Microcurrent Stimulation Triggers MAPK Signaling and TGF-β1 Release in Fibroblast and Osteoblast-Like Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Generation of Microcurrents
2.3. Western Blot Analysis
2.4. Cell Viability Assay
2.5. In Vitro Cell Migration Assay
2.6. Cell Proliferation
2.7. Quantitation of TGF- β1 Release
2.8. RNA Extraction and cDNA Synthesis
2.9. RNA Sequencing
2.9.1. Next Generation Sequencing
2.9.2. Short Read Mapping
2.9.3. Differential Expression Analysis
2.10. Real-Time PCR
2.11. Statistical Analysis
3. Results
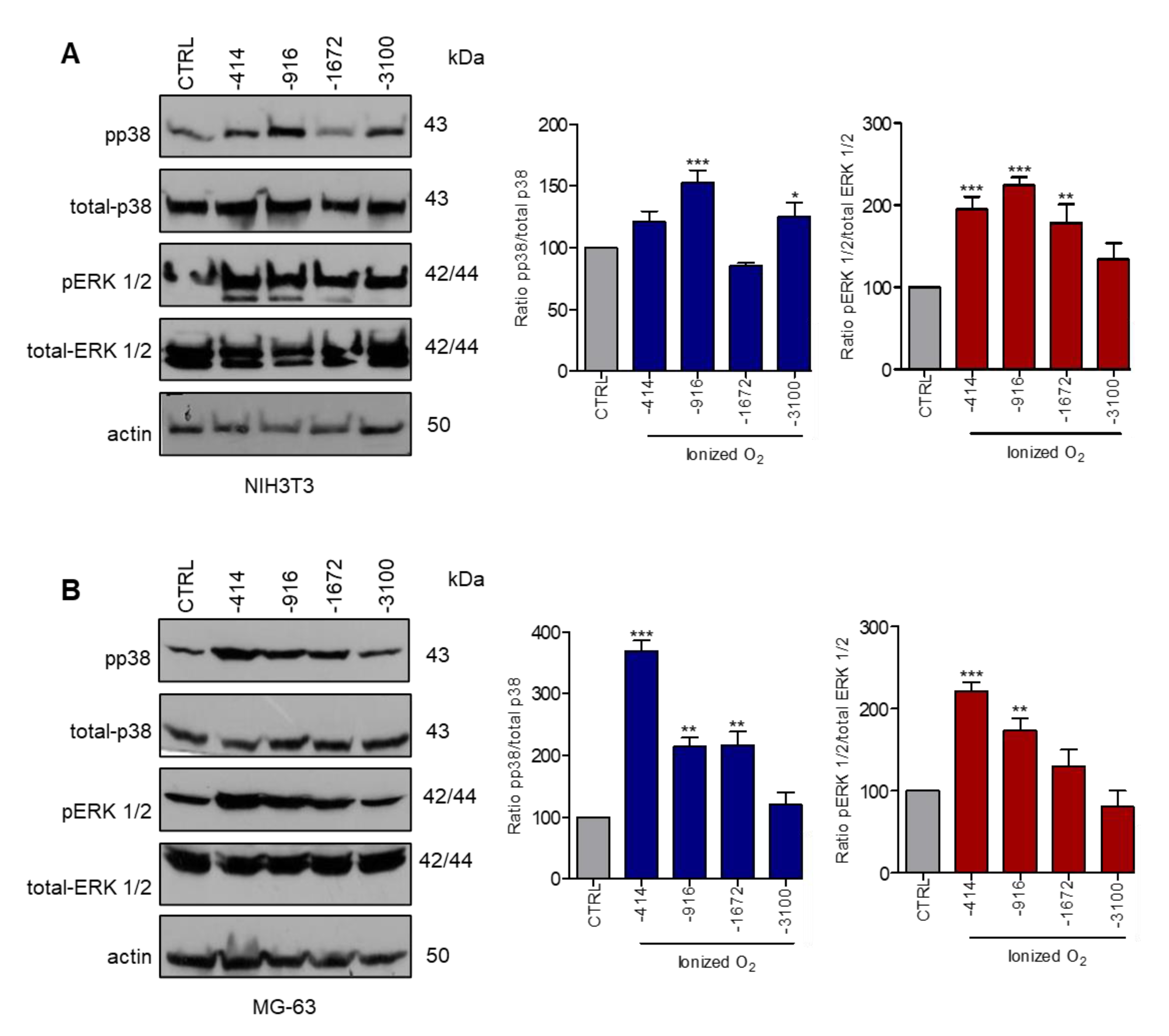
3.1. Stimulation with Microcurrents Activates ERK 1/2 and p38 MAP Kinases
3.2. Microcurrents Induce Wound Closure in an ERK 1/2- or p38-Dependent Manner In Vitro
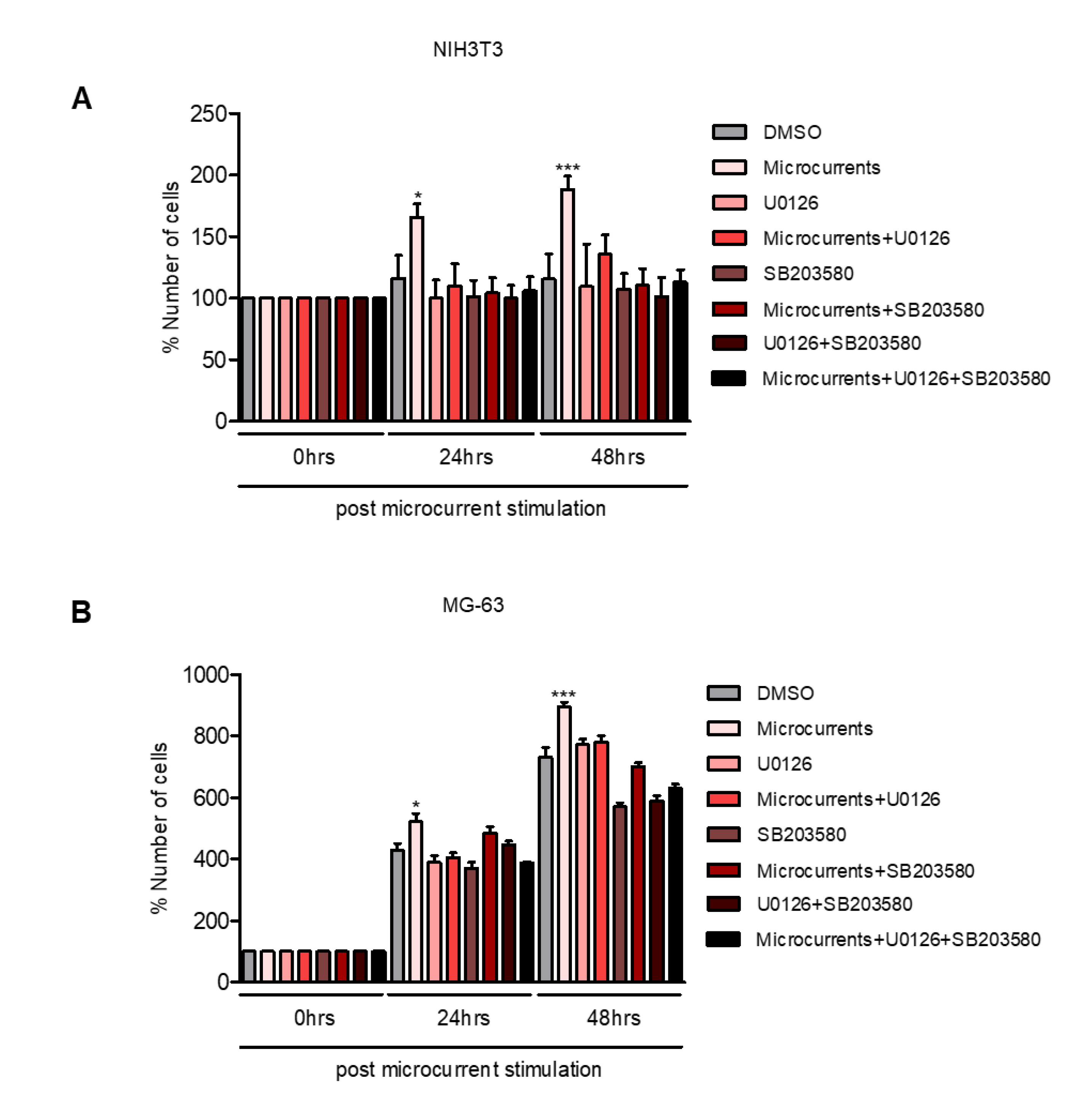
3.3. Microcurrents Enhance Cell Proliferation through ERK 1/2 and p38 Activation
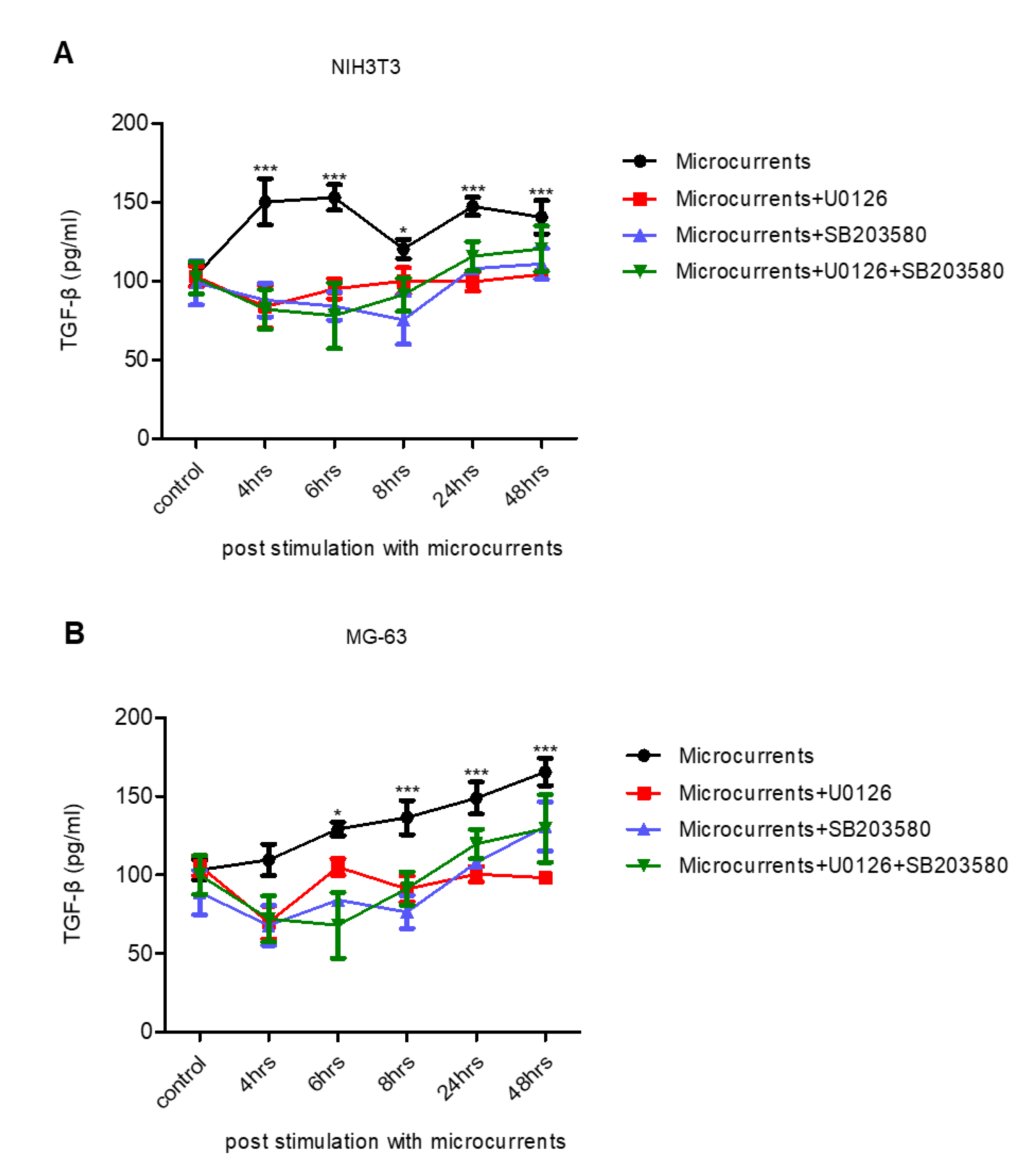
3.4. Treatment with Microcurrents Increases TGF-β1 Secretion
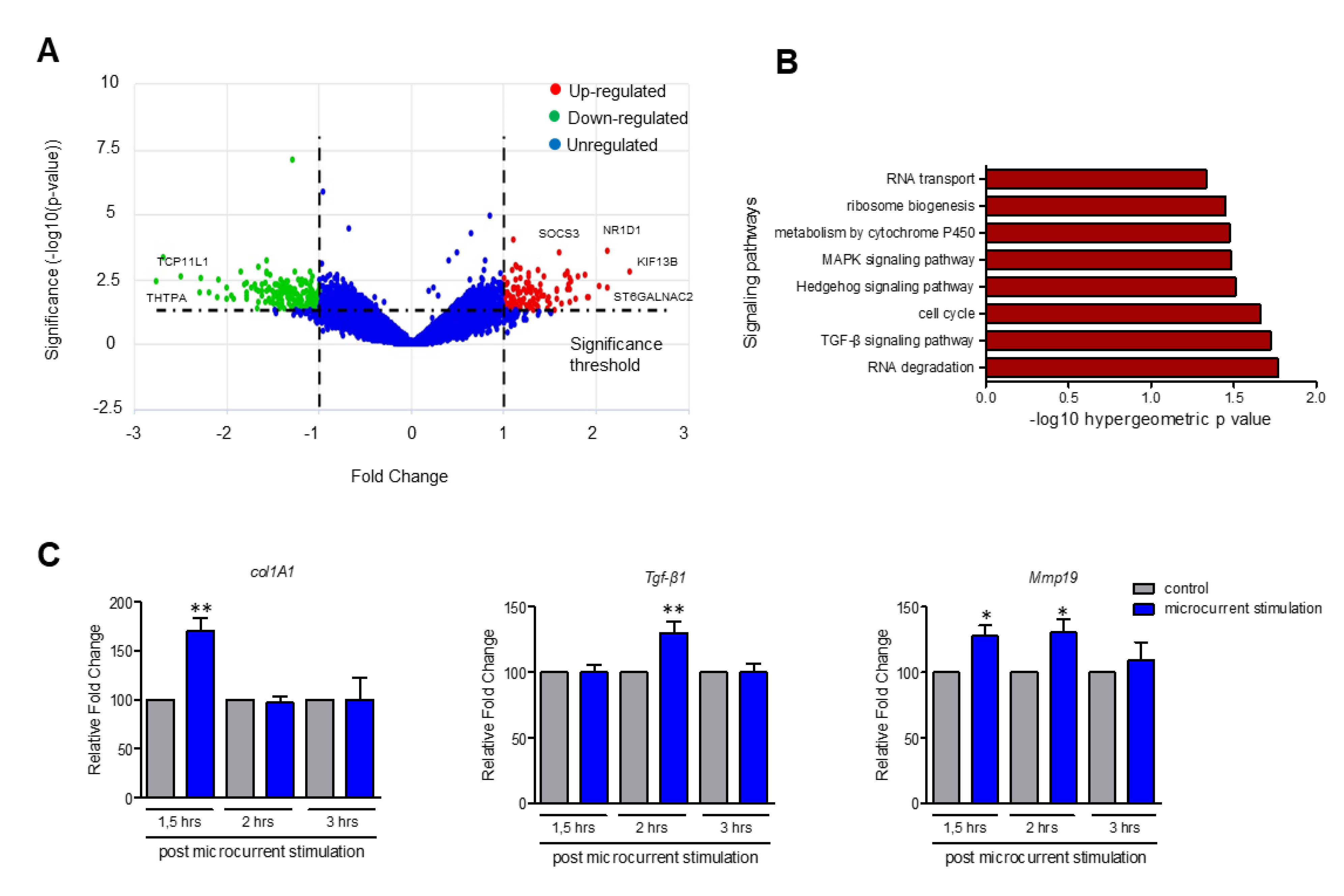
3.5. Upregulation of Genes Participating in TGF-β, MAPK and Hedgehog Signaling Pathways upon Stimulation with Microcurrents
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.; Johnson, C.; Burslem, F.; Martin, P. Wound healing and inflammation genes revealed by array analysis of “macrophageless” PU.1 null mice. Genome Biol. 2005, 6, 1–17. [Google Scholar]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef]
- Ud-Din, S.; Bayat, A. Electrical Stimulation and Cutaneous Wound Healing: A Review of Clinical Evidence. Healthcare 2014, 2, 445–467. [Google Scholar] [CrossRef] [Green Version]
- Ud-Din, S.; Sebastian, A.; Giddings, P.; Colthurst, J.; Whiteside, S.; Morris, J.; Nuccitelli, R.; Pullar, C.; Baguneid, M.; Bayat, A. Angiogenesis is induced and wound size is reduced by electrical stimulation in an acute wound healing model in human skin. PLoS ONE 2015, 10, e0124502. