Lessons from Bacillus Calmette-Guérin: Harnessing Trained Immunity for Vaccine Development
Abstract
:1. Introduction
2. BCG Vaccination and Tuberculosis Infection
3. Nonspecific and Enhancing Effects of BCG-Induced Trained Immunity
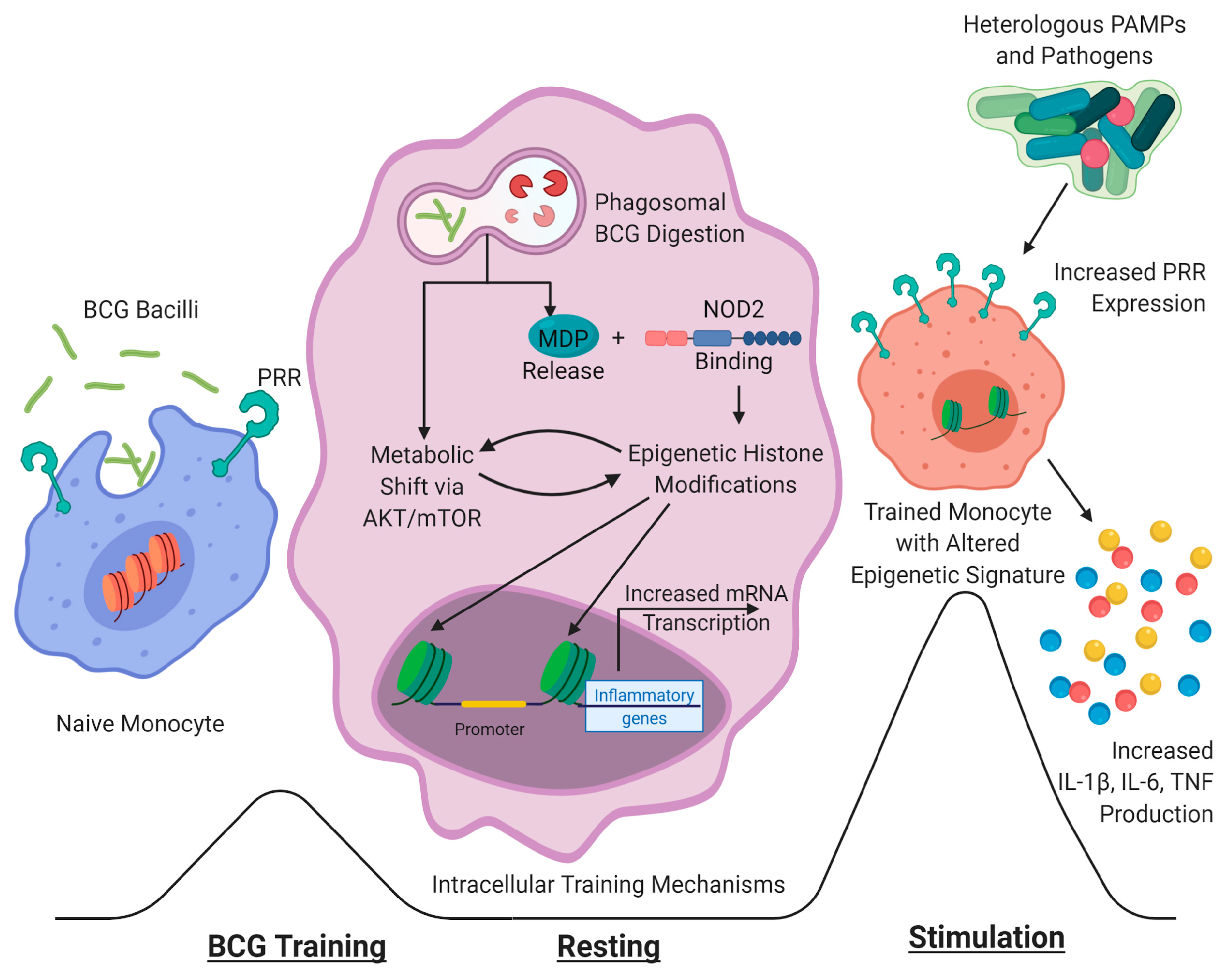
4. Mechanisms of BCG-Induced Protective and Trained Immunity
5. Optimizing BCG Formulation and Delivery to Augment Innate Responses
5.1. Vaccine Delivery
5.2. Vaccine Formulation
6. Harnessing and Improving BCG-Elicited Trained Immunity against Mtb and Beyond
7. Future Directions in Exploiting Trained Immunity
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Bree, L.; Koeken, V.A.C.M.; Joosten, L.A.; Aaby, P.; Benn, C.S.; van Crevel, R.; Netea, M.G.; De Bree, C.L. Non-specific effects of vaccines: Current evidence and potential implications. Semin. Immunol. 2018, 39, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Uthayakumar, D.; Paris, S.; Chapat, L.; Freyburger, L.; Poulet, H.; De Luca, K. Non-specific Effects of Vaccines Illustrated Through the BCG Example: From Observations to Demonstrations. Front. Immunol. 2018, 9, 2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khader, S.A.; Divangahi, M.; Hanekom, W.; Hill, P.C.; Maeurer, M.; Makar, K.W.; Mayer-Barber, K.D.; Mhlanga, M.M.; Nemes, E.; Schlesinger, L.S.; et al. Targeting innate immunity for tuberculosis vaccination. J. Clin. Investig. 2019, 129, 3482–3491. [Google Scholar] [CrossRef] [Green Version]
- Benévolo-De-Andrade, T.C.; Monteiro-Maia, R.; Cosgrove, C.; Castello-Branco, L.R.R. BCG Moreau Rio de Janeiro: An oral vaccine against tuberculosis—Review. Memórias Inst. Oswaldo Cruz 2005, 100, 459–465. [Google Scholar] [CrossRef]
- W.H.O. Global Tuberculosis Report. 2019. Available online: https://www.who.int/tb/publications/global_report/tb19_Exec_Sum_12Nov2019.pdf (accessed on 15 August 2020).
- Jeyanathan, M.; Yao, Y.; Afkhami, S.; Smaill, F.; Xing, Z. New Tuberculosis Vaccine Strategies: Taking Aim at Un-Natural Immunity. Trends Immunol. 2018, 39, 419–433. [Google Scholar] [CrossRef]
- Kalscheuer, R.; Palacios, A.; Anso, I.; Cifuente, J.O.; Anguita, J.; Jacobs, W.R., Jr.; Guerin, M.E.; Prados-Rosales, R.C. The Mycobacterium tuberculosis capsule: A cell structure with key implications in pathogenesis. Biochem. J. 2019, 476, 1995–2016. [Google Scholar] [CrossRef]
- Nemes, E.; Geldenhuys, H.; Rozot, V.; Rutkowski, K.T.; Ratangee, F.; Bilek, N.; Mabwe, S.; Makhethe, L.; Erasmus, M.; Toefy, A.; et al. Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N. Engl. J. Med. 2018, 379, 138–149. [Google Scholar] [CrossRef]
- Koeken, V.A.C.M.; Verrall, A.J.; Netea, M.G.; Hill, P.C.; Van Crevel, R. Trained innate immunity and resistance to Mycobacterium tuberculosis infection. Clin. Microbiol. Infect. 2019, 25, 1468–1472. [Google Scholar] [CrossRef] [Green Version]
- Verrall, A.J.; Alisjahbana, B.; Apriani, L.; Novianty, N.; Nurani, A.C.; Van Laarhoven, A.; Ussher, J.E.; Indrati, A.; Ruslami, R.; Netea, M.G.; et al. Early Clearance of Mycobacterium tuberculosis: The INFECT Case Contact Cohort Study in Indonesia. J. Infect. Dis. 2019, 221, 1351–1360. [Google Scholar] [CrossRef]
