Alterations in Progesterone Receptor Isoform Balance in Normal and Neoplastic Breast Cells Modulates the Stem Cell Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice, Treatments with Steroids and Tissue Preparations
2.2. Mammary Gland Primary Cultures
2.3. Cell Culture
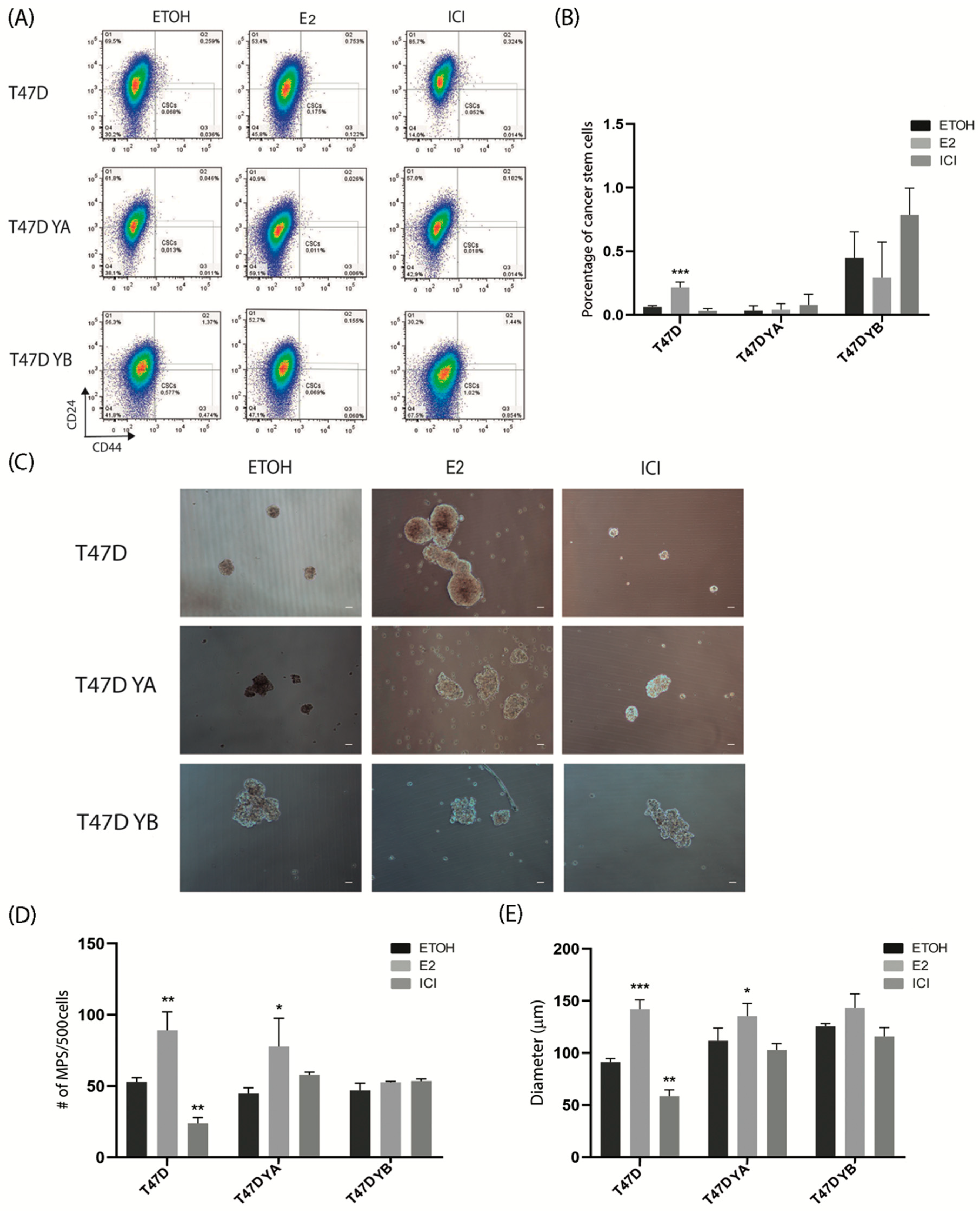
2.4. Mammosphere Assays
2.5. Flow Cytometry
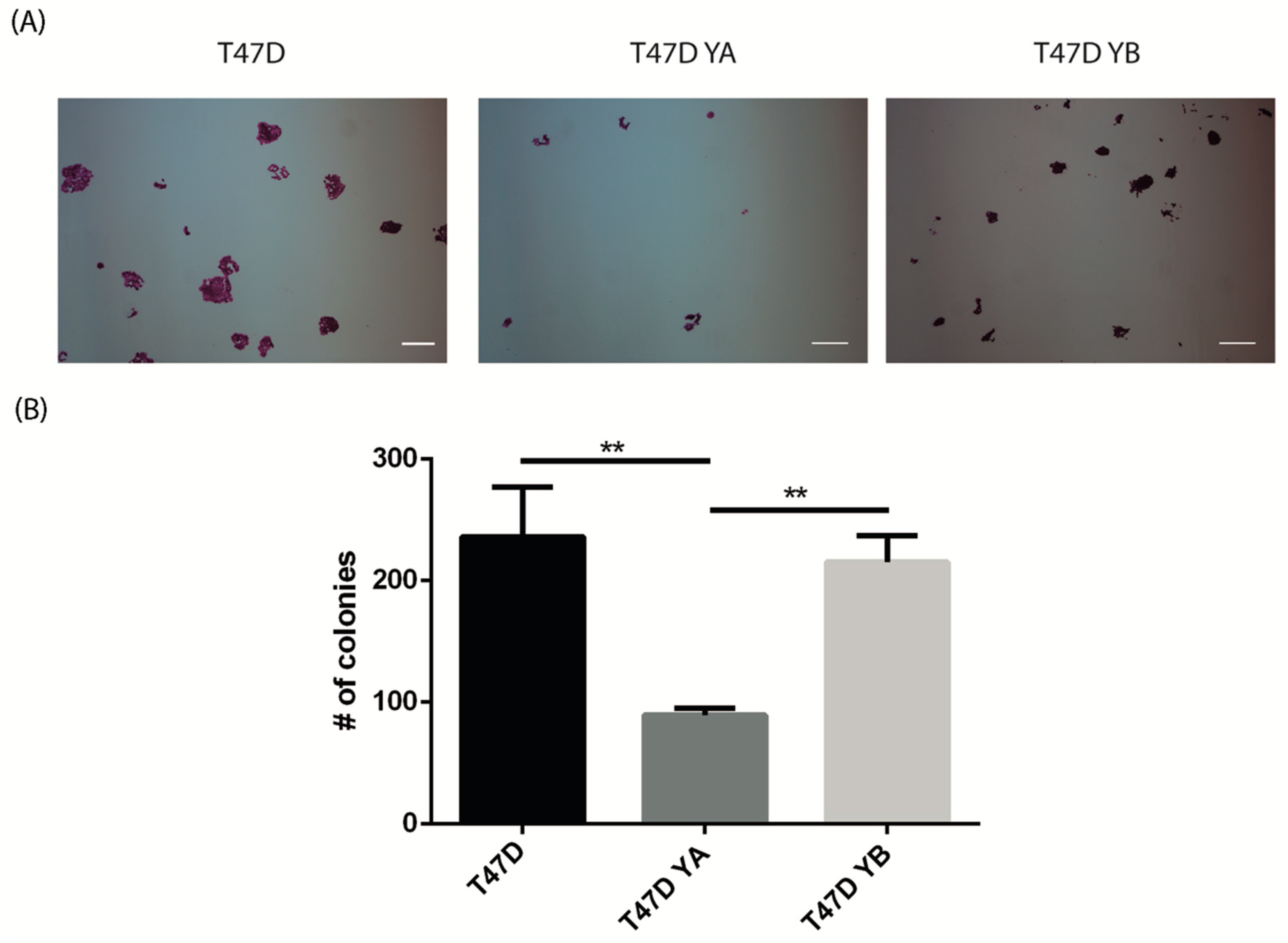
2.6. Clonogenic Assays
2.7. Immunofluorescence
2.8. Western Blots
2.9. Statistical Analysis
3. Results
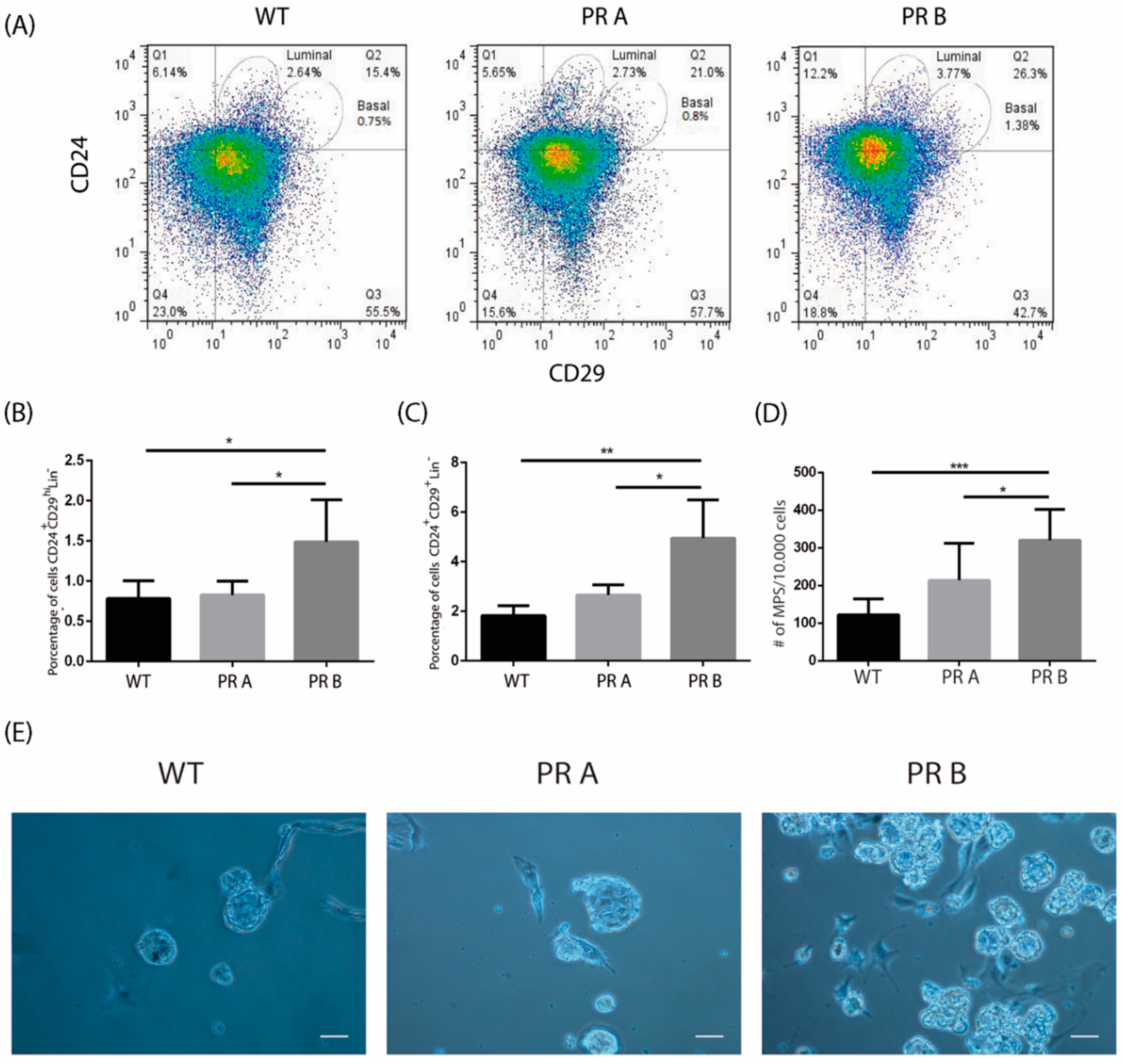
3.1. PRB Overexpression Alters the Luminal and Basal Cell Compartments in the Normal Mouse Mammary Gland
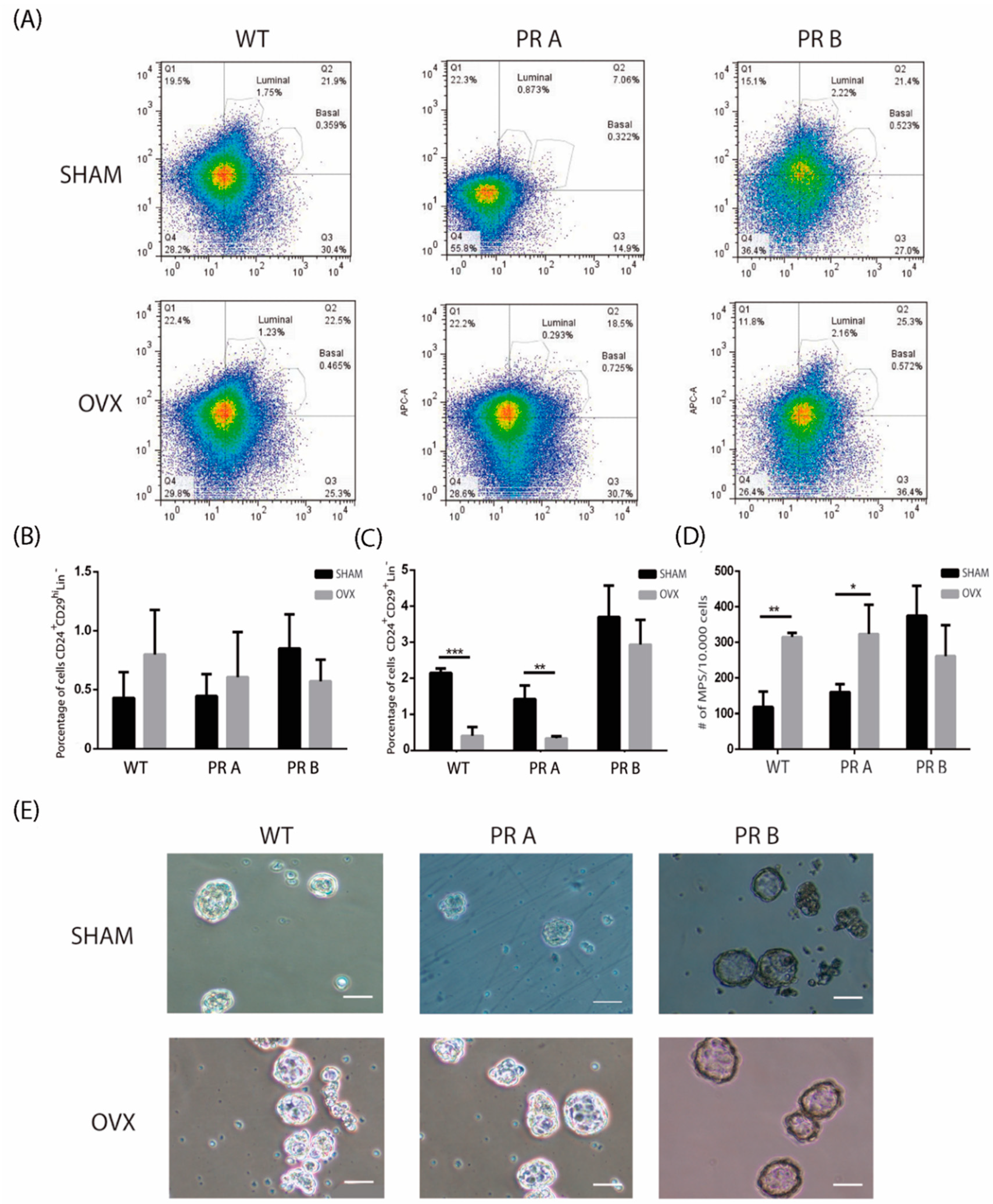
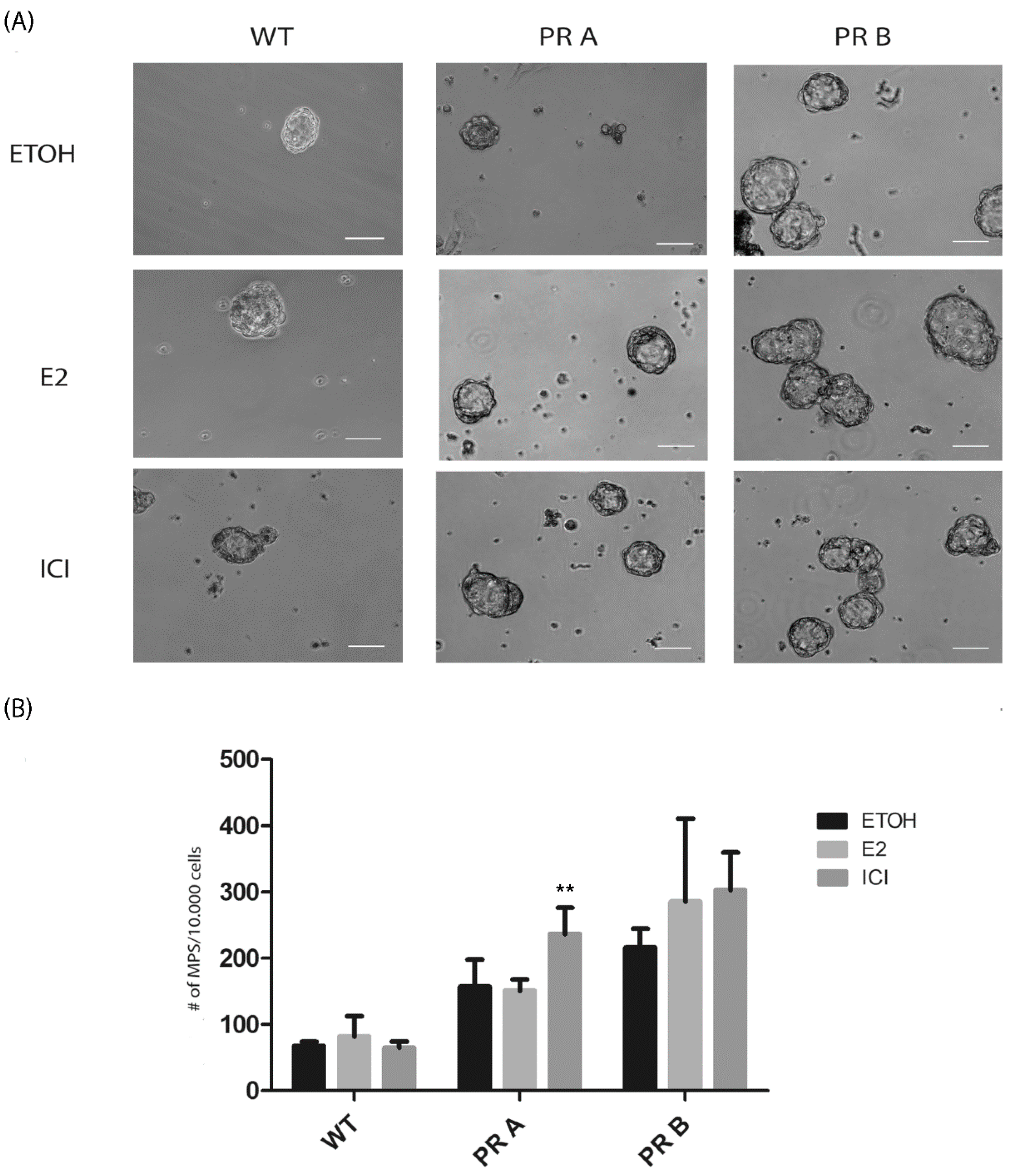
3.2. Hormonal Regulation of the Luminal and Basal Compartments in Mammary Glands of WT and PR Transgenic Mice
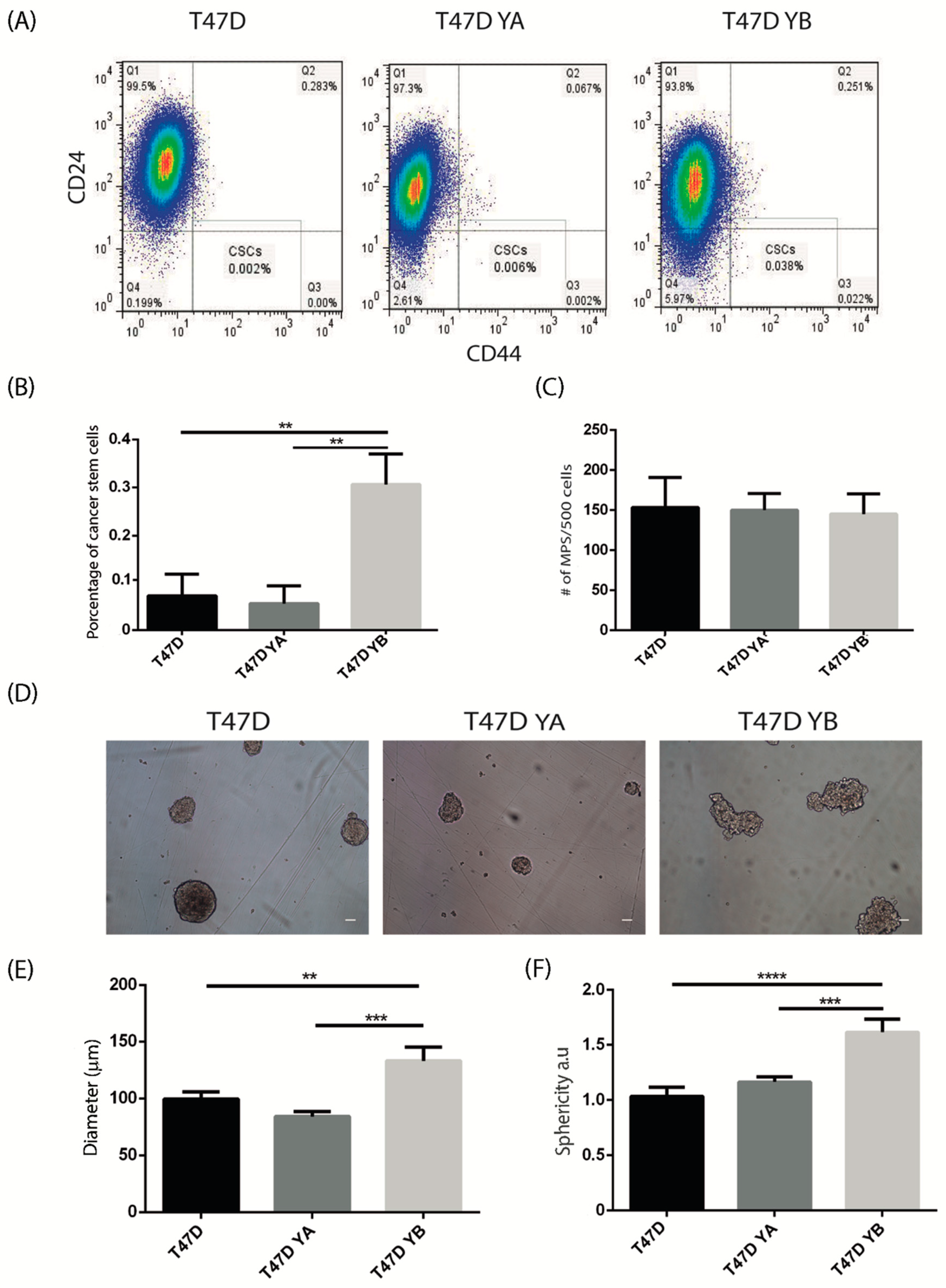
3.3. Changes in PR Isoform Balance in Breast Cancer Cells Impacts on the Stem Cell Population
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Visvader, J.E.; Stingl, J. Mammary stem cells and the differentiation hierarchy: Current status and perspectives. Genes Dev. 2014, 28, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- McNally, S.; Stein, T. Overview of Mammary Gland Development: A Comparison of Mouse and Human. Methods Mol. Biol. 2017, 1501, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Finlay-Schultz, J.; Sartorius, C.A. Steroid hormones, steroid receptors, and breast cancer stem cells. J. Mammary Gland Biol. Neoplasia 2015, 20, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Arnal, J.F.; Lenfant, F.; Metivier, R.; Flouriot, G.; Henrion, D.; Adlanmerini, M.; Fontaine, C.; Gourdy, P.; Chambon, P.; Katzenellenbogen, B.; et al. Membrane and nuclear estrogen receptor alpha actions: From tissue specificity to medical implications. Physiol. Rev. 2017, 97, 1045–1087. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.C. Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocr. Relat. Cancer 2014, 21, R235–R246. [Google Scholar] [CrossRef]
- Asselin-Labat, M.L.; Vaillant, F.; Sheridan, J.M.; Pal, B.; Wu, D.; Simpson, E.R.; Yasuda, H.; Smyth, G.K.; Martin, T.J.; Lindeman, G.J.; et al. Control of mammary stem cell function by steroid hormone signalling. Nature 2010, 465, 798–802. [Google Scholar] [CrossRef]
- Joshi, P.A.; Jackson, H.W.; Beristain, A.G.; Di Grappa, M.A.; Mote, P.A.; Clarke, C.L.; Stingl, J.; Waterhouse, P.D.; Khokha, R. Progesterone induces adult mammary stem cell expansion. Nature 2010, 465, 803–807. [Google Scholar] [CrossRef]
- Cittelly, D.M.; Finlay-Schultz, J.; Howe, E.N.; Spoelstra, N.S.; Axlund, S.D.; Hendricks, P.; Jacobsen, B.M.; Sartorius, C.A.; Richer, J.K. Progestin suppression of miR-29 potentiates dedifferentiation of breast cancer cells via KLF4. Oncogene 2013, 32, 2555–2564. [Google Scholar] [CrossRef]
- Axlund, S.D.; Yoo, B.H.; Rosen, R.B.; Schaack, J.; Kabos, P.; Labarbera, D.V.; Sartorius, C.A. Progesterone-inducible cytokeratin 5-positive cells in luminal breast cancer exhibit progenitor properties. Horm. Cancer 2013, 4, 36–49. [Google Scholar] [CrossRef]
- Raffo, D.; Berardi, D.E.; Pontiggia, O.; Todaro, L.; de Kier Joffe, E.B.; Simian, M. Tamoxifen selects for breast cancer cells with mammosphere forming capacity and increased growth rate. Breast Cancer Res. Treat. 2013, 142, 537–548. [Google Scholar] [CrossRef]
