Cytogenetic Analysis Did Not Reveal Differentiated Sex Chromosomes in Ten Species of Boas and Pythons (Reptilia: Serpentes)
Abstract
1. Introduction
2. Materials and Methods
2.1. Studied Material, Chromosome Preparations, and Staining
2.2. Fluorescence In Situ Hybridization with Telomeric Probe
2.3. Fluorescence In Situ Hybridization with rDNA Probe
2.4. Fluorescence In Situ Hybridization with (GATA)n Probe
2.5. Comparative Genomic Hybridization
2.6. Microscopy and Image Analyses
3. Results
3.1. Acrantophis Dumerili
3.2. Acrantophis Madagascariensis
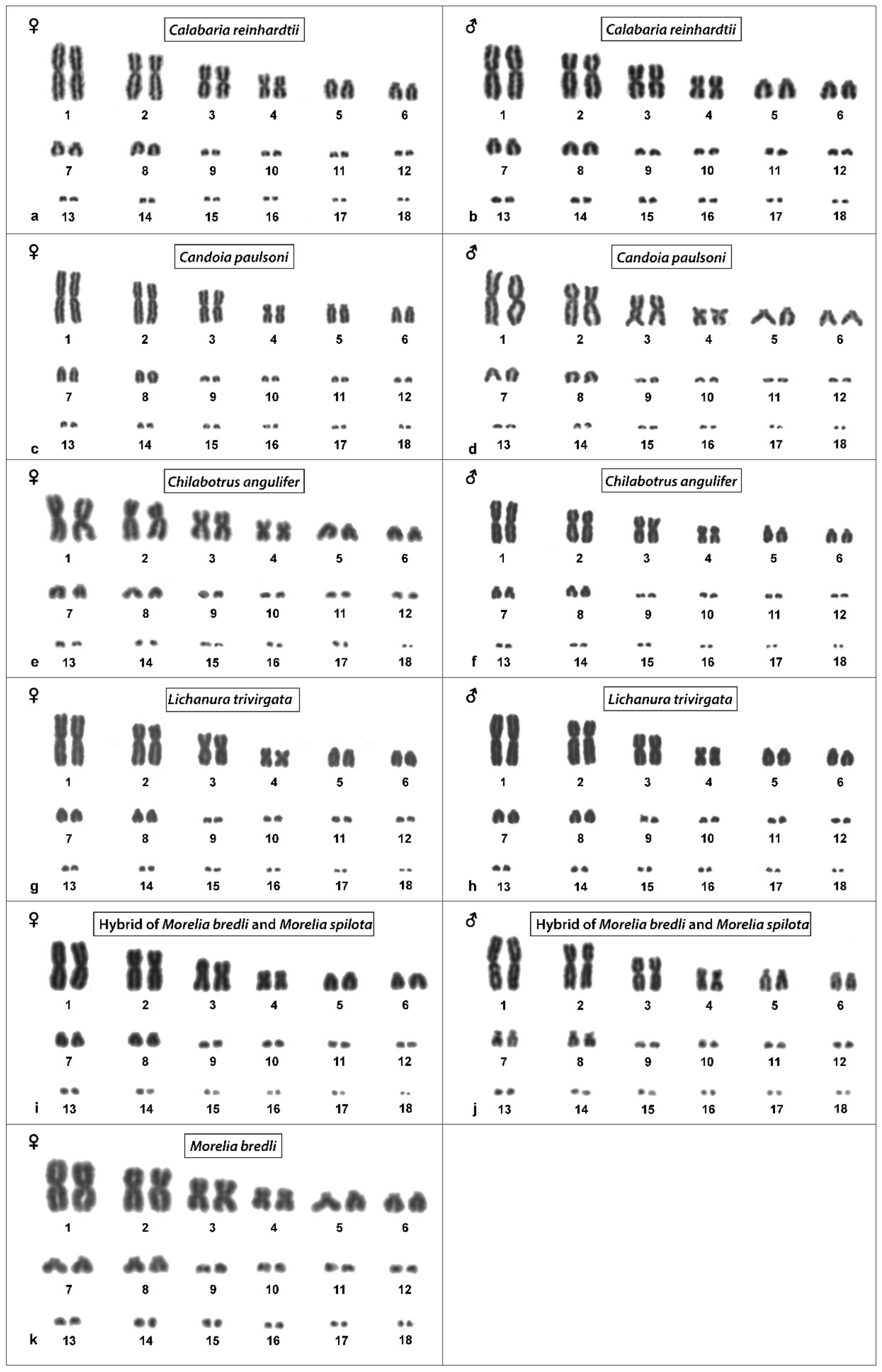
3.3. Calabaria Reinhardtii
3.4. Candoia Paulsoni
3.5. Chilabothrus Angulifer
3.6. Eunectes Notaeus
3.7. Lichanura Trivirgata
3.8. Morelia Bredli
3.9. Morelia Spilota
3.10. Morelia Bredli × Morelia Spilota Hybrid
3.11. Sanzinia Madagascariensis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uetz, P.; Freed, P.; Hošek, J. The Reptile Database. 2019. Available online: http://www.reptile-database.org (accessed on 6 October 2019).