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.C.D.O.; Andrade, Z.D.A.; Costa, T.F.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [Green Version]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Foulds, I.S.; Barker, A.T. Human skin battery potentials and their possible role in wound healing. Br. J. Dermatol. 1983, 109, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.; Zhao, M. The Electrical Response to Injury: Molecular Mechanisms and Wound Healing. Adv. Wound Care 2014, 3, 184–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Song, B.; Pu, J.; Wada, T.; Reid, B.; Tai, G.; Wang, F.; Guo, A.; Walczysko, P.; Gu, Y.; et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature 2006, 442, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Isseroff, R.R.; Dahle, S.E. Electrical Stimulation Therapy and Wound Healing: Where Are We Now? Adv. Wound Care 2012, 1, 238–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taghian, T.; Narmoneva, D.A.; Kogan, A.B. Modulation of cell function by electric field: A high-resolution analysis. J. R. Soc. Interface 2015, 12, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Ramadhinara, A.; Poulas, K. Use of wireless microcurrent stimulation for the treatment of diabetes-related wounds: 2 case reports. Adv. Ski. Wound Care 2013, 26, 1–4. [Google Scholar] [CrossRef]
- Hunckler, J.; de Mel, A. A current affair: Electrotherapy in wound healing. J. Multidiscip. Healthc. 2017, 10, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.Y.; Wendell, K.; Al-Waili, N.; Butler, G. Ultra-low microcurrent therapy: A novel approach for treatment of chronic resistant wounds. Adv. Ther. 2007, 24, 1202–1209. [Google Scholar] [CrossRef]
- Todd, I.; Clothier, R.H.; Huggins, M.L.; Patel, N.; Searle, K.C.; Jeyarajah, S.; Pradel, L.; Lacey, K.L. Electrical stimulation of transforming growth factor-β1 secretion by human dermal fibroblasts and the U937 human monocytic cell line. Atla Altern. Lab. Anim. 2001, 29, 693–701. [Google Scholar] [CrossRef] [PubMed]