- Simoes, B.M.; Vivanco, M.D. Cancer stem cells in the human mammary gland and regulation of their differentiation by estrogen. Future Oncol. 2011, 7, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S.L.; Hartig, S.M.; Edwards, D.P. Progesterone receptor signaling mechanisms. J. Mol. Biol. 2016, 428, 3831–3849. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, K.B.; Dye, W.W.; Harrell, J.C.; Kabos, P.; Sartorius, C.A. Rare steroid receptor-negative basal-like tumorigenic cells in luminal subtype human breast cancer xenografts. Proc. Natl. Acad. Sci. USA 2008, 105, 5774–5779. [Google Scholar] [CrossRef] [PubMed]
- Kabos, P.; Haughian, J.M.; Wang, X.; Dye, W.W.; Finlayson, C.; Elias, A.; Horwitz, K.B.; Sartorius, C.A. Cytokeratin 5 positive cells represent a steroid receptor negative and therapy resistant subpopulation in luminal breast cancers. Breast Cancer Res. Treat. 2011, 128, 45–55. [Google Scholar] [CrossRef]
- Kastner, P.; Krust, A.; Turcotte, B.; Stropp, U.; Tora, L.; Gronemeyer, H.; Chambon, P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990, 9, 1603–1614. [Google Scholar] [CrossRef]
- Shyamala, G.; Yang, X.; Silberstein, G.; Barcellos-Hoff, M.H.; Dale, E. Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc. Natl. Acad. Sci. USA 1998, 95, 696–701. [Google Scholar] [CrossRef]
- Simian, M.; Bissell, M.J.; Barcellos-Hoff, M.H.; Shyamala, G. Estrogen and progesterone receptors have distinct roles in the establishment of the hyperplastic phenotype in PR-A transgenic mice. Breast Cancer Res. 2009, 11, R72. [Google Scholar] [CrossRef]
- Shyamala, G.; Yang, X.; Cardiff, R.D.; Dale, E. Impact of progesterone receptor on cell-fate decisions during mammary gland development. Proc. Natl. Acad. Sci. USA 2000, 97, 3044–3049. [Google Scholar] [CrossRef]
- Lydon, J.P.; Ge, G.; Kittrell, F.S.; Medina, D.; O’Malley, B.W. Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res. 1999, 59, 4276–4284. [Google Scholar]