- Heise, P.J.; Maxson, L.R.; Dowling, H.G.; Hedges, S.B. Higher-level snake phylogeny inferred from mitochondrial DNA sequences of 12S rRNA and 16S rRNA genes. Mol. Biol. Evol. 1995, 12, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Pyron, R.A.; Burbrink, F.T.; Wiens, J.J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wiens, J.J. Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Mol. Phylogenet. Evol. 2016, 94, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Harrington, S.M.; Reeder, T.W. Phylogenetic inference and divergence dating of snakes using molecules, morphology and fossils: New insights into convergent evolution of feeding morphology and limb reduction. Biol. J. Linn. Soc. 2017, 121, 379–394. [Google Scholar] [CrossRef][Green Version]
- Pyron, R.A.; Hendry, C.R.; Chou, V.M.; Lemmon, E.M.; Lemmon, A.R.; Burbrink, F.T. Effectiveness of phylogenomic data and coalescent species-tree methods for resolving difficult nodes in the phylogeny of advanced snakes (Serpentes: Caenophidia). Mol. Phylogenet. Evol. 2014, 81, 221–231. [Google Scholar] [CrossRef]
- Olmo, E.; Signorino, G.G. Chromorep: A Reptile Chromosomes Database. 2005. Available online: http://chromorep.univpm.it (accessed on 6 October 2019).
- Oguiura, N.; Ferrarezzi, H.; Batistic, R.F. Cytogenetics and molecular data in snakes: A phylogenetic approach. Cytogenet. Genome Res. 2009, 127, 128–142. [Google Scholar] [CrossRef]
- Rovatsos, M.; Johnson Pokorná, M.; Kratochvíl, L. Differentiation of sex chromosomes and karyotype characterisation in the dragonsnake Xenodermus javanicus (Squamata: Xenodermatidae). Cytogenet. Genome Res. 2015, 147, 48–54. [Google Scholar] [CrossRef]
- Rovatsos, M.; Altmanová, M.; Johnson Pokorná, M.; Augstenová, B.; Kratochvíl, L. Cytogenetics of the Javan file snake (Acrochordus javanicus) and the evolution of snake sex chromosomes. J. Zool. Syst. Evol. Res. 2017, 56, 117–125. [Google Scholar] [CrossRef]
- O’Meally, D.; Patel, H.R.; Stiglec, R.; Sarre, S.D.; Georges, A.; Marshall Graves, J.A.; Ezaz, T. Non-homologous sex chromosomes of birds and snakes share repetitive sequences. Chromosome Res. 2010, 18, 787–800. [Google Scholar] [CrossRef]
- Augstenová, B.; Mazzoleni, S.; Kratochvíl, L.; Rovatsos, M. Evolutionary dynamics of the W chromosome in caenophidian snakes. Genes 2018, 9, 5. [Google Scholar] [CrossRef]
- Vicoso, B.; Emerson, J.J.; Zektser, Y.; Mahajan, S.; Bachtrog, D. Comparative sex chromosome genomics in snakes: Differentiation, evolutionary strata, and lack of global dosage compensation. PLoS Biol. 2013, 11, e1001643. [Google Scholar] [CrossRef] [PubMed]
- Rovatsos, M.; Vukić, J.; Lymberakis, P.; Kratochvíl, L. Evolutionary stability of sex chromosomes in snakes. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151992. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Purdom, F.; Jones, K.W. Satellite DNA and evolution of sex chromosomes. Chromosoma 1976, 59, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Purdom, I.F.; Jones, K.W. Sex chromosome associated satellite DNA: Evolution and conservation. Chromosoma 1980, 79, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Viana, P.F.; Ribeiro, L.B.; Souza, G.M.; Chalkidis, H.M.; Gross, M.C.; Feldberg, E. Is the karyotype of neotropical boid snakes really conserved? Cytotaxonomy, chromosomal rearrangements and karyotype organization in the Boidae family. PLoS ONE 2016, 11, e0160274. [Google Scholar] [CrossRef][Green Version]
- Ohno, S. Sex. Chromosomes and Sex-Linked Genes; Springer: Berlin, Germany, 1967; pp. 1–167. [Google Scholar]