- McCaig, C.D.; Song, B.; Rajnicek, A.M. Electrical dimensions in cell science. J. Cell Sci. 2009, 122, 4267–4276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayat, M.; Asgari-Moghadam, Z.; Maroufi, M.; Rezaie, F.S.; Bayat, M.; Rakhshan, M. Experimental wound healing using microamperage electrical stimulation in rabbits. J. Rehabil. Res. Dev. 2006, 43, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Castana, O.; Dimitrouli, A.; Argyrakos, T.; Theodorakopoulou, E.; Stampolidis, N.; Papadopoulos, E.; Pallantzas, A.; Stasinopoulos, I.; Poulas, K. Wireless electrical stimulation: An innovative powerful tool for the treatment of a complicated chronic ulcer. Int. J. Low. Extrem. Wounds 2013, 12, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Wirsing, P.G.; Habrom, A.D.; Zehnder, T.M.; Friedli, S.; Blatti, M. Wireless micro current stimulation—An innovative electrical stimulation method for the treatment of patients with leg and diabetic foot ulcers. Int. Wound J. 2015, 12, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.Q.; Taghian, T.; Hemingway, B.; Cho, H.; Kogan, A.B.; Narmoneva, D.A. Regulation of endothelial MAPK/ERK signalling and capillary morphogenesis by low-amplitude electric field. J. R. Soc. Interface 2013, 10. [Google Scholar] [CrossRef] [Green Version]
- Pearson, G.; Robinson, F.; Gibson, T.B.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-Activated Protein (MAP) Kinase Pathways: Regulation and Physiological Functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar]
- Pucihar, G.; Kotnik, T.; Kandušer, M.; Miklavčič, D. The influence of medium conductivity on electropermeabilization and survival of cells in vitro. Bioelectrochemistry 2001, 54, 107–115. [Google Scholar] [CrossRef]