- Joosten, S.A.; Van Meijgaarden, K.E.; Arend, S.M.; Prins, C.; Oftung, F.; Korsvold, G.E.; Kik, S.V.; Arts, R.J.; Van Crevel, R.; Netea, M.G.; et al. Mycobacterial growth inhibition is associated with trained innate immunity. J. Clin. Investig. 2018, 128, 1837–1851. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Soares-Weiser, K.; López-López, J.A.; Kakourou, A.; Chaplin, K.; Christensen, H.; Martin, N.K.; Sterne, J.A.C.; Reingold, A.L. Association of BCG, DTP, and measles containing vaccines with childhood mortality: Systematic review. BMJ 2016, 355, i5170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thysen, S.M.; Benn, C.S.; Gomes, V.F.; Rudolf, F.; Wejse, C.; Roth, A.; Kallestrup, P.; Aaby, P.; Fisker, A. Neonatal BCG vaccination and child survival in TB-exposed and TB-unexposed children: A prospective cohort study. BMJ Open 2020, 10, e035595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaby, P.; Roth, A.; Ravn, H.; Napirna, B.M.; Rodrigues, A.; Lisse, I.M.; Stensballe, L.; Diness, B.R.; Lausch, K.R.; Lund, N.; et al. Randomized Trial of BCG Vaccination at Birth to Low-Birth-Weight Children: Beneficial Nonspecific Effects in the Neonatal Period? J. Infect. Dis. 2011, 204, 245–252. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Tsilika, M.; Moorlag, S.; Antonakos, N.; Kotsaki, A.; Domínguez-Andrés, J.; Kyriazopoulou, E.; Gkavogianni, T.; Adami, M.-E.; Damoraki, G.; et al. Activate: Randomized Clinical Trial of BCG Vaccination Against Infection in the Elderly. Cell 2020. [Google Scholar] [CrossRef]
- Bekkering, S.; Blok, B.A.; Joosten, L.A.B.; Riksen, N.P.; Van Crevel, R.; Netea, M.G. In VitroExperimental Model of Trained Innate Immunity in Human Primary Monocytes. Clin. Vaccine Immunol. 2016, 23, 926–933. [Google Scholar] [CrossRef] [Green Version]
- Arts, R.J.; Blok, B.A.; Aaby, P.; Joosten, L.A.B.; De Jong, D.; Van Der Meer, J.W.M.; Benn, C.S.; Van Crevel, R.; Netea, M.G. Long-term in vitro and in vivo effects of γ-irradiated BCG on innate and adaptive immunity. J. Leukoc. Boil. 2015, 98, 995–1001. [Google Scholar] [CrossRef]
- Namakula, R.; De Bree, L.C.J.; Tvedt, T.H.A.; Netea, M.G.; Cose, S.; Hanevik, K. Monocytes from neonates and adults have a similar capacity to adapt their cytokine production after previous exposure to BCG and β-glucan. PLoS ONE 2020, 15, e0229287. [Google Scholar] [CrossRef] [Green Version]
- Freyne, B.; Donath, S.; Germano, S.; Gardiner, K.; Casalaz, D.; Robins-Browne, R.M.; Amenyogbe, N.; Messina, N.L.; Netea, M.G.; Flanagan, K.L.; et al. Neonatal BCG Vaccination Influences Cytokine Responses to Toll-like Receptor Ligands and Heterologous Antigens. J. Infect. Dis. 2018, 217, 1798–1808. [Google Scholar] [CrossRef]
- Jensen, K.J.; Larsen, N.; Biering-Sørensen, S.; Andersen, A.; Eriksen, H.B.; Monteiro, I.; Hougaard, D.; Aaby, P.; Netea, M.G.; Flanagan, K.L.; et al. Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: A randomized-controlled trial. J. Infect. Dis. 2014, 211, 956–967. [Google Scholar] [CrossRef]
