Cell Cycle-Dependent Control and Roles of DNA Topoisomerase II
Abstract
1. Introduction
2. Cell Cycle-Dependent Expression of Topo IIα in Vertebrates
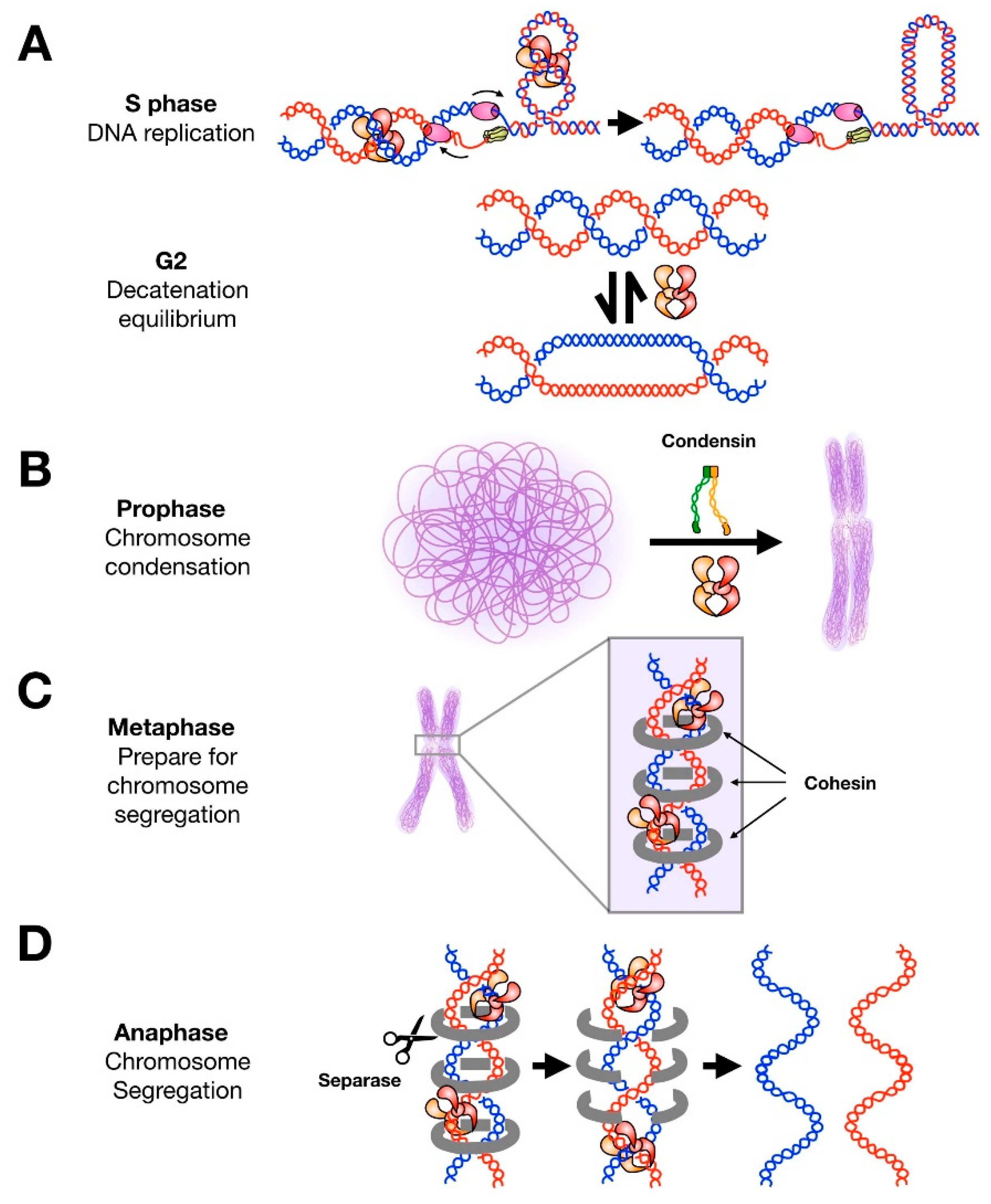
3. DNA Replication
4. Chromosome Condensation and Sister Chromatid Cohesion
5. Chromosome Segregation
6. Post-Translational Modifications
7. Decatenation Checkpoint
8. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meselson, M.; Stahl, F. The relication of DNA in E. coli. Proc. Natl. Acad. Sci. USA 1958, 44, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Postow, L.; Crisona, N.J.; Peter, B.J.; Hardy, C.D.; Cozzarelli, N.R. Topological challenges to DNA replication: Conformations at the fork. Proc. Natl. Acad. Sci. USA 2001, 98, 8219–8226. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.J.; Ullsperger, C.; Hiasa, H.; Marians, K.J.; Cozzarelli, N.R. The structure of supercoiled intermediates in DNA replication. Cell 1998, 94, 819–827. [Google Scholar] [CrossRef]
- Vos, S.M.; Tretter, E.M.; Schmidt, B.H.; Berger, J.M. All tangled up: How cells direct, manage and exploit topoisomerase function. Nat. Rev. Mol. Cell Biol. 2011, 12, 827–841. [Google Scholar] [CrossRef]
- Wang, J.C. Interaction between DNA and an Escherichia coli protein ω. J. Mol. Biol. 1971, 55, 523–533. [Google Scholar] [CrossRef]
- Champoux, J.J.; Dulbecco, R. An activity from mammalian cells that untwists superhelical DNA—A possible swivel for DNA replication (polyoma-ethidium bromide-mouse-embryo cells-dye binding assay). Proc. Natl. Acad. Sci. USA 1972, 69, 143–146. [Google Scholar] [CrossRef]
- Gellert, M.; Mizuuchi, K.; O’dea, M.H.; Nasht, H.A. DNA gyrase: An enzyme that introduces superhelical turns into DNA (Escherichia coli/ATP-dependent reaction/superhelix density). Proc. Natl. Acad. Sci. USA 1976, 73, 3872–3876. [Google Scholar] [CrossRef]
- Goto, T.; Wang, J.C. Yeast DNA Topoisomerase II. An ATP-dependent type II topoisomerase that catalyzes the catenation, decatenation, unknotting, and relaxation of double-stranded DNA rings. J. Biol. Chem. 1982, 257, 5866–58720. [Google Scholar]
- Kreuzer, K.N.; Cozzarelli, N.R. Formation and resolution of DNA catenanes by DNA gyrase. Cell 1980, 20, 245–254. [Google Scholar] [CrossRef]
- Tsai-Pflugfelder, M.; Liu, L.F.; Liu, A.A.; Tewey, K.M.; Whang-Peng, J.; Knutsen, T.; Huebner, K.; Croce, C.M.; Wang, J.C. Cloning and sequencing of cDNA encoding human DNA topoisomerase II and localization of the gene to chromosome region 17q21-22. Proc. Natl. Acad. Sci. USA 1988, 85, 7177–7181. [Google Scholar] [CrossRef]
- Tan, K.B.; Dorman, T.E.; Falls, K.M.; Chung, T.D.Y.; Mirabelli, C.K. Topoisomerase II alpha and topoisomerase II beta genes: Characterization and mapping to human chromosomes 17 and 3, respectively. Cancer Res. 1992, 52, 231–234. [Google Scholar] [PubMed]
- Grue, P.; Gräßer, A.; Sehested, M.; Jensen, P.B.; Uhse, A.; Straub, T.; Ness, W.; Boege, F. Essential mitotic functions of DNA topoisomerase IIα are not adopted by topoisomerase IIβ in human H69 cells. J. Biol. Chem. 1998, 273, 33660–33666. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.L.; Lin, C.-P.; Azarova, A.M.; Cai, L.; Wang, J.C.; Liu, L.F. Role of Topoisomerase II in the Expression of Developmentally Regulated Genes. Mol. Cell. Biol. 2006, 26, 7929–7941. [Google Scholar] [CrossRef] [PubMed]
- Ju, B.-G.; Lunyak, V.V.; Perissi, V.; Garcia-Bassets, I.; Rose, D.W.; Glass, C.K.; Rosenfeld, M.G. A Topoisomerase IIbeta-Mediated dsDNA Break Required for Regulated Transcription. Science 2006, 312, 1798–1802. [Google Scholar] [CrossRef] [PubMed]
- Madabhushi, R.; Gao, F.; Pfenning, A.R.; Pan, L.; Yamakawa, S.; Seo, J.; Rueda, R.; Phan, T.X.; Yamakawa, H.; Pao, P.C.; et al. Activity-Induced DNA Breaks Govern the Expression of Neuronal Early-Response Genes. Cell 2015, 161, 1592–1605. [Google Scholar] [CrossRef] [PubMed]
- Holm, C.; Goto, T.; Wang, J.C.; Botstein, D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell 1985, 41, 553–563. [Google Scholar] [CrossRef]
- Uemura, T.; Ohkura, H.; Adachi, Y.; Morino, K.; Shiozaki, K.; Yanagida, M. DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell 1987, 50, 917–925. [Google Scholar] [CrossRef]
- Heck, M.M.S.; Hittelman, W.N.; Earnshaw, W.C. Differential expression of DNA topoisomerases I and II during the eukaryotic cell cycle. Proc. Natl. Acad. Sci. USA 1988, 85, 1086–1090. [Google Scholar] [CrossRef]
- Woessner, R.D.; Mattern, M.R.; Mirabelli, C.K.; Johnson, R.K.; Drake, F.H. Proliferation- and Cell Cycle-dependent Differences in Expression of the 170 Kilodalton and 180 Kilodalton Forms of Topoisomerse II in NIH-3T3 Cells. Cell Growth Differ. 1991, 2, 209–214. [Google Scholar]
