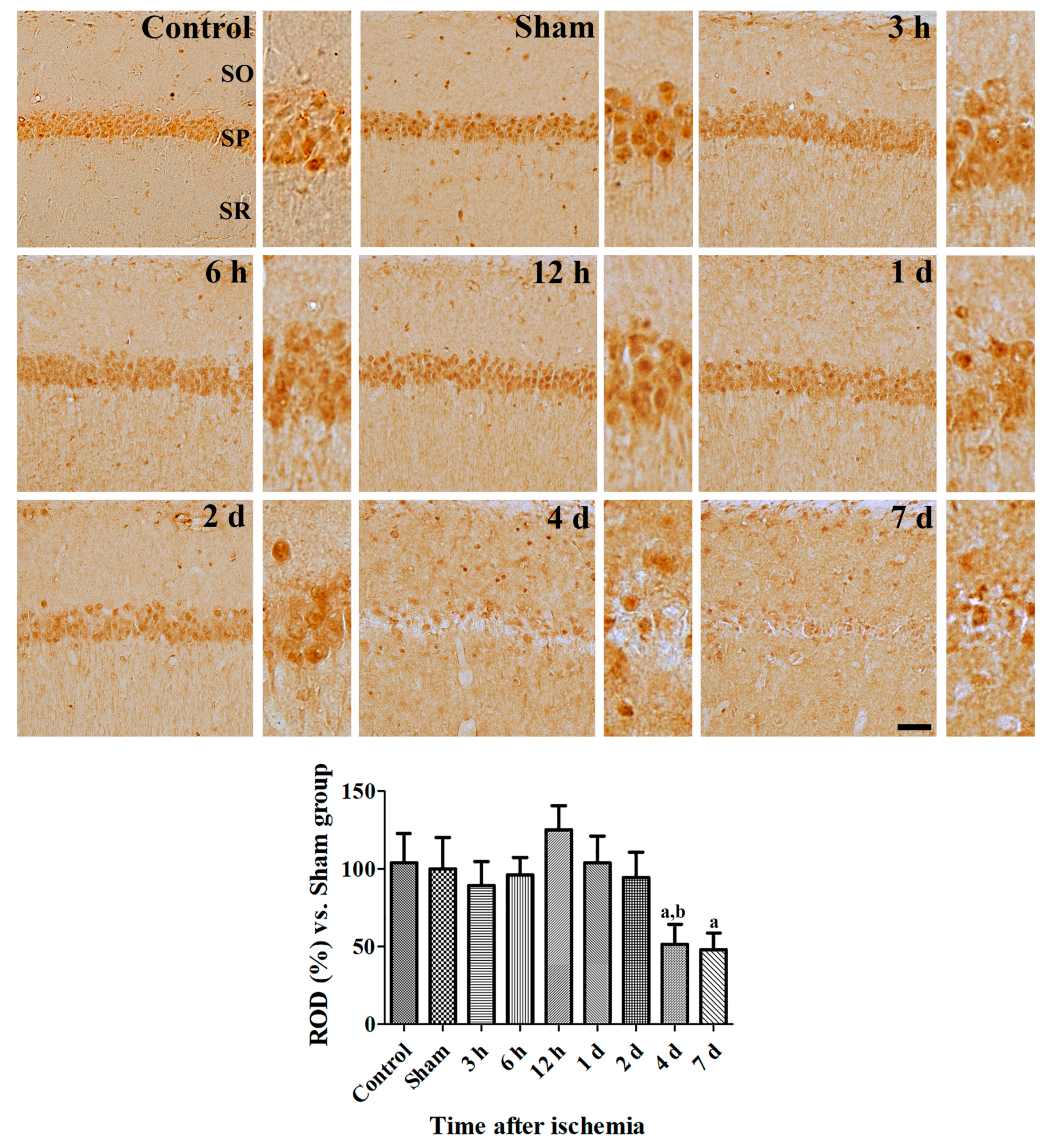
3.2.1. Changes of CRIP1a Immunoreactivity in the Hippocampal CA1 Region after Transient Forebrain Ischemia
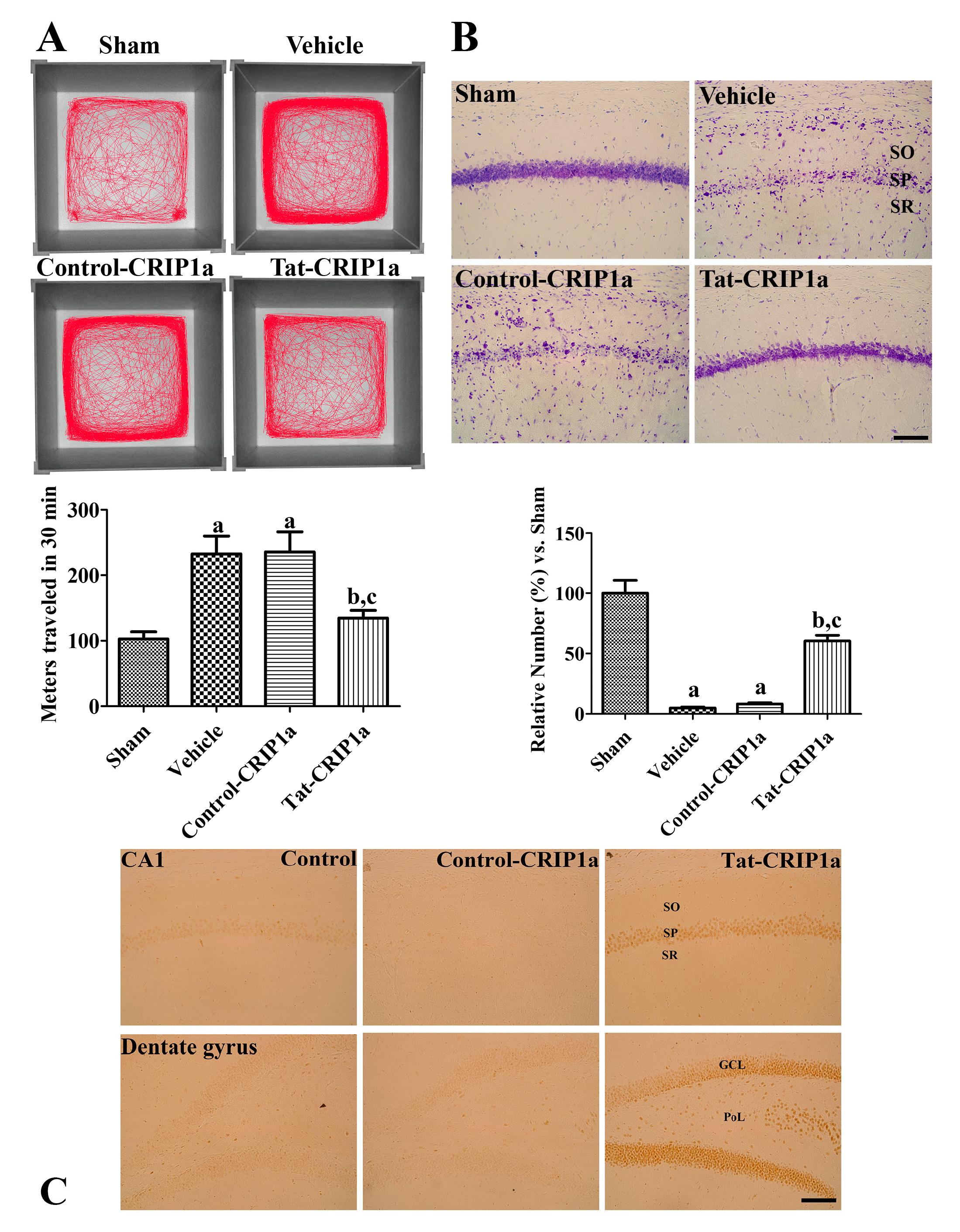
3.2.2. Effects of CRIP1a on Ischemia-Induced Motor Activity and Cell Death
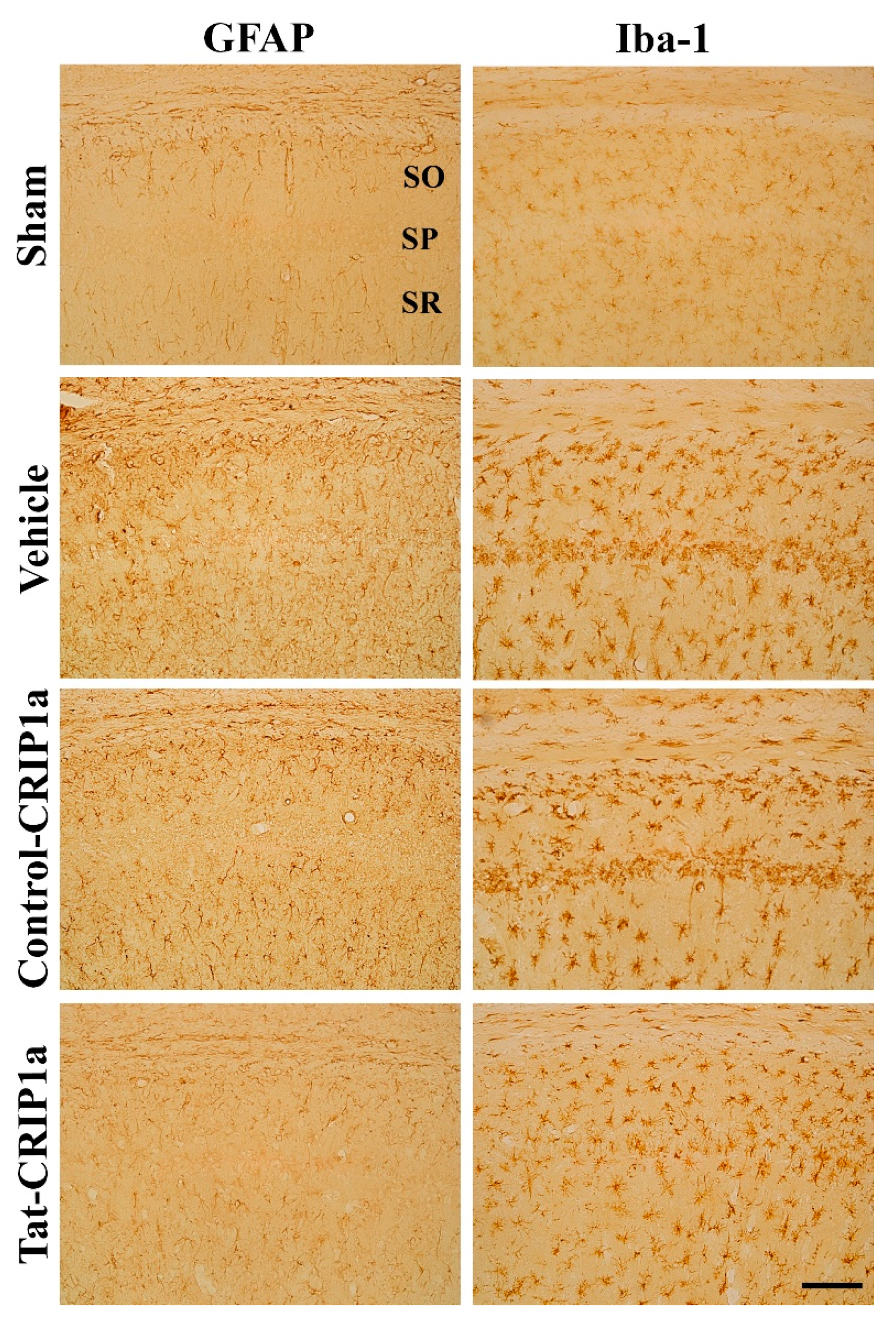
3.2.3. Roles of Tat-CRIP1a on Glial Activation in Hippocampal CA1 Region
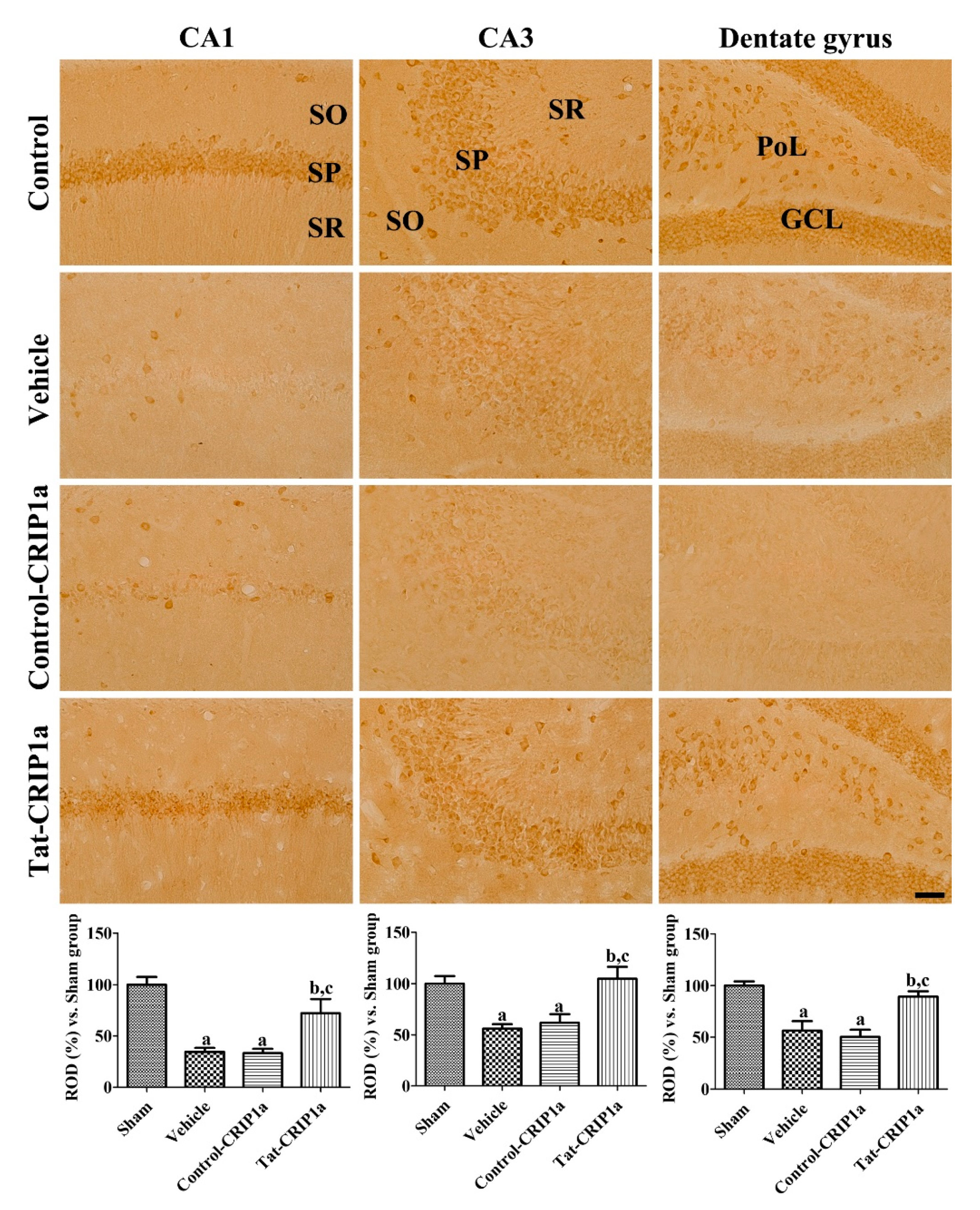
3.2.4. Effects of Tat-PDIA3 on 14-3-3-eta Expression in the Hippocampal Sub-Regions
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Cabral-Miranda, F.; Nicoloso-Simões, E.; Adão-Novaes, J.; Chiodo, V.; Hauswirth, W.W.; Linden, R.; Chiarini, L.B.; Petrs-Silva, H. rAAV8-733-mediated gene transfer of CHIP/Stub-1 prevents hippocampal neuronal death in experimental brain ischemia. Mol. Ther. 2017, 25, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.; MacWalter, R.; Doney, A. The cost of cerebral ischaemia. Neuropharmacology 2008, 55, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Denisova, J.V.; Kang, K.S.; Fontes, J.D.; Zhu, B.T.; Belousov, A.B. Neuronal Gap Junctions Are Required for NMDA Receptor–Mediated Excitotoxicity: Implications in Ischemic Stroke. J. Neurophysiol. 2010, 104, 3551–3556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hwang, I.K.; Yoo, K.Y.; An, S.J.; Li, H.; Lee, C.H.; Choi, J.H.; Lee, J.Y.; Lee, B.h.; Kim, Y.M.; Kwon, Y.G.; et al. Late expression of Na+/H+ exchanger 1 (NHE1) and neuroprotective effects of NHE inhibitor in the gerbil hippocampal CA1 region induced by transient ischemia. Exp. Neurol. 2008, 212, 314–323. [Google Scholar] [CrossRef]