- Smith, S.G.; Kleinnijenhuis, J.; Netea, M.G.; Dockrell, H.M. Whole Blood Profiling of Bacillus Calmette–Guérin-Induced Trained Innate Immunity in Infants Identifies Epidermal Growth Factor, IL-6, Platelet-Derived Growth Factor-AB/BB, and Natural Killer Cell Activation. Front. Immunol. 2017, 8, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; Van Loenhout, J.; De Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berendsen, M.L.; Bles, P.; De Bree, L.C.J.; Jensen, K.J.; Jensen, C.C.; Wejse, C.; Mendes, D.V.; Netea, M.G.; Benn, C.S. BCG Vaccination Induces Trained Innate Immunity in Adults Over 50 Years of Age: A Randomized Trial in Guinea-Bissau. Ssrn Electron. J. 2020. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.B.; Jacobs, C.; Van Loenhout, J.; Xavier, R.J.; Aaby, P.; Van Der Meer, J.W.M.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2013, 6, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Verma, D.; Parasa, V.R.; Raffetseder, J.; Martis, M.; Mehta, R.B.; Netea, M.; Lerm, M. Anti-mycobacterial activity correlates with altered DNA methylation pattern in immune cells from BCG-vaccinated subjects. Sci. Rep. 2017, 7, 12305. [Google Scholar] [CrossRef]
- Verrall, A.J.; Schneider, M.; Alisjahbana, B.; Apriani, L.; Van Laarhoven, A.; Koeken, V.A.C.M.; Van Dorp, S.; Diadani, E.; Utama, F.; Hannaway, R.F.; et al. Early clearance of Mycobacterium tuberculosis is associated with increased innate immune responses. J. Infect. Dis. 2019, 221, 1342–1350. [Google Scholar] [CrossRef]
- Arts, R.J.; Moorlag, S.J.; Novakovic, B.; Li, Y.; Wang, S.-Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.; et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Leentjens, J.; Kox, M.; Stokman, R.; Gerretsen, J.; Diavatopoulos, D.A.; Van Crevel, R.; Rimmelzwaan, G.F.; Pickkers, P.; Netea, M.G. BCG-vaccination enhances immunogenicity of subsequent influenza vaccination in healthy volunteers: A randomized placebo-controlled pilot study. J. Infect. Dis. 2015, 212, 1930–1938. [Google Scholar] [CrossRef] [Green Version]
- Walk, J.; De Bree, L.C.J.; Graumans, W.; Stoter, R.; Van Gemert, G.-J.; Van De Vegte-Bolmer, M.; Teelen, K.; Hermsen, C.C.; Arts, R.J.W.; Behet, M.C.; et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat. Commun. 2019, 10, 874. [Google Scholar] [CrossRef] [Green Version]
- Hamers, L.A.C.; Kox, M.; Arts, R.J.W.; Blok, B.; Leentjens, J.; Netea, M.G.; Pickkers, P. Gamma-Irradiated Bacille Calmette-Guérin Vaccination Does Not Modulate the Innate Immune Response during Experimental Human Endotoxemia in Adult Males. J. Immunol. Res. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Moliva, J.I.; Turner, J.; Torrelles, J.B. Immune Responses to Bacillus Calmette–Guérin Vaccination: Why Do They Fail to Protect against Mycobacterium tuberculosis? Front. Immunol. 2017, 8, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minassian, A.M.; Satti, I.; Poulton, I.D.; Meyer, J.; Hill, A.V.S.; McShane, H. A Human Challenge Model for Mycobacterium tuberculosis Using Mycobacterium bovis Bacille Calmette-Guérin. J. Infect. Dis. 2012, 205, 1035–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickett, T.E.; McLean, J.; Creissen, E.; Izzo, L.; Hagan, C.; Izzo, A.J.; Angulo, F.S.; Izzo, A.A. Characterizing the BCG Induced Macrophage and Neutrophil Mechanisms for Defense against Mycobacterium tuberculosis. Front. Immunol. 2020, 11, 1202. [Google Scholar] [CrossRef] [PubMed]