- Baxter, J.; Sen, N.; López Martínez, V.; Monturus De Carandini, M.E.; Schvartzman, J.B.; Diffley, J.F.X.; Aragón, L. Positive supercoiling of mitotic DNA drives decatenation by topoisomerase II in eukaryotes. Science 2011, 331, 1328–1332. [Google Scholar] [CrossRef]
- Kroll, D.J. Homologous and heterologous protein-protein interactions of human DNA topoisomerase. Arch. Biochem. Biophys. 1997, 345, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.C.G.; Farr, C.J. Topoisomerase II: Untangling its contribution at the centromere. Chromosom. Res. 2004, 12, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Downes, C.S.; Clarke, D.J.; Mullinger, A.M.; Giménez-Abián, J.F.; Creighton, A.M.; Johnson, R.T. A topoisomerase II-dependent G2 cycle checkpoint in mammalian cells. Nature 1994, 372, 467–470. [Google Scholar] [CrossRef]
- Chen, T.; Sun, Y.; Ji, P.; Kopetz, S.; Zhang, W. Topoisomerase IIα in chromosome instability and personalized cancer therapy. Oncogene 2015, 34, 4019–4031. [Google Scholar] [CrossRef]
- Kimura, K.; Saijo, M.; Ui, M.; Enomoto, T. Growth state- and cell cycle-dependent fluctuation in the expression of two forms of DNA topoisomerase II and possible specific modification of the higher molecular weight form in the M phase. J. Biol. Chem. 1994, 269, 1173–1176. [Google Scholar]
- Adachi, N.; Nomoto, M.; Kohno, K.; Koyama, H. Cell-cycle regulation of the DNA topoisomerase IIα promoter is mediated by proximal CCAAT boxes: Possible involvement of acetylation. Gene 2000, 245, 49–57. [Google Scholar] [CrossRef]
- Magan, N.; Szremska, A.P.; Isaacs, R.J.; Stowell, K.M. Modulation of DNA topoisomerase IIα promoter activity by members of the Sp (specificity protein) and NF-Y (nuclear factor Y) families of transcription factors. Biochem. J. 2003, 374, 723–729. [Google Scholar] [CrossRef]
- Hopfner, R.; Mousli, M.; Jeltsch, J.M.; Voulgaris, A.; Lutz, Y.; Marin, C.; Bellocq, J.P.; Oudet, P.; Bronner, C. ICBP90, a novel human CCAAT binding protein, involved in the regulation of topoisomerase IIα expression. Cancer Res. 2000, 60, 121–128. [Google Scholar]
- Falck, J.; Jensen, P.B.; Sehested, M. Evidence for repressional role of an inverted CCAAT box in cell cycle- dependent transcription of the human DNA topoisomerase IIα gene. J. Biol. Chem. 1999, 274, 18753–18758. [Google Scholar] [CrossRef]
- Goswami, P.C.; Roti Roti, J.L.; Hunt, C.R. The cell cycle-coupled expression of topoisomerase IIalpha during S phase is regulated by mRNA stability and is disrupted by heat shock or ionizing radiation. Mol. Cell. Biol. 1996, 16, 1500–1508. [Google Scholar] [CrossRef]
- Goswami, P.C.; Sheren, J.; Albee, L.D.; Parsian, A.; Sim, J.E.; Ridnour, L.A.; Higashikubo, R.; Gius, D.; Hunt, C.R.; Spitz, D.R. Cell cycle-coupled variation in topoisomerase IIα mRNA is regulated by the 3′-untranslated region: Possible role of redox-sensitive protein binding in mRNA accumulation. J. Biol. Chem. 2000, 275, 38384–38392. [Google Scholar] [CrossRef] [PubMed]
- Salmena, L.; Lam, V.; Peter McPherson, J.; Goldenberg, G.J. Role of proteasomal degradation in the cell cycle-dependent regulation of DNA topoisomerase IIα expression. Biochem. Pharmacol. 2001, 61, 795–802. [Google Scholar] [CrossRef]
- Fielding, A.B.; Concannon, M.; Darling, S.; Rusilowicz-Jones, E.V.; Sacco, J.J.; Prior, I.A.; Clague, M.J.; Urbé, S.; Coulson, J.M. The deubiquitylase USP15 regulates topoisomerase II alpha to maintain genome integrity. Oncogene 2018, 37, 2326–2342. [Google Scholar] [CrossRef]
- Eguren, M.; Álvarez-Fernández, M.; García, F.; López-Contreras, A.J.; Fujimitsu, K.; Yaguchi, H.; Luque-García, J.; Fernández-Capetillo, O.; Muñoz, J.; Yamano, H.; et al. A Synthetic Lethal Interaction between APC/C and Topoisomerase Poisons Uncovered by Proteomic Screens. Cell Rep. 2014, 6, 670–683. [Google Scholar] [CrossRef]
- Whalen, A.M.; McConnell, M.; Fisher, P.A. Developmental regulation of Drosophila DNA topoisomerase II. J. Cell Biol. 1991, 112, 203–213. [Google Scholar] [CrossRef]
- Bermejo, R.; Doksani, Y.; Capra, T.; Katou, Y.M.; Tanaka, H.; Shirahige, K.; Foiani, M. Top1- and Top2-mediated topological transitions at replication forks ensure fork progression and stability and prevent DNA damage checkpoint activation. Genes Dev. 2007, 21, 1921–1936. [Google Scholar] [CrossRef]
- Gonzalez, R.E.; Lim, C.U.; Cole, K.; Bianchini, C.H.; Schools, G.P.; Davis, B.E.; Wada, I.; Roninson, I.B.; Broude, E.V. Effects of conditional depletion of topoisomerase II on cell cycle progression in mammalian cells. Cell Cycle 2011, 10, 3505–3514. [Google Scholar] [CrossRef]
- Hossain, M.S.; Akimitsu, N.; Takaki, T.; Hirai, H.; Sekimizu, K. ICRF-193, a catalytic inhibitor of DNA topoisomerase II, inhibits re-entry into the cell division cycle from quiescent state in mammalian cells. Genes Cells 2002, 7, 285–294. [Google Scholar] [CrossRef]