- Szydlowska, K.; Tymianski, M. Calcium, ischemia and excitotoxicity. Cell Calcium 2010, 47, 122–129. [Google Scholar] [CrossRef]
- Ouyang, Y.B.; Xu, L.; Yue, S.; Liu, S.; Giffard, R.G. Neuroprotection by astrocytes in brain ischemia: Importance of microRNAs. Neurosci. Lett. 2014, 565, 53–58. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, Y.; Jia, J. Exercise preconditioning and brain ischemic tolerance. Neuroscience 2011, 177, 170–176. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Kawamura, Y.; Fukaya, M.; Maejima, T.; Yoshida, T.; Miura, E.; Watanabe, M.; Ohno-Shosaku, T.; Kano, M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J. Neurosci. 2006, 26, 2991–3001. [Google Scholar] [CrossRef]
- Busquets-Garcia, A.; Bains, J.; Marsicano, G. CB 1 receptor signaling in the brain: Extracting specificity from ubiquity. Neuropsychopharmacology 2018, 43, 4–20. [Google Scholar] [CrossRef]
- Niehaus, J.L.; Liu, Y.; Wallis, K.T.; Egertová, M.; Bhartur, S.G.; Mukhopadhyay, S.; Shi, S.; He, H.; Selley, D.E.; Howlett, A.C.; et al. CB1 cannabinoid receptor activity is modulated by the cannabinoid receptor interacting protein CRIP 1a. Mol. Pharmacol. 2007, 72, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Guggenhuber, S.; Alpar, A.; Chen, R.; Schmitz, N.; Wickert, M.; Mattheus, T.; Harasta, A.E.; Purrio, M.; Kaiser, N.; Elphick, M.R.; et al. Cannabinoid receptor-interacting protein Crip1a modulates CB1 receptor signaling in mouse hippocampus. Brain Struct. Funct. 2016, 221, 2061–2074. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.H.; Blume, L.C.; Straiker, A.; Cox, J.O.; David, B.G.; Secor McVoy, J.R.; Sayers, K.W.; Poklis, J.L.; Abdullah, R.A.; Egertová, M.; et al. Cannabinoid receptor-interacting protein 1a modulates CB1 receptor signaling and regulation. Mol. Pharmacol. 2015, 87, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Abush, H.; Akirav, I. Cannabinoids modulate hippocampal memory and plasticity. Hippocampus 2010, 20, 1126–1138. [Google Scholar] [CrossRef]
- Bojnik, E.; Turunç, E.; Armağan, G.; Kanıt, L.; Benyhe, S.; Yalçın, A.; Borsodi, A. Changes in the cannabinoid (CB1) receptor expression level and G-protein activation in kainic acid induced seizures. Epilepsy Res. 2012, 99, 64–68. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Moro, M.A.; Martínez-Orgado, J. Cannabinoids in neurodegenerative disorders and stroke/brain trauma: From preclinical models to clinical applications. Neurotherapeutics 2015, 12, 793–806. [Google Scholar]
- Mukhopadhyay, P.; Rajesh, M.; Pan, H.; Patel, V.; Mukhopadhyay, B.; Bátkai, S.; Gao, B.; Haskó, G.; Pacher, P. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic. Biol. Med. 2010, 48, 457–467. [Google Scholar] [CrossRef]
- Panikashvili, D.; Mechoulam, R.; Beni, S.M.; Alexandrovich, A.; Shohami, E. CB1 cannabinoid receptors are involved in neuroprotection via NF-κB inhibition. J. Cereb. Blood Flow Metab. 2005, 25, 477–484. [Google Scholar] [CrossRef]
- Panikashvili, D.; Simeonidou, C.; Ben-Shabat, S.; Hanuš, L.; Breuer, A.; Mechoulam, R.; Shohami, E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature 2001, 413, 527–531. [Google Scholar] [CrossRef]