- Soares dos Santos, M.P.; Coutinho, J.; Marote, A.; Sousa, B.; Ramos, A.; Ferreira, J.A.F.; Bernardo, R.; Rodrigues, A.; Marques, A.T.; da Cruz e Silva, O.A.B.; et al. Capacitive technologies for highly controlled and personalized electrical stimulation by implantable biomedical systems. Sci. Rep. 2019, 9, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Harrington, C.A.; Michael, W.; Michelle, M.G. Use of bioanalyzer electropherograms for quality control and target evaluation in microarray expression profiling studies of ocular tissues. J. Ocul. Biol. Dis. Inform. 2009, 2, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Moulos, P.; Hatzis, P. Systematic integration of RNA-Seq statistical algorithms for accurate detection of differential gene expression patterns. Nucleic Acids Res. 2015, 43, 1–12. [Google Scholar] [CrossRef]
- De Crignis, E.; Carofiglio, F.; Moulos, P.; Verstegen, M.M.A.; Romal, S.; Mubashir Khalid, M.; Pourfarzad, F.; Koutsothanassis, C.; Gehart, H.; Wai Kan, T.; et al. Human liver organoids; a patient-derived primary model for HBV Infection and Related Hepatocellular Carcinoma. bioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Arvaniti, E.; Moulos, P.; Vakrakou, A.; Chatziantoniou, C.; Chadjichristos, C.; Kavvadas, P.; Charonis, A.; Politis, P.K. Whole-transcriptome analysis of UUO mouse model of renal fibrosis reveals new molecular players in kidney diseases. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karagianni, P.; Moulos, P.; Schmidt, D.; Odom, D.T.; Talianidis, I. Bookmarking by Non-pioneer Transcription Factors during Liver Development Establishes Competence for Future Gene Activation. Cell Rep. 2020, 30, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Moulos, P.; Samiotaki, M.; Panayotou, G.; Dedos, S.G. Combinatory annotation of cell membrane receptors and signalling pathways of Bombyx mori prothoracic glands. Sci. Data 2016, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Desjardins-Park, H.E.; Foster, D.S.; Longaker, M.T. Fibroblasts and wound healing: An update. Regen. Med. 2018, 13, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Pautke, C.; Schieker, M.; Tischer, T.; Kolk, A.; Neth, P.; Mutschler, W.; Milz, S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004, 24, 3743–3748. [Google Scholar]