- Hossain, M.S.; Kurokawa, K.; Akimitsu, N.; Sekimizu, K. DNA topoisomerase II is required for the G0-to-S phase transition in Drosophila Schneider cells, but not in yeast. Genes Cells 2004, 9, 905–917. [Google Scholar] [CrossRef]
- Lucas, I.; Germe, T.; Chevrier-Miller, M.; Hyrien, O. Topoisomerase II can unlink replicating DNA by precatenane removal. EMBO J. 2001, 20, 6509–6519. [Google Scholar] [CrossRef]
- Charbin, A.; Bouchoux, C.; Uhlmann, F. Condensin aids sister chromatid decatenation by topoisomerase II. Nucleic Acids Res. 2014, 42, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Fachinetti, D.; Bermejo, R.; Cocito, A.; Minardi, S.; Katou, Y.; Kanoh, Y.; Shirahige, K.; Azvolinsky, A.; Zakian, V.A.; Foiani, M. Replication Termination at Eukaryotic Chromosomes Is Mediated by Top2 and Occurs at Genomic Loci Containing Pausing Elements. Mol. Cell 2010, 39, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Snapka, R.M.; Powelson, M.A.; Strayer, J.M. Swiveling and Decatenatio of Replicating Simian Virus 40 Genomes in Vivo. Mol. Cell. Biol. 1988, 8, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Strausfeld, U. Effects of VM26 (teniposide), a specific inhibitor of type II DNA topoisomerase, on SV40 chromatin replication in vitro. Nucleic Acids Res. 1987, 15, 3455–3468. [Google Scholar] [CrossRef]
- Dewar, J.M.; Budzowska, M.; Walter, J.C. The mechanism of DNA replication termination in vertebrates. Nature 2015, 525, 345–350. [Google Scholar] [CrossRef]
- Deegan, T.D.; Baxter, J.; Ortiz Bazán, M.Á.; Yeeles, J.T.P.; Labib, K.P.M. Pif1-Family Helicases Support Fork Convergence during DNA Replication Termination in Eukaryotes. Mol. Cell 2019, 74, 231–244. [Google Scholar] [CrossRef]
- Gilson, E.; Géli, V. How telomeres are replicated. Nat. Rev. Mol. Cell Biol. 2007, 8, 825–838. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Z.; Li, F.; Hu, Q.; Liu, H.; Tang, M.; Ma, W.; Huang, J.; Songyang, Z.; Rong, Y.; et al. Looping-out mechanism for resolution of replicative stress at telomeres. EMBO Rep. 2017, 18, 1412–1428. [Google Scholar] [CrossRef]
- Ye, J.; Lenain, C.; Bauwens, S.; Rizzo, A.; Saint-Léger, A.; Poulet, A.; Benarroch, D.; Magdinier, F.; Morere, J.; Amiard, S.; et al. TRF2 and Apollo Cooperate with Topoisomerase 2α to Protect Human Telomeres from Replicative Damage. Cell 2010, 142, 230–242. [Google Scholar] [CrossRef]
- Yekezare, M.; Gó mez-González, B.; Diffley, J.F.X. Controlling DNA replication origins in response to DNA damage—Inhibit globally, activate locally. J. Cell Sci. 2013, 126, 1297–1306. [Google Scholar] [CrossRef]
- Truong, L.N.; Wu, X. Prevention of DNA re-replication in eukaryotic cells. J. Mol. Cell Biol. 2011, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Cuvier, O.; Stanojcic, S.; Lemaitre, J.M.; Mechali, M. A topoisomerase II-dependent mechanism for resetting replicons at the S-M-phase transition. Genes Dev. 2008, 22, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Earnshaw, W.C.; Halligan, B.; Cooke, C.A.; Heck, M.M.S.; Liu, L.F. Topoisomerase II is a structural component of mitotic chromosome scaffolds. J. Cell Biol. 1985, 100, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Gasser, S.M.; Laroche, T.; Falquet, J.; Boy de la Tour, E.; Laemmli, U.K. Metaphase chromosome structure. Involvement of topoisomerase II. J. Mol. Biol. 1986, 188, 613–629. [Google Scholar] [CrossRef]
- Hirano, T.; Mitchison, T.J. Topoisomerase II does not play a scaffolding role in the organization of mitotic chromosomes assembled in Xenopus egg extracts. J. Cell Biol. 1993, 120, 601–612. [Google Scholar] [CrossRef]
- Toyoda, Y.; Yanigada, M. Coordinated Requirements of Human Topo II and Cohesin for Metaphase Centromere Alignment under Mad2-dependent Spindle Checkpoint Surveillance. Mol. Biol. Cell 2006, 17, 2287–2302. [Google Scholar] [CrossRef]
- Wang, L.H.C.; Mayer, B.; Stemmann, O.; Nigg, E.A. Centromere DNA decatenation depends on cohesin removal and is required for mammalian cell division. J. Cell Sci. 2010, 123, 806–813. [Google Scholar] [CrossRef]
- Cuvier, O.; Hirano, T. A role of topoisomerase II in linking DNA replication to chromosome condensation. J. Cell Biol. 2003, 160, 645–655. [Google Scholar] [CrossRef]
- Chang, C.J.; Goulding, S.; Earnshaw, W.C.; Carmena, M. RNAi analysis reveals an unexpected role for topoisomerase II in chromosome arm congression to a metaphase plate. J. Cell Sci. 2003, 116, 4715–4726. [Google Scholar] [CrossRef]
- Samejima, K.; Samejima, I.; Vagnarelli, P.; Ogawa, H.; Vargiu, G.; Kelly, D.A.; De Lima Alves, F.; Kerr, A.; Green, L.C.; Hudson, D.F.; et al. Mitotic chromosomes are compacted laterally by KIF4 and condensin and axially by topoisomerase IIα. J. Cell Biol. 2012, 199, 755–770. [Google Scholar] [CrossRef]