- Koeken, V.A.C.M.; Van Der Pasch, E.S.; Leijte, G.P.; Mourits, V.P.; De Bree, L.C.J.; Moorlag, S.J.; Budnick, I.; Idh, N.; Lerm, M.; Kox, M.; et al. The effect of BCG vaccination on alveolar macrophages obtained from induced sputum from healthy volunteers. Cytokine 2020, 133, 155135. [Google Scholar] [CrossRef]
- Inohara, N.; Ogura, Y.; Fontalba, A.; Gutierrez, O.; Pons, F.; Crespo, J.; Fukase, K.; Inamura, S.; Kusumoto, S.; Hashimoto, M.; et al. Host Recognition of Bacterial Muramyl Dipeptide Mediated through NOD2. J. Boil. Chem. 2003, 278, 5509–5512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourits, V.P.; Koeken, V.A.C.M.; De Bree, L.C.J.; Moorlag, S.J.C.F.M.; Chu, W.C.; Xu, X.; Dijkstra, H.; Lemmers, H.; Joosten, L.A.B.; Wang, Y.; et al. BCG-Induced Trained Immunity in Healthy Individuals: The Effect of Plasma Muramyl Dipeptide Concentrations. J. Immunol. Res. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.; Carvalho, A.; La Rocca, C.; Palma, C.; Rodrigues, F.; Silvestre, R.; Kleinnijenhuis, J.; Lachmandas, E.; Gonçalves, L.G.; Belinha, A.; et al. Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep. 2016, 17, 2562–2571. [Google Scholar] [CrossRef] [Green Version]
- Arts, R.J.; Blok, B.A.; Van Crevel, R.; Joosten, L.A.B.; Aaby, P.; Benn, C.S.; Netea, M.G. Vitamin A induces inhibitory histone methylation modifications and down-regulates trained immunity in human monocytes. J. Leukoc. Boil. 2015, 98, 129–136. [Google Scholar] [CrossRef]
- Cirovic, B.; De Bree, L.C.J.; Groh, L.; Blok, B.A.; Chan, J.; Van Der Velden, W.J.; Bremmers, M.; Van Crevel, R.; Händler, K.; Picelli, S.; et al. BCG Vaccination in Humans Elicits Trained Immunity via the Hematopoietic Progenitor Compartment. Cell Host Microbe 2020, 28, 322–334. [Google Scholar] [CrossRef]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonça, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.-C.; et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018, 172, 176–190. [Google Scholar] [CrossRef] [Green Version]
- Angelidou, A.; Conti, M.-G.; Diray-Arce, J.; Benn, C.S.; Shann, F.; Netea, M.G.; Liu, M.; Potluri, L.P.; Sanchez-Schmitz, G.; Husson, R.N.; et al. Licensed Bacille Calmette-Guérin (BCG) formulations differ markedly in bacterial viability, RNA content and innate immune activation. Vaccine 2020, 38, 2229–2240. [Google Scholar] [CrossRef]
- Price, D.N.; Kusewitt, D.F.; Lino, C.A.; McBride, A.A.; Muttil, P. Oral Tolerance to Environmental Mycobacteria Interferes with Intradermal, but Not Pulmonary, Immunization against Tuberculosis. PLoS Pathog. 2016, 12, e1005614. [Google Scholar] [CrossRef]