- More, S.V.; Choi, D.K. Promising cannabinoid-based therapies for Parkinson’s disease: Motor symptoms to neuroprotection. Mol. Neurodegener. 2015, 10, 17. [Google Scholar] [CrossRef]
- Cerri, S.; Levandis, G.; Ambrosi, G.; Montepeloso, E.; Antoninetti, G.F.; Franco, R.; Lanciego, J.L.; Baqi, Y.; Müller, C.E.; Pinna, A.; et al. Neuroprotective potential of adenosine A2A and cannabinoid CB1 receptor antagonists in an animal model of Parkinson disease. J. Neuropathol. Exp. Neurol. 2014, 73, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, Y.; Papadopoulos, V. The role of the 14-3-3 protein family in health, disease, and drug development. Drug Discov. Today 2016, 21, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Smani, D.; Sarkar, S.; Raymick, J.; Kanungo, J.; Paule, M.G.; Gu, Q. Downregulation of 14-3-3 proteins in a kainic acid-induced neurotoxicity model. Mol. Neurobiol. 2018, 55, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lee, C.T.; Errico, S.L.; Becker, K.G.; Freed, W.J. Increases in expression of 14-3-3 eta and 14-3-3 zeta transcripts during neuroprotection induced by Δ9-tetrahydrocannabinol in AF5 cells. J. Neurosci. Res. 2007, 85, 1724–1733. [Google Scholar] [CrossRef]
- Jung, H.; Park, I.; Ghil, S. Cannabinoid receptor activation inhibits cell cycle progression by modulating 14-3-3β. Cell. Mol. Biol. Lett. 2014, 19, 347–360. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Cho, S.B.; Jung, H.Y.; Kim, W.; Lee, K.Y.; Kim, J.W.; Moon, S.M.; Won, M.H.; Choi, J.H.; Yoon, Y.S.; et al. Protein disulfide-isomerase A3 significantly reduces ischemia-induced damage by reducing oxidative and endoplasmic reticulum stress. Neurochem. Int. 2019, 122, 19–30. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Cho, S.B.; Jung, H.Y.; Kim, W.; Choi, G.M.; Won, M.H.; Kim, D.W.; Hwang, I.K.; Choi, S.Y.; Moon, S.M. Tat-protein disulfide-isomerase A3: A possible candidate for preventing ischemic damage in the spinal cord. Cell Death Dis. 2017, 8, e3075. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Lee, K.Y.; Park, J.H.; Jung, H.Y.; Kim, J.W.; Yoon, Y.S.; Won, M.H.; Choi, J.H.; Hwang, I.K. Glucose metabolism and neurogenesis in the gerbil hippocampus after transient forebrain ischemia. Neural Regen. Res. 2016, 11, 1254–1259. [Google Scholar]
- Kim, D.W.; Lee, S.H.; Jeong, M.S.; Sohn, E.J.; Kim, M.J.; Jeong, H.J.; An, J.J.; Jang, S.H.; Won, M.H.; Hwang, I.K.; et al. Transduced Tat-SAG fusion protein protects against oxidative stress and brain ischemic insult. Free Radic. Biol. Med. 2010, 48, 969–977. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Cho, S.B.; Jung, H.Y.; Kim, W.; Nam, S.M.; Kim, J.W.; Moon, S.M.; Yoon, Y.S.; Kim, D.W.; Choi, S.Y.; et al. Differential roles of exogenous protein disulfide isomerase A3 on proliferating cell and neuroblast numbers in the normal and ischemic gerbils. Brain Behav. 2020, 10, e01534. [Google Scholar] [CrossRef]
- Marsicano, G.; Moosmann, B.; Hermann, H.; Lutz, B.; Behl, C. Neuroprotective properties of cannabinoids against oxidative stress: Role of the cannabinoid receptor CB1. J. Neurochem. 