- Lohmann, C.H.; Schwartz, Z.; Liu, Y.; Guerkov, H.; Dean, D.D.; Simon, B.; Boyan, B.D. Pulsed electromagnetic field stimulation of MG63 osteoblast-like cells affects differentiation and local factor production. J. Orthop. Res. 2000, 18, 637–646. [Google Scholar] [CrossRef]
- Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K. The role of the extracellular matrix components in cutaneous wound healing. BioMed Res. Int. 2014, 2014, 747584. [Google Scholar] [CrossRef] [Green Version]
- Le, H.; Kleinerman, R.; Lerman, O.Z.; Brown, D.; Galiano, R.; Gurtner, G.C.; Warren, S.M.; Levine, J.P.; Saadeh, P.B. Hedgehog signaling is essential for normal wound healing. Wound Repair Regen. 2008, 16, 768–773. [Google Scholar] [CrossRef]
- Zhu, Z.X.; Sun, C.C.; Ting Zhu, Y.; Wang, Y.; Wang, T.; Chi, L.S.; Cai, W.H.; Zheng, J.Y.; Zhou, X.; Cong, W.T.; et al. Hedgehog signaling contributes to basic fibroblast growth factor-regulated fibroblast migration. Exp. Cell Res. 2017, 355, 83–94. [Google Scholar] [CrossRef]
- Di Mauro, C.; Rosa, R.; D’Amato, V.; Ciciola, P.; Servetto, A.; Marciano, R.; Orsini, R.C.; Formisano, L.; De Falco, S.; Cicatiello, V.; et al. Hedgehog signalling pathway orchestrates angiogenesis in triple-negative breast cancers. Br. J. Cancer 2017, 116, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Messerli, M.A.; Graham, D.M. Extracellular electrical fields direct wound healing and regeneration. Biol. Bull. 2011, 221, 79–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaron, R.K.; Ciombor, D.M.K.; Simon, B.J. Treatment of Nonunions with Electric and Electromagnetic Fields. Clin. Orthop. Relat. Res. 2004, 02906, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ciombor, D.M.K.; Aaron, R.K. The role of electrical stimulation in bone repair. Foot Ankle Clin. 2005, 10, 579–593. [Google Scholar] [CrossRef]