- Sharpe, S.A.; White, A.; Sarfas, C.; Sibley, L.; Gleeson, F.C.; McIntyre, A.; Basaraba, R.; Clark, S.; Hall, G.; Rayner, E.; et al. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: Protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis 2016, 101, 174–190. [Google Scholar] [CrossRef] [Green Version]
- Darrah, P.A.; Zeppa, J.J.; Maiello, P.; Hackney, J.A.; Ii, M.H.W.; Hughes, T.K.; Pokkali, S.; Ii, P.A.S.; Grant, N.L.; Rodgers, M.A.; et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 2020, 577, 95–102. [Google Scholar] [CrossRef]
- Tejera-Alhambra, M.; Palomares, O.; De Diego, R.P.; Díaz-Lezcano, I.; Sánchez-Ramón, S. New Biological Insights in the Immunomodulatory Effects of Mucosal Polybacterial Vaccines in Clinical Practice. Curr. Pharm. Des. 2016, 22, 6283–6293. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, E.; Paul, M.J.; Reljic, R.; McShane, H. Mucosal delivery of tuberculosis vaccines: A review of current approaches and challenges. Expert Rev. Vaccines 2019, 18, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Hoft, D.F.; Xia, M.; Zhang, G.L.; Blazevic, A.; Tennant, J.; Kaplan, C.; Matuschak, G.; Dube, T.J.; Hill, H.; Schlesinger, L.S.; et al. PO and ID BCG vaccination in humans induce distinct mucosal and systemic immune responses and CD4+ T cell transcriptomal molecular signatures. Mucosal Immunol. 2017, 11, 486–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra-Maupome, M.; Vang, D.X.; McGill, J.L. Aerosol vaccination with Bacille Calmette-Guerin induces a trained innate immune phenotype in calves. PLoS ONE 2019, 14, e0212751. [Google Scholar] [CrossRef] [Green Version]
- Dijkman, K.; Sombroek, C.C.; Vervenne, R.A.W.; Hofman, S.O.; Boot, C.; Remarque, E.J.; Kocken, C.H.M.; Ottenhoff, T.H.M.; Kondova, I.; Khayum, M.A.; et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat. Med. 2019, 25, 255–262. [Google Scholar] [CrossRef]
- Jensen, K.J.; Biering-Sørensen, S.; Ursing, J.; Kofoed, P.-E.L.; Aaby, P.; Benn, C.S. Seasonal variation in the non-specific effects of BCG vaccination on neonatal mortality: Three randomised controlled trials in Guinea-Bissau. BMJ Glob. Heal. 2020, 5, e001873. [Google Scholar] [CrossRef] [Green Version]
- De Bree, L.C.J.; Mourits, V.P.; Koeken, V.A.C.M.; Moorlag, S.J.; Janssen, R.; Folkman, L.; Barreca, D.; Krausgruber, T.; Fife-Gernedl, V.; Novakovic, B.; et al. Circadian rhythm influences induction of trained immunity by BCG vaccination. J. Clin. Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sartono, E.; Lisse, I.M.; Terveer, E.M.; Van De Sande, P.J.M.; Whittle, H.; Fisker, A.B.; Roth, A.; Aaby, P.; Yazdanbakhsh, M.; Benn, C.S. Oral Polio Vaccine Influences the Immune Response to BCG Vaccination. A Natural Experiment. PLoS ONE 2010, 5, e10328. [Google Scholar] [CrossRef] [PubMed]
- Blok, B.A.; De Bree, L.C.J.; Diavatopoulos, D.A.; Langereis, J.D.; Joosten, L.A.B.; Aaby, P.; Van Crevel, R.; Benn, C.S.; Netea, M.G. Interacting, Nonspecific, Immunological Effects of Bacille Calmette-Guérin and Tetanus-diphtheria-pertussis Inactivated Polio Vaccinations: An Explorative, Randomized Trial. Clin. Infect. Dis. 2019, 70, 455–463. [Google Scholar] [CrossRef]