- Matsubara, K.; Tarui, H.; Toriba, M.; Yamada, K.; Nishida-Umehara, C.; Agata, K.; Matsuda, Y. Evidence for different origin of sex chromosomes in snakes, birds, and mammals and step-wise differentiation of snake sex chromosomes. Proc. Natl. Acad. Sci. USA 2006, 103, 18190–18195. [Google Scholar] [CrossRef]
- Booth, W.; Million, L.; Reynolds, R.G.; Burghardt, G.M.; Vargo, E.L.; Schal, C.; Tzika, A.C.; Schuett, G.W. Consecutive virgin births in the New World boid snake, the Colombian rainbow boa, Epicrates maurus. J. Hered. 2011, 102, 759–763. [Google Scholar] [CrossRef][Green Version]
- Booth, W.; Johnson, D.H.; Moore, S.; Schal, C.; Vargo, E.L. Evidence for viable, non-clonal but fatherless Boa constrictors. Biol. Lett. 2011, 7, 253–256. [Google Scholar] [CrossRef]
- Kinney, M.E.; Wack, R.F.; Grahn, R.A.; Lyons, L. Parthenogenesis in a Brazilian rainbow boa (Epicrates cenchria cenchria). Zoo Biol. 2013, 32, 172–176. [Google Scholar] [CrossRef]
- Booth, W.; Schuett, G.W.; Ridgway, A.; Buxton, D.W.; Castoe, T.A.; Bastone, G.; Bennett, C.; McMahan, W. New insights on facultative parthenogenesis in pythons. Biol. J. Linn. Soc. 2014, 112, 461–468. [Google Scholar] [CrossRef]
- Mallery, C.S., Jr.; Carrillo, M.M. A case study of sex-linkage in Python regius (Serpentes: Boidae), with new insights into sex determination in Henophidia. Phyllomedusa 2016, 15, 29–42. [Google Scholar] [CrossRef]
- Gamble, T.; Castoe, T.A.; Nielsen, S.V.; Banks, J.L.; Card, D.C.; Schield, D.R.; Schuett, G.W.; Booth, W. The discovery of XY sex chromosomes in a boa and python. Curr. Biol. 2017, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mengden, G.A.; Stock, A.D. Chromosomal evolution in Serpentes; a comparison of G and C chromosome banding patterns of some colubrid and boid genera. Chromosoma 1980, 79, 53–64. [Google Scholar] [CrossRef]
- Augstenová, B.; Johnson Pokorná, M.; Altmanová, M.; Frynta, D.; Rovatsos, M.; Kratochvíl, L. ZW, XY, and yet ZW: Sex chromosome evolution in snakes even more complicated. Evolution 2018, 72, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Kumazawa, Y.; Ota, H.; Nishida, C.; Matsuda, Y. Karyotype analysis of four blind snake species (Reptilia: Squamata: Scolecophidia) and karyotypic changes in Serpentes. Cytogenet. Genome Res. 2019, 157, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, N.; Badenhorst, D.; Montiel, E.E.; Literman, R. Molecular cytogenetic search for cryptic sex chromosomes in painted turtles Chrysemys picta. Cytogenet. Genome Res. 2014, 144, 39–46. [Google Scholar] [CrossRef]
- Matsubara, K.; Nishida, C.; Matsuda, Y.; Kumazawa, Y. Sex chromosome evolution in snakes inferred from divergence patterns of two gametologous genes and chromosome distribution of sex chromosome-linked repetitive sequences. Zool. Lett. 2016, 2, 19. [Google Scholar] [CrossRef]
- Matsubara, K.; O’Meally, D.; Azad, B.; Georges, A.; Sarre, S.D.; Graves, J.A.M.; Matsuda, Y.; Ezaz, T. Amplification of microsatellite repeat motifs is associated with the evolutionary differentiation and heterochromatinization of sex chromosomes in Sauropsida. Chromosoma 2016, 125, 111–123. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Augstenová, B.; Clemente, L.; Auer, M.; Fritz, U.; Praschag, P.; Protiva, T.; Velenský, P.; Kratochvíl, L.; Rovatsos, M. Turtles of the genera Geoemyda and Pangshura (Testudines: Geoemydidae) lack differentiated sex chromosomes: The end of a 40-year error cascade for Pangshura. PeerJ 2019, 7, e6241. [Google Scholar] [CrossRef]
- Sumner, A.T. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 1972, 75, 304–306. [Google Scholar] [CrossRef]