- Baxter, J.; Aragón, L. A model for chromosome condensation based on the interplay between condensin and topoisomerase II. Trends Genet. 2012, 28, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Hirano, T. ATP-dependent positive supercoiling of DNA by 13S condensin: A biochemical implication for chromosome condensation. Cell 1997, 90, 625–634. [Google Scholar] [CrossRef]
- Kimura, K.; Rybenkov, V.V.; Crisona, N.J.; Hirano, T.; Cozzarelli, N.R. 13S Condensin Actively Reconfigures DNA by Introducing Global Positive Writhe. Cell 1999, 98, 239–248. [Google Scholar] [CrossRef]
- Takahashi, N.; Quimbaya, M.; Schubert, V.; Lammens, T.; Vandepoele, K.; Schubert, I.; Matsui, M.; Inzé, D.; Berx, G.; De Veylder, L. The MCM-binding protein ETG1 aids sister chromatid cohesion required for postreplicative homologous recombination repair. PLoS Genet. 2010, 6, e1000817. [Google Scholar] [CrossRef]
- Krasnow, M.A.; Cozzarelli, N.R. Catenation of DNA rings by topoisomerases. Mechanism of control by spermidine. J. Biol. Chem. 1982, 257, 2687–2693. [Google Scholar]
- Rybenkov, V.V.; Ullsperger, C.; Vologodskii, A.V.; Cozzarelli, N.R. Simplification of DNA topology below equilibrium values by type II topoisomerases. Science 1997, 277, 690–693. [Google Scholar] [CrossRef]
- Piskadlo, E.; Tavares, A.; Oliveira, R.A. Metaphase chromosome structure is dynamically maintained by condensin I-directed DNA (de)catenation. Elife 2017, 6, e26120. [Google Scholar] [CrossRef]
- Sen, N.; Leonard, J.; Torres, R.; Garcia-Luis, J.; Palou-Marin, G.; Aragón, L. Physical Proximity of Sister Chromatids Promotes Top2-Dependent Intertwining. Mol. Cell 2016, 64, 134–147. [Google Scholar] [CrossRef]
- Bauer, D.L.V.; Marie, R.; Rasmussen, K.H.; Kristensen, A.; Mir, K.U. DNA catenation maintains structure of human metaphase chromosomes. Nucleic Acids Res. 2012, 40, 11428–11434. [Google Scholar] [CrossRef]
- Nielsen, C.F.; Huttner, D.; Bizard, A.H.; Hirano, S.; Li, T.N.; Palmai-Pallag, T.; Bjerregaard, V.A.; Liu, Y.; Nigg, E.A.; Wang, L.H.C.; et al. PICH promotes sister chromatid disjunction and co-operates with topoisomerase II in mitosis. Nat. Commun. 2015, 6, 8962. [Google Scholar] [CrossRef]
- Daniloski, Z.; Bisht, K.K.; McStay, B.; Smith, S. Resolution of human ribosomal DNA occurs in anaphase, dependent on tankyrase 1, condensin II, and topoisomerase IIα. Genes Dev. 2019, 33, 276–281. [Google Scholar] [CrossRef] [PubMed]
- D’Alcontres, M.S.; Palacios, J.A.; Mejias, D.; Blasco, M.A. TopoIIα prevents telomere fragility and formation of ultra thin DNA bridges during mitosis through TRF1-dependent binding to telomeres. Cell Cycle 2014, 13, 1463–1481. [Google Scholar] [CrossRef] [PubMed]
- Rattner, J.B.; Hendzel, M.J.; Furbee, C.S.; Muller, M.T.; Bazett-Jones, D.P. Topoisomerase II α is associated with the mammalian centromere in a cell cycle- and species-specific manner and is required for proper centromere/kinetochore structure. J. Cell Biol. 1996, 134, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.B.; Giménez-Abián, J.F.; Clarke, D.J. A novel chromatin tether domain controls topoisomerase IIα dynamics and mitotic chromosome formation. J. Cell Biol. 2013, 203, 471–486. [Google Scholar] [CrossRef]
- Andersen, C.L.; Wandall, A.; Kjeldsen, E.; Mielke, C.; Koch, J. Active, but not inactive, human centromeres display topoisomerase II activity in vivo. Chromosom. Res. 2002, 10, 305–312. [Google Scholar] [CrossRef]
- Farcas, A.M.; Uluocak, P.; Helmhart, W.; Nasmyth, K. Cohesin’s Concatenation of Sister DNAs Maintains Their Intertwining. Mol. Cell 2011, 44, 97–107. [Google Scholar] [CrossRef]
- Tapia-Alveal, C.; Outwin, E.A.; Trempolec, N.; Dziadkowiec, D.; Murray, J.M.; O’Connell, M.J. SMC complexes and topoisomerase II work together so that sister chromatids can work apart. Cell Cycle 2010, 9, 2065–2070. [Google Scholar] [CrossRef][Green Version]
- Peters, J.M. The anaphase promoting complex/cyclosome: A machine designed to destroy. Nat. Rev. Mol. Cell Biol. 2006, 7, 644–656. [Google Scholar] [CrossRef]
- Batty, P.; Gerlich, D.W. Mitotic Chromosome Mechanics: How Cells Segregate Their Genome. Trends Cell Biol. 2019, 29, 717–726. [Google Scholar] [CrossRef]
- Nagasaka, K.; Hossain, M.J.; Roberti, M.J.; Ellenberg, J.; Hirota, T. Sister chromatid resolution is an intrinsic part of chromosome organization in prophase. Nat. Cell Biol. 2016, 18, 692–699. [Google Scholar] [CrossRef]