- Espinosa-Cueto, P.; Magallanes-Puebla, A.; Mancilla, R. Phosphate starvation enhances phagocytosis of Mycobacterium bovis/BCG by macrophages. BMC Immunol. 2020, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moliva, J.I.; Hossfeld, A.P.; Canan, C.H.; Dwivedi, V.; Wewers, M.D.; Beamer, G.; Turner, J.; Torrelles, J.B. Exposure to human alveolar lining fluid enhances Mycobacterium bovis BCG vaccine efficacy against Mycobacterium tuberculosis infection in a CD8+ T-cell-dependent manner. Mucosal Immunol. 2017, 11, 968–978. [Google Scholar] [CrossRef]
- Moliva, J.I.; Hossfeld, A.P.; Sidiki, S.; Canan, C.H.; Dwivedi, V.; Beamer, G.; Turner, J.; Torrelles, J.B. Selective delipidation of Mycobacterium bovis BCG enables direct pulmonary vaccination and enhances protection against Mycobacterium tuberculosis. Mucosal Immunol. 2019, 12, 805–815. [Google Scholar] [CrossRef]
- Arca, H. Çiğdem; Günbeyaz, M.; Şenel, S. Chitosan-based systems for the delivery of vaccine antigens. Expert Rev. Vaccines 2009, 8, 937–953. [Google Scholar] [CrossRef]
- Caetano, L.A.; Figueiredo, L.; Almeida, A.J.; Gonçalves, L. BCG-loaded chitosan microparticles: Interaction with macrophages and preliminaryin vivostudies. J. Microencapsul. 2017, 34, 203–217. [Google Scholar] [CrossRef]
- Speth, M.T.; Repnik, U.; Müller, E.; Spanier, J.; Kalinke, U.; Corthay, A.; Griffiths, G. Poly(I:C)-Encapsulating Nanoparticles Enhance Innate Immune Responses to the Tuberculosis Vaccine Bacille Calmette–Guérin (BCG) via Synergistic Activation of Innate Immune Receptors. Mol. Pharm. 2017, 14, 4098–4112. [Google Scholar] [CrossRef]
- Ning, H.; Wang, L.; Zhou, J.; Lu, Y.; Kang, J.; Ding, T.; Shen, L.; Xu, Z.; Bai, Y. Recombinant BCG With Bacterial Signaling Molecule Cyclic di-AMP as Endogenous Adjuvant Induces Elevated Immune Responses After Mycobacterium tuberculosis Infection. Front. Immunol. 2019, 10, 1519. [Google Scholar] [CrossRef] [Green Version]
- Tarancón, R.; Domínguez-Andrés, J.; Uranga, S.; Ferreira, A.V.; Groh, L.A.; Domenech, M.; González-Camacho, F.; Riksen, N.P.; Aguilo, N.; Yuste, J.; et al. New live attenuated tuberculosis vaccine MTBVAC induces trained immunity and confers protection against experimental lethal pneumonia. PLoS Pathog. 2020, 16, e1008404. [Google Scholar] [CrossRef] [PubMed]
- Prabowo, S.A.; Painter, H.; Zelmer, A.; Smith, S.G.; Seifert, K.; Amat, M.; Cardona, P.-J.; Fletcher, H.A. RUTI Vaccination Enhances Inhibition of Mycobacterial Growth ex vivo and Induces a Shift of Monocyte Phenotype in Mice. Front. Immunol. 2019, 10, 894. [Google Scholar] [CrossRef] [Green Version]
- Soudi, S.; Hosseini, A.Z.; Hashemi, S.M. Co-administration of rectal BCG and autoclaved Leishmania major induce protection in susceptible BALB/c mice. Parasite Immunol. 2011, 33, 561–571. [Google Scholar] [CrossRef]