- Poole, A.J.; Li, Y.; Kim, Y.; Lin, S.C.; Lee, W.H.; Lee, E.Y. Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science 2006, 314, 1467–1470. [Google Scholar] [CrossRef]
- Gonzalez-Suarez, E.; Jacob, A.P.; Jones, J.; Miller, R.; Roudier-Meyer, M.P.; Erwert, R.; Pinkas, J.; Branstetter, D.; Dougall, W.C. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 2010, 468, 103–107. [Google Scholar] [CrossRef]
- Schramek, D.; Leibbrandt, A.; Sigl, V.; Kenner, L.; Pospisilik, J.A.; Lee, H.J.; Hanada, R.; Joshi, P.A.; Aliprantis, A.; Glimcher, L.; et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 2010, 468, 98–102. [Google Scholar] [CrossRef]
- Hopp, T.A.; Weiss, H.L.; Hilsenbeck, S.G.; Cui, Y.; Allred, D.C.; Horwitz, K.B.; Fuqua, S.A. Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin. Cancer Res. 2004, 10, 2751–2760. [Google Scholar] [CrossRef]
- Mote, P.A.; Bartow, S.; Tran, N.; Clarke, C.L. Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res. Treat. 2002, 72, 163–172. [Google Scholar] [CrossRef]
- Graham, J.D.; Yeates, C.; Balleine, R.L.; Harvey, S.S.; Milliken, J.S.; Bilous, A.M.; Clarke, C.L. Characterization of progesterone receptor A and B expression in human breast cancer. Cancer Res. 1995, 55, 5063–5068. [Google Scholar]
- Bonneterre, J.; Bosq, J.; Jamme, P.; Valent, A.; Gilles, E.M.; Zukiwski, A.A.; Fuqua, S.A.; Lange, C.A.; O’Shaughnessy, J. Tumour and cellular distribution of activated forms of PR in breast cancers: A novel immunohistochemical analysis of a large clinical cohort. ESMO Open 2016, 1, e000072. [Google Scholar] [CrossRef]