- Titos, I.; Ivanova, T.; Mendoza, M. Chromosome length and perinuclear attachment constrain resolution of DNA intertwines. J. Cell Biol. 2014, 206, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.A.; Queiroz-Machado, J.; Sunkel, C.E. Condensin-dependent localisation of topoisomerase II to an axial chromosomal structure is required for sister chromatid resolution during mitosis. J. Cell Sci. 2003, 116, 4763–4776. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.M.; Phua, H.H.; Mills, W.; Carpenter, A.J.; Porter, A.C.G.; Farr, C.J. Depletion of topoisomerase IIα leads to shortening of the metaphase interkinetochore distance and abnormal persistence of PICH-coated anaphase threads. J. Cell Sci. 2007, 120, 3952–3964. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, C.; Kelly, G.; Shirahige, K.; Uhlmann, F. Condensin-Dependent rDNA Decatenation Introduces a Temporal Pattern to Chromosome Segregation. Curr. Biol. 2008, 18, 1084–1089. [Google Scholar] [CrossRef]
- Leonard, J.; Sen, N.; Torres, R.; Sutani, T.; Jarmuz, A.; Shirahige, K.; Aragón, L. Condensin Relocalization from Centromeres to Chromosome Arms Promotes Top2 Recruitment during Anaphase. Cell Rep. 2015, 13, 2336–2344. [Google Scholar] [CrossRef]
- Bizard, A.H.; Allemand, J.F.; Hassenkam, T.; Paramasivam, M.; Sarlós, K.; Singh, M.I.; Hickson, I.D. PICH and TOP3A cooperate to induce positive DNA supercoiling. Nat. Struct. Mol. Biol. 2019, 26, 267–274. [Google Scholar] [CrossRef]
- Fernández-Casañas, M.; Chan, K.L. The unresolved problem of DNA bridging. Genes 2018, 9, 623. [Google Scholar] [CrossRef]
- Bhalla, N.; Biggins, S.; Murray, A.W. Mutation of YCS4, a Budding Yeast Condensin Subunit, Affects Mitotic and Nonmitotic Chromosome Behavior. Mol. Biol. Cell 2002, 13, 632–645. [Google Scholar] [CrossRef]
- Bhat, M.A.; Philp, A.V.; Glover, D.M.; Bellen, H.J. Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with topoisomerase II. Cell 1996, 87, 1103–1114. [Google Scholar] [CrossRef]
- Uemura, T.; Yanagida, M. Mitotic spindle pulls but fails to separate chromosomes in type II DNA topoisomerase mutants: Uncoordinated mitosis. EMBO J. 1986, 5, 1003–1010. [Google Scholar] [CrossRef]
- Maeshima, K.; Laemmli, U.K. A Two-step scaffolding model for mitotic chromosome assembly. Dev. Cell 2003, 4, 467–480. [Google Scholar] [CrossRef]
- Roca, J.; Wang, J.C. The probabilities of supercoil removal and decatenation by yeast DNA topoisomerase II. Genes Cells 1996, 1, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Hirano, M.; Kobayashi, R.; Hirano, T. Phosphorylation and activation of 13S condensin by Cdc2 in vitro. Science 1998, 282, 487–490. [Google Scholar] [CrossRef] [PubMed]
- McClendon, A.K.; Rodriguez, A.C.; Osheroff, N. Human topoisomerase IIα rapidly relaxes positively supercoiled DNA: Implications for enzyme action ahead of replication forks. J. Biol. Chem. 2005, 280, 39337–39345. [Google Scholar] [CrossRef]
- Mankouri, H.W.; Hickson, I.D. The RecQ helicase-topoisomerase III-Rmi1 complex: A DNA structure-specific “dissolvasome”? Trends Biochem. Sci. 2007, 32, 538–546. [Google Scholar] [CrossRef]
- Nakayama, M.; Yamaguchi, S.; Sagisu, Y.; Sakurai, H.; Ito, F.; Kawasaki, K. Loss of RecQ5 leads to spontaneous mitotic defects and chromosomal aberrations in Drosophila melanogaster. DNA Repair 2009, 8, 232–241. [Google Scholar] [CrossRef]
- Sakurai, H.; Okado, M.; Ito, F.; Kawasaki, K. Anaphase DNA bridges induced by lack of RecQ5 in Drosophila syncytial embryos. FEBS Lett. 2011, 585, 1923–1928. [Google Scholar] [CrossRef]
- Watt, P.M.; Louis, E.J.; Borts, R.H.; Hickson, I.D. Sgs1: A eukaryotic homolog of E. coil RecQ that interacts with topoisomerase II in vivo and is required for faithful chromosome segregation. Cell 1995, 81, 253–260. [Google Scholar] [CrossRef]
- Ramamoorthy, M.; Tadokoro, T.; Rybanska, I.; Ghosh, A.K.; Wersto, R.; May, A.; Kulikowicz, T.; Sykora, P.; Croteau, D.L.; Bohr, V.A. RECQL5 cooperates with Topoisomerase II alpha in DNA decatenation and cell cycle progression. Nucleic Acids Res. 2012, 40, 1621–1635. [Google Scholar] [CrossRef]
- Bachant, J.; Alcasabas, A.; Blat, Y.; Kleckner, N.; Elledge, S.J. The SUMO-1 isopeptidase Smt4 is linked to centromeric cohesion through SUMO-1 modification of DNA topoisomerase II. Mol. Cell 2002, 9, 1169–1182. [Google Scholar] [CrossRef]
- Ryu, H.; Furuta, M.; Kirkpatrick, D.; Gygi, S.P.; Azuma, Y. PIASy-dependent SUMOylation regulates DNA topoisomerase IIα activity. J. Cell Biol. 2010, 191, 783–784. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, M.; Santos, V.; Ferreira, F.; Costa, R.; Cardoso, J.; Pinheiro, I.; Rino, J.; Jaffray, E.; Hay, R.T.; Ferreira, J. Conjugation of human topoisomerase 2α with small ubiquitin-like modifiers 2/3 in response to topoisomerase inhibitors: Cell cycle stage and chromosome domain specificity. Cancer Res. 2008, 68, 2409–2418. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.; Arnaoutov, A.; Anan, T.; Dasso, M. PIASy mediates SUMO-2 conjugation of Topoisomerase-II on mitotic chromosomes. EMBO J. 2005, 24, 2172–2182. [Google Scholar] [CrossRef]