2002, 80, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Bechara, C.; Sagan, S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013, 587, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, A.; Xu, R.; Tao, X.; Dong, Y.; Lv, X.; Wei, D. Cell-penetrating and endoplasmic reticulum-locating TAT-IL-24-KDEL fusion protein induces tumor apoptosis. J. Cell. Physiol. 2016, 231, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.P.; Lv, Q.L.; Lin, Q.; Kang, K.; Jin, K.Y.; Hai, J. The cannabinoid receptor agonist WIN55, 212-2 ameliorates hippocampal neuronal damage after chronic cerebral hypoperfusion possibly through inhibiting oxidative stress and ASK1-p38 signaling. Neurotox. Res. 2019, 37, 847–856. [Google Scholar] [CrossRef]
- Shen, M.; Thayer, S.A. Cannabinoid receptor agonists protect cultured rat hippocampal neurons from excitotoxicity. Mol. Pharmacol. 1998, 54, 459–462. [Google Scholar] [CrossRef]
- Hansen, H.H.; Azcoitia, I.; Pons, S.; Romero, J.; García-Segura, L.M.; Ramos, J.A.; Hansen, H.S.; Fernández-Ruiz, J. Blockade of cannabinoid CB1 receptor function protects against in vivo disseminating brain damage following NMDA-induced excitotoxicity. J. Neurochem. 2002, 82, 154–158. [Google Scholar] [CrossRef]
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef]
- Mascia, F.; Klotz, L.; Lerch, J.; Ahmed, M.H.; Zhang, Y.; Enz, R. CRIP 1a inhibits endocytosis of G-protein coupled receptors activated by endocannabinoids and glutamate by a common molecular mechanism. J. Neurochem. 2017, 141, 577–591. [Google Scholar] [CrossRef]
- Ahn, K.H.; Mahmoud, M.M.; Shim, J.Y.; Kendall, D.A. Distinct roles of β-arrestin 1 and β-arrestin 2 in ORG27569-induced biased signaling and internalization of the cannabinoid receptor 1 (CB1). J. Biol. Chem. 2013, 288, 9790–9800. [Google Scholar] [CrossRef]