- Ijdo, J.W.; Baldini, A.; Ward, D.C.; Reeders, S.T.; Wells, R.A. Origin of human chromosome 2: An ancestral telomere-telomere fusion. Proc. Natl. Acad. Sci. USA 1991, 88, 9051–9055. [Google Scholar] [CrossRef] [PubMed]
- Rovatsos, M.; Johnson Pokorná, M.; Altmanová, M.; Kratochvíl, L. Female heterogamety in Madagascar chameleons (Squamata: Chamaeleonidae: Furcifer): Differentiation of sex and neo-sex chromosomes. Sci. Rep. 2015, 5, 13196. [Google Scholar] [CrossRef] [PubMed]
- Endow, S.A. Polytenization of the ribosomal genes on the X and Y chromosomes of Drosophila melanogaster. Genetics 1982, 100, 375–385. [Google Scholar] [PubMed]
- Rovatsos, M.; Altmanová, M.; Johnson Pokorná, M.; Velenský, P.; Sánchez Baca, A.; Kratochvíl, L. Evolution of karyotypes in chameleons. Genes 2017, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Mengden, G.A. Chromosomal Evolution and the Phylogeny of Elapid Snakes. Ph.D. Thesis, Australian National University, Canberra, Australia, 1982; pp. 1–221. [Google Scholar] [CrossRef]
- Reynolds, R.G.; Niemiler, M.L.; Revell, L.J. Toward a Tree-of-Life for the boas and pythons: Multi locus species-level phylogeny with unprecedented taxon sampling. Mol. Phylogenet. Evol. 2014, 71, 201–213. [Google Scholar] [CrossRef]
- Matsubara, K.; Uno, Y.; Srikulnath, K.; Matsuda, Y.; Miller, E.; Olsson, M. No interstitial telomeres on autosomes but remarkable amplification of telomeric repeats on the W sex chromosome in the sand lizard (Lacerta agilis). J. Hered. 2015, 106, 753–757. [Google Scholar] [CrossRef]
- Pokorná, M.; Rens, W.; Rovatsos, M.; Kratochvíl, L. A ZZ/ZW sex chromosome system in the thick-tailed gecko (Underwoodisaurus milii; Squamata: Gekkota: Carphodactylidae), a member of the ancient gecko lineage. Cytogenet. Genome Res. 2014, 142, 190–196. [Google Scholar] [CrossRef]
- Lee, L.; Montiel, E.E.; Valenzuela, N. Discovery of putative XX/XY male heterogamety in Emydura subglobosa turtles exposes a novel trajectory of sex chromosome evolution in Emydura. Cytogenet. Genome Res. 2019, 158, 160–169. [Google Scholar] [CrossRef]
- Kawai, A.; Nishida-Umehara, C.; Ishijima, J.; Tsuda, Y.; Ota, H.; Matsuda, Y. Different origins of bird and reptile sex chromosomes inferred from comparative mapping of chicken Z-linked genes. Cytogenet. Genome Res. 2007, 117, 92–102. [Google Scholar] [CrossRef]
- Literman, R.; Badenhorst, D.; Valenzuela, N. qPCR-based molecular sexing by copy number variation in rRNA genes and its utility for sex identification in soft-shell turtles. Methods Ecol. Evol. 2014, 5, 872–880. [Google Scholar] [CrossRef]
- Viana, P.F.; Ezaz, T.; Cioffi, M.B.; Almeida, B.J.; Feldberg, E. Evolutionary insights of the ZW sex chromosomes in snakes: A new chapter added by the amazonian puffing snakes of the genus Spilotes. Genes 2019, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, B. The evolution of sex chromosomes. Science 1991, 251, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Pokorná, M.; Kratochvíl, L. Phylogeny of sex-determining mechanisms in squamate reptiles: Are sex chromosomes an evolutionary trap? Zool. J. Linn. Soc. 2009, 156, 168–183. [Google Scholar] [CrossRef]
- Gorman, G.C.; Gress, F. Chromosome cytology of four boid snakes and a varanid lizard, with comments on the cytosystematics of primitive snakes. Herpetologica 1970, 26, 308–317. [Google Scholar]
- Mezzasalma, M.; Andreone, F.; Glaw, F.; Petraccioli, A.; Odierna, G.; Guarino, F.M. A karyological study of three typhlopid species with some inferences on chromosome evolution in blindsnakes (Scolecophidia). Zool. Anz. 2016, 264, 34–40. [Google Scholar] [CrossRef]