- Azuma, Y.; Arnaoutov, A.; Dasso, M. SUMO-2/3 regulates topoisomerase II in mitosis. J. Cell Biol. 2003, 163, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Yong-Gonzalez, V.; Kikuchi, Y.; Strunnikov, A. SIZ1/SIZ2 control of chromosome transmission fidelity is mediated by the sumoylation of topoisomerase II. Genetics 2006, 172, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Dawlaty, M.M.; Malureanu, L.; Jeganathan, K.B.; Kao, E.; Sustmann, C.; Tahk, S.; Shuai, K.; Grosschedl, R.; Van Deursen, J.M. Resolution of Sister Centromeres Requires RanBP2-Mediated SUMOylation of Topoisomerase IIα. Cell 2008, 133, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Verver, D.E.; Zheng, Y.; Speijer, D.; Hoebe, R.; Dekker, H.L.; Repping, S.; Stap, J.; Hamer, G. Non-SMC element 2 (NSMCE2) of the SMC5/6 complex helps to resolve topological stress. Int. J. Mol. Sci. 2016, 17, 1782. [Google Scholar] [CrossRef] [PubMed]
- Deiss, K.; Lockwood, N.; Howell, M.; Segeren, H.A.; Saunders, R.E.; Chakravarty, P.; Soliman, T.N.; Martini, S.; Rocha, N.; Semple, R.; et al. A genome-wide RNAi screen identifies the SMC5/6 complex as a non-redundant regulator of a Topo2a-dependent G2 arrest. Nucleic Acids Res. 2019, 47, 2906–2921. [Google Scholar] [CrossRef]
- Antoniou-Kourounioti, M.; Mimmack, M.L.; Porter, A.C.G.; Farr, C.J. The impact of the C-terminal region on the interaction of topoisomerase ii alpha with mitotic chromatin. Int. J. Mol. Sci. 2019, 20, 1238. [Google Scholar] [CrossRef]
- Yoshida, M.M.; Ting, L.; Gygi, S.P.; Azuma, Y. SUMOylation of DNA topoisomerase IIα regulates histone H3 kinase Haspin and H3 phosphorylation in mitosis. J. Cell Biol. 2016, 213, 665–678. [Google Scholar] [CrossRef]
- Edgerton, H.; Johansson, M.; Keifenheim, D.; Mukherjee, S.; Chacón, J.M.; Bachant, J.; Gardner, M.K.; Clarke, D.J. A noncatalytic function of the topoisomerase II CTD in Aurora B recruitment to inner centromeres during mitosis. J. Cell Biol. 2016, 213, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Krenn, V.; Musacchio, A. The Aurora B kinase in chromosome bi-orientation and spindle checkpoint signaling. Front. Oncol. 2015, 5, 225. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kumagai, A.; Dunphy, W.G. Claspin, a Chk1-regulatory protein, monitors DNA replication on chromatin independently of RPA, ATR, and Rad17. Mol. Cell 2003, 11, 329–340. [Google Scholar] [CrossRef]
- Ryu, H.; Yoshida, M.M.; Sridharan, V.; Kumagai, A.; Dunphy, W.G.; Dasso, M.; Azuma, Y. SUMOylation of the C-terminal domain of DNA topoisomerase IIα regulates the centromeric localization of Claspin. Cell Cycle 2015, 14, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- Guturi, K.K.N.; Bohgaki, M.; Bohgaki, T.; Srikumar, T.; Ng, D.; Kumareswaran, R.; El Ghamrasni, S.; Jeon, J.; Patel, P.; Eldin, M.S.; et al. RNF168 and USP10 regulate topoisomerase IIα function via opposing effects on its ubiquitylation. Nat. Commun. 2016, 7, 12638. [Google Scholar] [CrossRef]
- Lou, Z.; Minter-Dykhouse, K.; Chen, J. BRCA1 participates in DNA decatenation. Nat. Struct. Mol. Biol. 2005, 12, 589–593. [Google Scholar] [CrossRef]
- Heck, M.M.S.; Hittelman, W.N.; Earnshaw, W.C. In vivo phosphorylation of the 170-kDa form of eukaryotic DNA topoisomerase II. Cell cycle analysis. J. Biol. Chem. 1989, 264, 15161–15164. [Google Scholar]
- Cardenas, M.E.; Dang, Q.; Glover, C.V.; Gasser, S.M. Casein kinase II phosphorylates the eukaryote-specific C-terminal domain of topoisomerase II in vivo. EMBO J. 1992, 11, 1785–1796. [Google Scholar] [CrossRef]
- Wells, N.J.; Addison, C.M.; Fry, A.M.; Ganapathi, R.; Hickson, I.D. Serine 1524 is a major site of phosphorylation on human topoisomerase IIα protein in vivo and is a substrate for casein kinase II in vitro. J. Biol. Chem. 1994, 269, 29746–29751. [Google Scholar]
- Ackerman, P.; Glover, C.V.C.; Osheroff, N. Phosphorylation of DNA topoisomerase II by casein kinase II: Modulation of eukaryotic topoisomerase II activity in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 3164–3168. [Google Scholar] [CrossRef]
- Sahyoun, N.; Wolf, M.; Besterman, J.; Hsieht, T.; Sandert, M.; Iii, H.L.; Chang, K.; Cuatrecasas, P. Protein kinase C phosphorylates topoisomerase II: Topoisomerase activation and its possible role in phorbol ester-induced differentiation of HL-60 Cells. Proc. Natl. Acad. Sci. USA 1986, 83, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Escargueil, A.E.; Plisov, S.Y.; Filhol, O.; Cochet, C.; Larsen, A.K. Mitotic phosphorylation of DNA topoisomerase II α by protein kinase CK2 creates the MPM-2 phosphoepitope on Ser-1469. J. Biol. Chem. 2000, 275, 34710–34718. [Google Scholar] [CrossRef] [PubMed]