- Rojas, P.A.; May, M.; Sequeira, G.R.; Elia, A.; Alvarez, M.; Martinez, P.; Gonzalez, P.; Hewitt, S.; He, X.; Perou, C.M.; et al. Progesterone receptor isoform ratio: A Breast cancer prognostic and predictive factor for antiprogestin responsiveness. J. Natl. Cancer Inst. 2017, 109, djw317. [Google Scholar] [CrossRef]
- Carlini, M.J.; Recouvreux, M.S.; Simian, M.; Nagai, M.A. Gene expression profile and cancer-associated pathways linked to progesterone receptor isoform a (PRA) predominance in transgenic mouse mammary glands. BMC Cancer 2018, 18, 682. [Google Scholar] [CrossRef]
- Pontiggia, O.; Sampayo, R.; Raffo, D.; Motter, A.; Xu, R.; Bissell, M.J.; de Kier Joffe, E.B.; Simian, M. The tumor microenvironment modulates tamoxifen resistance in breast cancer: A role for soluble stromal factors and fibronectin through beta1 integrin. Breast Cancer Res. Treat. 2012, 133, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Dontu, G.; Abdallah, W.M.; Foley, J.M.; Jackson, K.W.; Clarke, M.F.; Kawamura, M.J.; Wicha, M.S. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003, 17, 1253–1270. [Google Scholar] [CrossRef]
- Shackleton, M.; Vaillant, F.; Simpson, K.J.; Stingl, J.; Smyth, G.K.; Asselin-Labat, M.L.; Wu, L.; Lindeman, G.J.; Visvader, J.E. Generation of a functional mammary gland from a single stem cell. Nature 2006, 439, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Ito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Asselin-Labat, M.L.; Shackleton, M.; Stingl, J.; Vaillant, F.; Forrest, N.C.; Eaves, C.J.; Visvader, J.E.; Lindeman, G.J. Steroid hormone receptor status of mouse mammary stem cells. J. Natl. Cancer Inst. 2006, 98, 1011–1014. [Google Scholar] [CrossRef]