- Gamble, T.; Zarkower, D. Identification of sex-specific molecular markers using restriction site-associated DNA sequencing. Mol. Ecol. Res. 2014, 14, 902–913. [Google Scholar] [CrossRef]
- Gamble, T.; Coryell, J.; Ezaz, T.; Lynch, J.; Scantlebury, D.P.; Zarkower, D. Restriction site-associated DNA sequencing (RAD-seq) reveals an extraordinary number of transitions among gecko sex-determining systems. Mol. Biol. Evol. 2015, 32, 1296–1309. [Google Scholar] [CrossRef]
- Wilson, C.A.; Titus, T.; Batzel, P.; Postlethwait, J.H.; Raman, R. A search for sex-linked loci in the agamid lizard, Calotes versicolor. Sex. Dev. 2019, 13, 143–150. [Google Scholar] [CrossRef]
- Watts, P.C.; Buley, K.R.; Sanderson, S.; Boardman, W.; Ciofi, C.; Gibson, R. Parthenogenesis in Komodo dragons. Nature 2006, 444, 1021–1022. [Google Scholar] [CrossRef]
- Solari, A.J. Sex. Chromosomes and Sex. Determination in Vertebrates; CRC Press: London, UK, 1993; pp. 1–336. [Google Scholar]
- Valenzuela, N.; Lance, V.A. Temperature-Dependent Sex Determination in Vertebrates; Smithsonian Books: Washington, DC, USA, 2004; pp. 1–194. [Google Scholar]
- Burger, J.; Zappalorti, R.T. Effects of incubation temperature on sex ratios in pine snakes: Differential vulnerability of males and females. Am. Natur. 1988, 132, 492–505. [Google Scholar] [CrossRef]
- Dunlap, K.D.; Lang, J.W. Offspring sex ratio varies with maternal size in the common garter snake, Thamnophis sirtalis. Copeia 1990, 1990, 568–570. [Google Scholar] [CrossRef]
- Reichling, S.B.; Gutzke, W.H. Phenotypic consequences of incubation environment in the African elapid genus Aspidelaps. Zoo Biol. 1996, 15, 301–308. [Google Scholar] [CrossRef]






| Family | Species | ♀ | ♂ |
|---|---|---|---|
| Boidae | Chilabothrus angulifer | 2 | 1 |
| Eunectes notaeus | 1 | 2 | |
| Calabariidae | Calabaria reinhardtii | 1 | 2 |
| Candoiidae | Candoia paulsoni | 1 | 1 |
| Charinidae | Lichanura trivirgata | 1 | 1 |
| Sanziniidae | Acrantophis dumerili | 7 | 3 |
| Acrantophis madagascariensis | 2 | 1 | |
| Sanzinia madagascariensis | 1 | 1 | |
| Pythonidae | Morelia bredli | 1 | 0 |
| Morelia spilota | 3 | 4 | |
| Morelia bredli × Morelia spilota hybrid | 3 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augstenová, B.; Mazzoleni, S.; Kostmann, A.; Altmanová, M.; Frynta, D.; Kratochvíl, L.; Rovatsos, M. Cytogenetic Analysis Did Not Reveal Differentiated Sex Chromosomes in Ten Species of Boas and Pythons (Reptilia: Serpentes). Genes 2019, 10, 934. https://doi.org/10.3390/genes10110934
Augstenová B, Mazzoleni S, Kostmann A, Altmanová M, Frynta D, Kratochvíl L, Rovatsos M. Cytogenetic Analysis Did Not Reveal Differentiated Sex Chromosomes in Ten Species of Boas and Pythons (Reptilia: Serpentes). Genes. 2019; 10(11):934. https://doi.org/10.3390/genes10110934
Chicago/Turabian StyleAugstenová, Barbora, Sofia Mazzoleni, Alexander Kostmann, Marie Altmanová, Daniel Frynta, Lukáš Kratochvíl, and Michail Rovatsos. 2019. "Cytogenetic Analysis Did Not Reveal Differentiated Sex Chromosomes in Ten Species of Boas and Pythons (Reptilia: Serpentes)" Genes 10, no. 11: 934. https://doi.org/10.3390/genes10110934
APA StyleAugstenová, B., Mazzoleni, S., Kostmann, A., Altmanová, M., Frynta, D., Kratochvíl, L., & Rovatsos, M. (2019). Cytogenetic Analysis Did Not Reveal Differentiated Sex Chromosomes in Ten Species of Boas and Pythons (Reptilia: Serpentes). Genes, 10(11), 934. https://doi.org/10.3390/genes10110934