- Grozav, A.G.; Chikamori, K.; Kozuki, T.; Grabowski, D.R.; Bukowski, R.M.; Willard, B.; Kinter, M.; Andersen, A.H.; Ganapathi, R.; Ganapathi, M.K. Casein kinase I delta;/ε phosphorylates topoisomerase IIα at serine-1106 and modulates DNA cleavage activity. Nucleic Acids Res. 2009, 37, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Liu, X. Plk1-dependent phosphorylation regulates functions of DNA topoisomerase IIα in cell cycle progression. J. Biol. Chem. 2008, 283, 6209–6221. [Google Scholar] [CrossRef]
- Gardner, L.; Malik, R.; Shimizu, Y.; Mullins, N.; ElShamy, W.M. Geminin overexpression prevents the completion of topoisomerase IIα chromosome decatenation, leading to aneuploidy in human mammary epithelial cells. Breast Cancer Res. 2011, 13, R53. [Google Scholar] [CrossRef]
- Cardenas, M.E.; Gasser, S.M. Regulation of topoisomerase II by phosphorylation: A role for casein kinase II. J. Cell Sci. 1993, 104, 219–225. [Google Scholar]
- Nakazawa, N.; Arakawa, O.; Ebe, M.; Yanagida, M. Casein kinase II– dependent phosphorylation of DNA topoisomerase II suppresses the effect of a catalytic topo II inhibitor, ICRF-193, in fission yeast. J. Biol. Chem. 2019, 294, 3772–3782. [Google Scholar] [CrossRef]
- Corbett, A.H.; Fernald, A.W.; Osheroff, N. Protein Kinase C Modulates the Catalytic Activity of Topoisomerase II by Enhancing the Rate of ATP Hydrolysis: Evidence for a Common Mechanism of Regulation by Phosphorylation. Biochemistry 1993, 32, 2090–2097. [Google Scholar] [CrossRef]
- Wells, N.J.; Fry, A.M.; Guano, F.; Norbury, C.; Hickson, I.D. Cell cycle phase-specific phosphorylation of human topoisomerase IIα. Evidence of a role for protein kinase C. J. Biol. Chem. 1995, 270, 28357–28363. [Google Scholar]
- Luo, K.; Yuan, J.; Chen, J.; Lou, Z. Topoisomerase IIα controls the decatenation checkpoint. Nat. Cell Biol. 2009, 11, 204–210. [Google Scholar] [CrossRef]
- Kurz, E.U.; Leader, K.B.; Kroll, D.J.; Clark, M.; Gieseler, F. Modulation of human DNA topoisomerase IIα function by interaction with 14-3-3ε. J. Biol. Chem. 2000, 275, 13948–13954. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Ren, X.; Liu, D.; Deng, X.; Qin, X.; Zhao, X.; Wang, E.; Yu, B. 14-3-3 epsilon prevents G2/M transition of fertilized mouse eggs by binding with CDC25B. BMC Dev. Biol. 2014, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.J.; Vas, A.C.; Andrews, C.A.; Díaz-Martínez, L.A.; Giménez-Abián, J.F. Topoisomerase II checkpoints: Universal mechanisms that regulate mitosis. Cell Cycle 2006, 5, 1925–1928. [Google Scholar] [CrossRef]
- Andrews, C.A.; Vas, A.C.; Meier, B.; Giménez-Abián, J.F.; Díaz-Martínez, L.A.; Green, J.; Erickson, S.L.; VanderWaal, K.E.; Hsu, W.S.; Clarke, D.J. A mitotic topoisomerase II checkpoint in budding yeast is required for genome stability but acts independently of Pds1/securin. Genes Dev. 2006, 20, 1162–1174. [Google Scholar] [CrossRef]
- Giménez-Abián, J.F.; Clarke, D.J.; Devlin, J.; Giménez-Abián, M.I.; De La Torre, C.; Johnson, R.T.; Mullinger, A.M.; Downes, C.S. Premitotic chromosome individualization in mammalian cells depends on topoisomerase II activity. Chromosoma 2000, 109, 235–244. [Google Scholar] [CrossRef]
- Bower, J.J.; Zhou, Y.; Zhou, T.; Simpson, D.A.; Arlander, S.J.; Paules, R.S.; Cordeiro-Stone, M.; Kaufmann, W.K. Revised genetic requirements for the decatenation G2 checkpoint: The role of ATM. Cell Cycle 2010, 9, 1617–1628. [Google Scholar] [CrossRef][Green Version]
- Baxter, J.; Diffley, J.F.X. Topoisomerase II Inactivation Prevents the Completion of DNA Replication in Budding Yeast. Mol. Cell 2008, 30, 790–802. [Google Scholar] [CrossRef]
- Carpenter, A.J.; Porter, A.C.G. Construction, Characterization, and Complementation of a Conditional-Lethal DNA Topoisomerase IIalpha Mutant Human Cell Line. Mol. Biol. Cell 2004, 15, 5700–5711. [Google Scholar] [CrossRef]
- Kozuki, T.; Chikamori, K.; Surleac, M.D.; Micluta, M.A.; Petrescu, A.J.; Norris, E.J.; Elson, P.; Hoeltge, G.A.; Grabowski, D.R.; Porter, A.C.G.; et al. Roles of the C-terminal domains of topoisomerase IIα’ and topoisomerase IIβ in regulation of the decatenation checkpoint. Nucleic Acids Res. 2017, 45, 5995–6010. [Google Scholar] [CrossRef]
- Watt, P.M.; Hickson, I.D. Structure and function of type II DNA topoisomerases. Biochem. J. 1994, 303, 681–695. [Google Scholar] [CrossRef]