- Scheid, A.; Borriello, F.; Pietrasanta, C.; Christou, H.; Diray-Arce, J.; Pettengill, M.A.; Joshi, S.; Li, N.; Bergelson, I.; Kollmann, T.R.; et al. Adjuvant Effect of Bacille Calmette–Guérin on Hepatitis B Vaccine Immunogenicity in the Preterm and Term Newborn. Front. Immunol. 2018, 9, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covián, C.; Fernández-Fierro, A.; Retamal-Díaz, A.; Díaz, F.E.; Vasquez, A.E.; Lay, M.K.; Riedel, C.A.; González, P.A.; Bueno, S.M.; Kalergis, A.M. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front. Immunol. 2019, 10, 2806. [Google Scholar] [CrossRef]
- Moorlag, S.J.; Van Deuren, R.C.; Van Werkhoven, C.H.; Jaeger, M.; Debisarun, P.; Taks, E.; Mourits, V.P.; Koeken, V.A.; De Bree, L.C.J.; Doesschate, T.T.; et al. Safety and COVID-19 symptoms in individuals recently vaccinated with BCG: A retrospective cohort study. Cell Rep. Med. 2020, 1, 100073. [Google Scholar] [CrossRef]
- Escobar, L.E.; Molina-Cruz, A.; Barillas-Mury, C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc. Natl. Acad. Sci. USA 2020, 117, 17720–17726. [Google Scholar] [CrossRef]
- Spencer, J.C.; Ganguly, R.; Waldman, R.H. Nonspecific Protection of Mice against Influenza Virus Infection by Local or Systemic Immunization with Bacille Calmette-Guerin. J. Infect. Dis. 1977, 136, 171–175. [Google Scholar] [CrossRef]
- De Bree, C.L.C.J.; Marijnissen, R.J.; Kel, J.M.; Huber, S.K.R.; Aaby, P.; Benn, C.S.; Wijnands, M.V.W.; Diavatopoulos, D.A.; Van Crevel, R.; Joosten, L.A.B.; et al. Bacillus Calmette–Guérin-Induced Trained Immunity Is Not Protective for Experimental Influenza A/Anhui/1/2013 (H7N9) Infection in Mice. Front. Immunol. 2018, 9, 869. [Google Scholar] [CrossRef]
- Mukherjee, S.; Subramaniam, R.; Chen, H.; Smith, A.; Keshava, S.; Shams, H. Boosting efferocytosis in alveolar space using BCG vaccine to protect host against influenza pneumonia. PLoS ONE 2017, 12, e0180143. [Google Scholar] [CrossRef]
- Sánchez-Ramón, S.; Conejero, L.; Netea, M.G.; Sancho, D.; Palomares, Ó.; Subiza, J.L. Trained Immunity-Based Vaccines: A New Paradigm for the Development of Broad-Spectrum Anti-infectious Formulations. Front. Immunol. 2018, 9, 2936. [Google Scholar] [CrossRef]
- Sui, Y.; Lewis, G.K.; Wang, Y.; Berckmueller, K.; Frey, B.; Dzutsev, A.; Vargas-Inchaustegui, D.; Mohanram, V.; Musich, T.; Shen, X.; et al. Mucosal vaccine efficacy against intrarectal SHIV is independent of anti-Env antibody response. J. Clin. Investig. 2019, 129, 1314–1328. [Google Scholar] [CrossRef] [PubMed]
- Bigot, J.; Guillot, L.; Guitard, J.; Ruffin, M.; Corvol, H.; Chignard, M.; Hennequin, C.; Balloy, V. Respiratory Epithelial Cells Can Remember Infection: A Proof of Concept Study. J. Infect. Dis. 2019, 221, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Reuschl, A.-K.; Edwards, M.R.; Parker, R.; Connell, D.W.; Hoang, L.; Halliday, A.; Jarvis, H.; Siddiqui, N.; Wright, C.; Bremang, S.; et al. Innate activation of human primary epithelial cells broadens the host response to Mycobacterium tuberculosis in the airways. PLoS Pathog. 2017, 13, e1006577. [Google Scholar] [CrossRef]