- Nishimura, K.Y.; Isseroff, R.R.; Nucciteili, R. Human keratinocytes migrate to the negative pole in direct current electric fields comparable to those measured in mammalian wounds. J. Cell Sci. 1996, 109, 199–207. [Google Scholar]
- Gentzkow, G.D. Electrical Stimulation to Heal Dermal Wounds. J. Dermatol. Surg. Oncol. 1993, 19, 753–758. [Google Scholar] [CrossRef]
- Sun, Y.S. Electrical Stimulation for Wound-Healing: Simulation on the Effect of Electrode Configurations. Biomed. Res. Int. 2017. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Bai, H.; Wang, E.; Forrester, J.V.; McCaig, C.D. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J. Cell Sci. 2004, 117, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Lagoumintzis, G.; Zagoriti, Z.; Jensen, M.S.; Argyrakos, T.; Koutsojannis, C.; Poulas, K. Wireless direct microampere current in wound healing: Clinical and immunohistological data from two single case reports. Biosensors 2019, 9, 107. [Google Scholar] [CrossRef] [Green Version]
- Wang, E.; Zhao, M.; Forrester, J.V.; McCaig, C.D. Electric fields and MAP kinase signaling can regulate early wound healing in lens epithelium. Investig. Ophthalmol. Vis. Sci. 2003, 44, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Jacobson, K.; Schaller, M.D. MAP kinases and cell migration. J. Cell Sci. 2004, 117, 4619–4628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos-Silva, M.A.; Trajano, E.T.L.; Schanuel, F.S.; Monte-Alto-Costa, A. Heat delays skin wound healing in mice. Exp. Biol. Med. 2017, 242, 258–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinter, M.; Sieghart, W.; Schmid, M.; Dauser, B.; Prager, G.; Dienes, H.P.; Trauner, M.; Peck-Radosavljevic, M. Hedgehog inhibition reduces angiogenesis by downregulation of tumoral VEGF-A expression in hepatocellular carcinoma. United