- Skoufias, D.A.; Lacroix, F.B.; Andreassen, P.R.; Wilson, L.; Margolis, R.L. Inhibition of DNA decatenation, but not DNA damage, arrests cells at metaphase. Mol. Cell 2004, 15, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.M.R.; Bratlie-Thoresen, S.; Brown, R.; Gillespie, D.A.F. Chk1 is required for G2/M checkpoint response induced by the catalytic topoisomerase II inhibitor ICRF-193. Cell Cycle 2007, 6, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Deming, P.B.; Cistulli, C.A.; Zhao, H.; Graves, P.R.; Piwnica-Worms, H.; Paules, R.S.; Downes, C.S.; Kaufmann, W.K. The human decatenation checkpoint. Proc. Natl. Acad. Sci. USA 2001, 98, 12044–12049. [Google Scholar] [CrossRef] [PubMed]
- Greer Card, D.A.; Sierant, M.L.; Davey, S. Rad9A is required for G2 decatenation checkpoint and to prevent endoreduplication in response to topoisomerase II inhibition. J. Biol. Chem. 2010, 285, 15653–15661. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, A.; Shinohara, M.; Rieder, C.L. Topoisomerase II and histone deacetylase inhibitors delay the G2/M transition by triggering the p38 MAPK checkpoint pathway. J. Cell Biol. 2004, 166, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Rodríguez-Bravo, V.; Medema, R.H. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J. Cell Biol. 2009, 185, 193–202. [Google Scholar] [CrossRef]
- Lara-Gonzalez, P.; Westhorpe, F.G.; Taylor, S.S. Review- The Spindle Assembly Checkpoint (Higher Eukaryotes). Curr. Biol. 2012, 22, R966–R980. [Google Scholar] [CrossRef]
- Bower, J.J.; Karaca, G.F.; Zhou, Y.; Simpson, D.A.; Cordeiro-Stone, M.; Kaufmann, W.K. Topoisomerase IIα maintains genomic stability through decatenation G 2 checkpoint signaling. Oncogene 2010, 29, 4787–4799. [Google Scholar] [CrossRef]
- Jensen, L.H.; Nitiss, K.C.; Rose, A.; Dong, J.; Zhou, J.; Hu, T.; Osheroff, N.; Jensen, P.B.; Sehested, M.; Nitiss, J.L. A novel mechanism of cell killing by anti-topoisomerase II bisdioxopiperazines. J. Biol. Chem. 2000, 275, 2137–2146. [Google Scholar] [CrossRef]
- Huang, K.C.; Gao, H.; Yamasaki, E.F.; Grabowski, D.R.; Liu, S.; Shen, L.L.; Chan, K.K.; Ganapathi, R.; Snapka, R.M. Topoisomerase II Poisoning by ICRF-193. J. Biol. Chem. 2001, 276, 44488–44494. [Google Scholar] [CrossRef]
- Park, I.; Avraham, H.K. Cell cycle-dependent DNA damage signaling induced by ICRF-193 involves ATM, ATR, CHK2, and BRCA1. Exp. Cell Res. 2006, 312, 1996–2008. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Hayashita, Y.; Maeno, K.; Masuda, A.; Sugito, N.; Osada, H.; Yanagisawa, K.; Ebi, H.; Shimokata, K.; Takahashi, T. Identification of decatenation G2 checkpoint impairment independently of DNA damage G2 checkpoint in human lung cancer cell lines. Cancer Res. 2004, 64, 4826–4832. [Google Scholar] [CrossRef] [PubMed]
- Giam, M.; Rancati, G. Aneuploidy and chromosomal instability in cancer: A jackpot to chaos. Cell Div. 2015, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.; Chia, K.M.; Spoerri, L.; Mukhopadhyay, P.; Wigan, M.; Stark, M.; Pavey, S.; Gabrielli, B. Defective decatenation checkpoint function is a common feature of melanoma. J. Investig. Dermatol. 2014, 134, 150–158. [Google Scholar] [CrossRef]
- Jain, C.K.; Roychoudhury, S.; Majumder, H.K. Selective killing of G2 decatenation checkpoint defective colon cancer cells by catalytic topoisomerase II inhibitor. Biochim. Biophys. Acta-Mol. Cell Res. 2015, 1853, 1195–1204. [Google Scholar] [CrossRef]
- Damelin, M.; Sun, Y.E.; Sodja, V.B.; Bestor, T.H. Decatenation checkpoint deficiency in stem and progenitor cells. Cancer Cell 2005, 8, 479–484. [Google Scholar] [CrossRef]
- Dykhuizen, E.C.; Hargreaves, D.C.; Miller, E.L.; Cui, K.; Korshunov, A.; Kool, M.; Pfister, S.; Cho, Y.J.; Zhao, K.; Crabtree, G.R. BAF complexes facilitate decatenation of DNA by topoisomerase IIα. Nature 2013, 497, 624–627. [Google Scholar] [CrossRef]
- Kadoch, C.; Hargreaves, D.C.; Hodges, C.; Elias, L.; Ho, L.; Ranish, J.; Crabtree, G.R. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 2013, 45, 592–601. [Google Scholar] [CrossRef]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef]
- Haggarty, S.J.; Koeller, K.M.; Kau, T.R.; Silver, P.A.; Roberge, M.; Schreiber, S.L. Small Molecule Modulation of the Human Chromatid Decatenation Checkpoint. Chem. Biol. 2003, 10, 1267–1279. [Google Scholar] [CrossRef][Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Berger, J.M. Cell Cycle-Dependent Control and Roles of DNA Topoisomerase II. Genes 2019, 10, 859. https://doi.org/10.3390/genes10110859
Lee JH, Berger JM. Cell Cycle-Dependent Control and Roles of DNA Topoisomerase II. Genes. 2019; 10(11):859. https://doi.org/10.3390/genes10110859
Chicago/Turabian StyleLee, Joyce H., and James M. Berger. 2019. "Cell Cycle-Dependent Control and Roles of DNA Topoisomerase II" Genes 10, no. 11: 859. https://doi.org/10.3390/genes10110859
APA StyleLee, J. H., & Berger, J. M. (2019). Cell Cycle-Dependent Control and Roles of DNA Topoisomerase II. Genes, 10(11), 859. https://doi.org/10.3390/genes10110859