- Tenland, E.; Håkansson, G.; Alaridah, N.; Lutay, N.; Rönnholm, A.; Hallgren, O.; Westergren-Thorsson, G.; Godaly, G. Innate Immune Responses after Airway Epithelial Stimulation with Mycobacterium bovis Bacille-Calmette Guérin. PLoS ONE 2016, 11, e0164431. [Google Scholar] [CrossRef] [Green Version]
- Ryndak, M.B.; Laal, S. Mycobacterium tuberculosis Primary Infection and Dissemination: A Critical Role for Alveolar Epithelial Cells. Front. Microbiol. 2019, 9, 299. [Google Scholar] [CrossRef]
- Lorenzo-Gómez, M.F.; Padilla-Fernãndez, B.; Garcia-Cenador, M.B.; Virseda-Rodríguez, Á.J.; Martín-García, I.; Sã¡nchez-Escudero, A.; Vicente-Arroyo, M.J.; Mirón-Canelo, J.A. Comparison of sublingual therapeutic vaccine with antibiotics for the prophylaxis of recurrent urinary tract infections. Front. Microbiol. 2015, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Nikolova, M.; Stankulova, D.; Taskov, H.; Nenkov, P.; Maximov, V.; Petrunov, B. Polybacterial immunomodulator Respivax restores the inductive function of innate immunity in patients with recurrent respiratory infections. Int. Immunopharmacol. 2009, 9, 425–432. [Google Scholar] [CrossRef]
- Rial, A.; Ferrara, F.; Suárez, N.; Scavone, P.; Marqués, J.M.; Chabalgoity, J.A. Intranasal administration of a polyvalent bacterial lysate induces self-restricted inflammation in the lungs and a Th1/Th17 memory signature. Microbes Infect. 2016, 18, 747–757. [Google Scholar] [CrossRef]
- Emmerich, B.; Emslander, H.P.; Milatović, D.; Hallek, M.; Pachmann, K. Effects of a bacterial extract on local immunity of the lung in patients with chronic bronchitis. Lung 1990, 168, 726–731. [Google Scholar] [CrossRef]
- Emmerich, B.; Emslander, H.P.; Pachmann, K.; Hallek, M.; Milatovic, D.; Busch, R. Local Immunity in Patients with Chronic Bronchitis and the Effects of a Bacterial Extract, Broncho-Vaxom®, on T Lymphocytes, Macrophages, Gamma-Interferon and Secretory Immunoglobulin A in Bronchoalveolar Lavage Fluid and Other Variables. Respiration 1990, 57, 90–99. [Google Scholar] [CrossRef]
- Mauël, J.; Van Pham, T.; Kreis, B.; Bauer, J. Stimulation by a bacterial extract (broncho-vaxom) of the metabolic and functional activities of murine macrophages. Int. J. Immunopharmacol. 1989, 11, 637–645. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasco, S.T.; Anguita, J. Lessons from Bacillus Calmette-Guérin: Harnessing Trained Immunity for Vaccine Development. Cells 2020, 9, 2109. https://doi.org/10.3390/cells9092109
Pasco ST, Anguita J. Lessons from Bacillus Calmette-Guérin: Harnessing Trained Immunity for Vaccine Development. Cells. 2020; 9(9):2109. https://doi.org/10.3390/cells9092109
Chicago/Turabian StylePasco, Samuel T., and Juan Anguita. 2020. "Lessons from Bacillus Calmette-Guérin: Harnessing Trained Immunity for Vaccine Development" Cells 9, no. 9: 2109. https://doi.org/10.3390/cells9092109
APA StylePasco, S. T., & Anguita, J. (2020). Lessons from Bacillus Calmette-Guérin: Harnessing Trained Immunity for Vaccine Development. Cells, 9(9), 2109. https://doi.org/10.3390/cells9092109