- Traboulsi, T.; El Ezzy, M.; Gleason, J.L.; Mader, S. Antiestrogens: Structure-activity relationships and use in breast cancer treatment. J. Mol. Endocrinol. 2017, 58, R15–R31. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, C.A.; Groshong, S.D.; Miller, L.A.; Powell, R.L.; Tung, L.; Takimoto, G.S.; Horwitz, K.B. New T47D breast cancer cell lines for the independent study of progesterone B- and A-receptors: Only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 1994, 54, 3868–3877. [Google Scholar] [PubMed]
- Kenny, P.A.; Lee, G.Y.; Myers, C.A.; Neve, R.M.; Semeiks, J.R.; Spellman, P.T.; Lorenz, K.; Lee, E.H.; Barcellos-Hoff, M.H.; Petersen, O.W.; et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol. 2007, 1, 84–96. [Google Scholar] [CrossRef]
- Graham, J.D.; Mote, P.A.; Salagame, U.; van Dijk, J.H.; Balleine, R.L.; Huschtscha, L.I.; Reddel, R.R.; Clarke, C.L. DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology 2009, 150, 3318–3326. [Google Scholar] [CrossRef]
- Kariagina, A.; Aupperlee, M.D.; Haslam, S.Z. Progesterone receptor isoform functions in normal breast development and breast cancer. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 11–33. [Google Scholar] [CrossRef]
- Aupperlee, M.D.; Smith, K.T.; Kariagina, A.; Haslam, S.Z. Progesterone receptor isoforms A and B: Temporal and spatial differences in expression during murine mammary gland development. Endocrinology 2005, 146, 3577–3588. [Google Scholar] [CrossRef]
- Mulac-Jericevic, B.; Lydon, J.P.; DeMayo, F.J.; Conneely, O.M. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc. Natl. Acad. Sci. USA 2003, 100, 9744–9749. [Google Scholar] [CrossRef]
- Shiah, Y.J.; Tharmapalan, P.; Casey, A.E.; Joshi, P.A.; McKee, T.D.; Jackson, H.W.; Beristain, A.G.; Chan-Seng-Yue, M.A.; Bader, G.D.; Lydon, J.P.; et al. A progesterone-CXCR4 axis controls mammary progenitor cell fate in the adult gland. Stem Cell Rep. 2015, 4, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.H.; Dwyer, A.R.; Diep, C.H.; Hu, H.; Hagen, K.M.; Lange, C.A. Phosphorylated progesterone receptor isoforms mediate opposing stem cell and proliferative breast cancer cell fates. Endocrinology 2019, 160, 430–446. [Google Scholar] [CrossRef]