Eur. Gastroenterol. J. 2013, 1, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Laurendeau, I.; Ferrer, M.; Garrido, D.; D’Haene, N.; Ciavarelli, P.; Basso, A.; Vidaud, M.; Bieche, I.; Salmon, I.; Szijan, I. Gene Expression Profiling of the Hedgehog Signaling Pathway in Human Meningiomas. Mol. Med. 2010, 16, 262–270. [Google Scholar] [CrossRef]
- Janda, E.; Lehmann, K.; Killisch, I.; Jechlinger, M.; Herzig, M.; Downward, J.; Beug, H.; Grünert, S. Ras and TGFβ cooperatively regulate epithelial cell plasticity and metastasis: Dissection of Ras signaling pathways. J. Cell Biol. 2002, 156, 299–313. [Google Scholar] [CrossRef]
- Medicherla, S.; Wadsworth, S.; Cullen, B.; Silcock, D.; Ma, J.Y.; Mangadu, R.; Kerr, I.; Chakravarty, S.; Luedtke, G.L.; Dugar, S.; et al. p38 MAPK inhibition reduces diabetes-induced impairment of wound healing. Diabetesmetab. Syndr. Obes. Targets Ther. 2009, 2, 91–100. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinou, E.; Zagoriti, Z.; Pyriochou, A.; Poulas, K. Microcurrent Stimulation Triggers MAPK Signaling and TGF-β1 Release in Fibroblast and Osteoblast-Like Cell Lines. Cells 2020, 9, 1924. https://doi.org/10.3390/cells9091924
Konstantinou E, Zagoriti Z, Pyriochou A, Poulas K. Microcurrent Stimulation Triggers MAPK Signaling and TGF-β1 Release in Fibroblast and Osteoblast-Like Cell Lines. Cells. 2020; 9(9):1924. https://doi.org/10.3390/cells9091924
Chicago/Turabian StyleKonstantinou, Evangelia, Zoi Zagoriti, Anastasia Pyriochou, and Konstantinos Poulas. 2020. "Microcurrent Stimulation Triggers MAPK Signaling and TGF-β1 Release in Fibroblast and Osteoblast-Like Cell Lines" Cells 9, no. 9: 1924. https://doi.org/10.3390/cells9091924
APA StyleKonstantinou, E., Zagoriti, Z., Pyriochou, A., & Poulas, K. (2020). Microcurrent Stimulation Triggers MAPK Signaling and TGF-β1 Release in Fibroblast and Osteoblast-Like Cell Lines. Cells, 9(9), 1924. https://doi.org/10.3390/cells9091924