- Booth, W.T.; Walker, N.B.; Lowther, W.T.; Howlett, A.C. Cannabinoid receptor interacting protein 1a (CRIP1a): Function and structure. Molecules 2019, 24, 3672. [Google Scholar] [CrossRef]
- Blume, L.C.; Leone-Kabler, S.; Luessen, D.J.; Marrs, G.S.; Lyons, E.; Bass, C.E.; Chen, R.; Selley, D.E.; Howlett, A.C. Cannabinoid receptor interacting protein suppresses agonist-driven CB 1 receptor internalization and regulates receptor replenishment in an agonist-biased manner. J. Neurochem. 2016, 139, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Masters, S.C.; Fu, H. 14-3-3 proteins mediate an essential anti-apoptotic signal. J. Biol. Chem. 2001, 276, 45193–45200. [Google Scholar] [CrossRef]
- Morrison, D.K. The 14-3-3 proteins: Integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 2009, 19, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Yacoubian, T.A.; Slone, S.R.; Harrington, A.J.; Hamamichi, S.; Schieltz, J.M.; Caldwell, K.A.; Caldwell, G.A.; Standaert, D.G. Differential neuroprotective effects of 14-3-3 proteins in models of Parkinson’s disease. Cell Death Dis. 2010, 11, e2. [Google Scholar] [CrossRef] [PubMed]
- Steinacker, P.; Aitken, A.; Otto, M. 14-3-3 proteins in neurodegeneration. Semin. Cell Dev. Biol. 2011, 22, 696–704. [Google Scholar] [CrossRef]
- Qiao, Y.; Hu, T.; Yang, B.; Li, H.; Chen, T.; Yin, D.; He, H.; He, M. Capsaicin Alleviates the Deteriorative Mitochondrial Function by Upregulating 14-3-3η in Anoxic or Anoxic/Reoxygenated Cardiomyocytes. Oxid. Med. Cell Longev. 2020, 2020. [Google Scholar] [CrossRef]