- Hines, W.C.; Su, Y.; Kuhn, I.; Polyak, K.; Bissell, M.J. Sorting out the FACS: A devil in the details. Cell Rep. 2014, 6, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Obradovic, M.M.S.; Hoffmann, M.; Harper, K.L.; Sosa, M.S.; Werner-Klein, M.; Nanduri, L.K.; Werno, C.; Ehrl, C.; Maneck, M.; et al. Early dissemination seeds metastasis in breast cancer. Nature 2016, 540, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Harper, K.L.; Sosa, M.S.; Entenberg, D.; Hosseini, H.; Cheung, J.F.; Nobre, R.; Avivar-Valderas, A.; Nagi, C.; Girnius, N.; Davis, R.J.; et al. Mechanism of early dissemination and metastasis in Her2(+) mammary cancer. Nature 2016, 540, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, C.A.; Shen, T.; Horwitz, K.B. Progesterone receptors A and B differentially affect the growth of estrogen-dependent human breast tumor xenografts. Breast Cancer Res. Treat. 2003, 79, 287–299. [Google Scholar] [CrossRef]
- Pathiraja, T.N.; Shetty, P.B.; Jelinek, J.; He, R.; Hartmaier, R.; Margossian, A.L.; Hilsenbeck, S.G.; Issa, J.P.; Oesterreich, S. Progesterone receptor isoform-specific promoter methylation: Association of PRA promoter methylation with worse outcome in breast cancer patients. Clin. Cancer Res. 2011, 17, 4177–4186. [Google Scholar] [CrossRef]
- Wargon, V.; Riggio, M.; Giulianelli, S.; Sequeira, G.R.; Rojas, P.; May, M.; Polo, M.L.; Gorostiaga, M.A.; Jacobsen, B.; Molinolo, A.; et al. Progestin and antiprogestin responsiveness in breast cancer is driven by the PRA/PRB ratio via AIB1 or SMRT recruitment to the CCND1 and MYC promoters. Int. J. Cancer 2015, 136, 2680–2692. [Google Scholar] [CrossRef]
- Singhal, H.; Greene, M.E.; Zarnke, A.L.; Laine, M.; Al Abosy, R.; Chang, Y.F.; Dembo, A.G.; Schoenfelt, K.; Vadhi, R.; Qiu, X.; et al. Progesterone receptor isoforms, agonists and antagonists differentially reprogram estrogen signaling. Oncotarget 2018, 9, 4282–4300. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recouvreux, M.S.; Diaz Bessone, M.I.; Taruselli, A.; Todaro, L.; Lago Huvelle, M.A.; Sampayo, R.G.; Bissell, M.J.; Simian, M. Alterations in Progesterone Receptor Isoform Balance in Normal and Neoplastic Breast Cells Modulates the Stem Cell Population. Cells 2020, 9, 2074. https://doi.org/10.3390/cells9092074
Recouvreux MS, Diaz Bessone MI, Taruselli A, Todaro L, Lago Huvelle MA, Sampayo RG, Bissell MJ, Simian M. Alterations in Progesterone Receptor Isoform Balance in Normal and Neoplastic Breast Cells Modulates the Stem Cell Population. Cells. 2020; 9(9):2074. https://doi.org/10.3390/cells9092074
Chicago/Turabian StyleRecouvreux, María Sol, María Inés Diaz Bessone, Agustina Taruselli, Laura Todaro, María Amparo Lago Huvelle, Rocío G. Sampayo, Mina J. Bissell, and Marina Simian. 2020. "Alterations in Progesterone Receptor Isoform Balance in Normal and Neoplastic Breast Cells Modulates the Stem Cell Population" Cells 9, no. 9: 2074. https://doi.org/10.3390/cells9092074
APA StyleRecouvreux, M. S., Diaz Bessone, M. I., Taruselli, A., Todaro, L., Lago Huvelle, M. A., Sampayo, R. G., Bissell, M. J., & Simian, M. (2020). Alterations in Progesterone Receptor Isoform Balance in Normal and Neoplastic Breast Cells Modulates the Stem Cell Population. Cells, 9(9), 2074. https://doi.org/10.3390/cells9092074