- Muratake, T.; Hayashi, S.; Ichikawa, T.; Kumanishi, T.; Ichimura, Y.; Kuwano, R.; Isobe, T.; Wang, Y.; Minoshima, S.; Shimizu, N.; et al. Structural organization and chromosomal assignment of the human 14-3-3 η chain gene (YWHAH). Genomics 1996, 36, 63–69. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.J.; Kim, D.-S.; Kim, W.; Jung, H.Y.; Yu, Y.H.; Ju, Y.I.; Park, D.-K.; Hwang, I.K.; Kim, D.W.; Yoo, D.Y. Tat-Cannabinoid Receptor Interacting Protein Reduces Ischemia-Induced Neuronal Damage and Its Possible Relationship with 14-3-3η. Cells 2020, 9, 1827. https://doi.org/10.3390/cells9081827
Kwon HJ, Kim D-S, Kim W, Jung HY, Yu YH, Ju YI, Park D-K, Hwang IK, Kim DW, Yoo DY. Tat-Cannabinoid Receptor Interacting Protein Reduces Ischemia-Induced Neuronal Damage and Its Possible Relationship with 14-3-3η. Cells. 2020; 9(8):1827. https://doi.org/10.3390/cells9081827
Chicago/Turabian StyleKwon, Hyun Jung, Duk-Soo Kim, Woosuk Kim, Hyo Young Jung, Yeon Hee Yu, Young In Ju, Dae-Kyoon Park, In Koo Hwang, Dae Won Kim, and Dae Young Yoo. 2020. "Tat-Cannabinoid Receptor Interacting Protein Reduces Ischemia-Induced Neuronal Damage and Its Possible Relationship with 14-3-3η" Cells 9, no. 8: 1827. https://doi.org/10.3390/cells9081827
APA StyleKwon, H. J., Kim, D.-S., Kim, W., Jung, H. Y., Yu, Y. H., Ju, Y. I., Park, D.-K., Hwang, I. K., Kim, D. W., & Yoo, D. Y. (2020). Tat-Cannabinoid Receptor Interacting Protein Reduces Ischemia-Induced Neuronal Damage and Its Possible Relationship with 14-3-3η. Cells, 9(8), 1827. https://doi.org/10.3390/cells9081827





