Regulation of AMH, AMHR-II, and BMPs (2,6) Genes of Bovine Granulosa Cells Treated with Exogenous FSH and Their Association with Protein Hormones
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Tissue Collection
2.2. Granulosa Cells and Follicular Fluid Collection
2.3. Culturing of Granulosa Cells
2.4. Cell Proliferation Assay
2.5. Apoptotic Assay
2.6. RNA Purification, cDNA Preparation and Real-Time Quantitative PCR (qRT-PCR)
2.7. Hormonal Assay
2.8. Statistical Analysis
3. Results
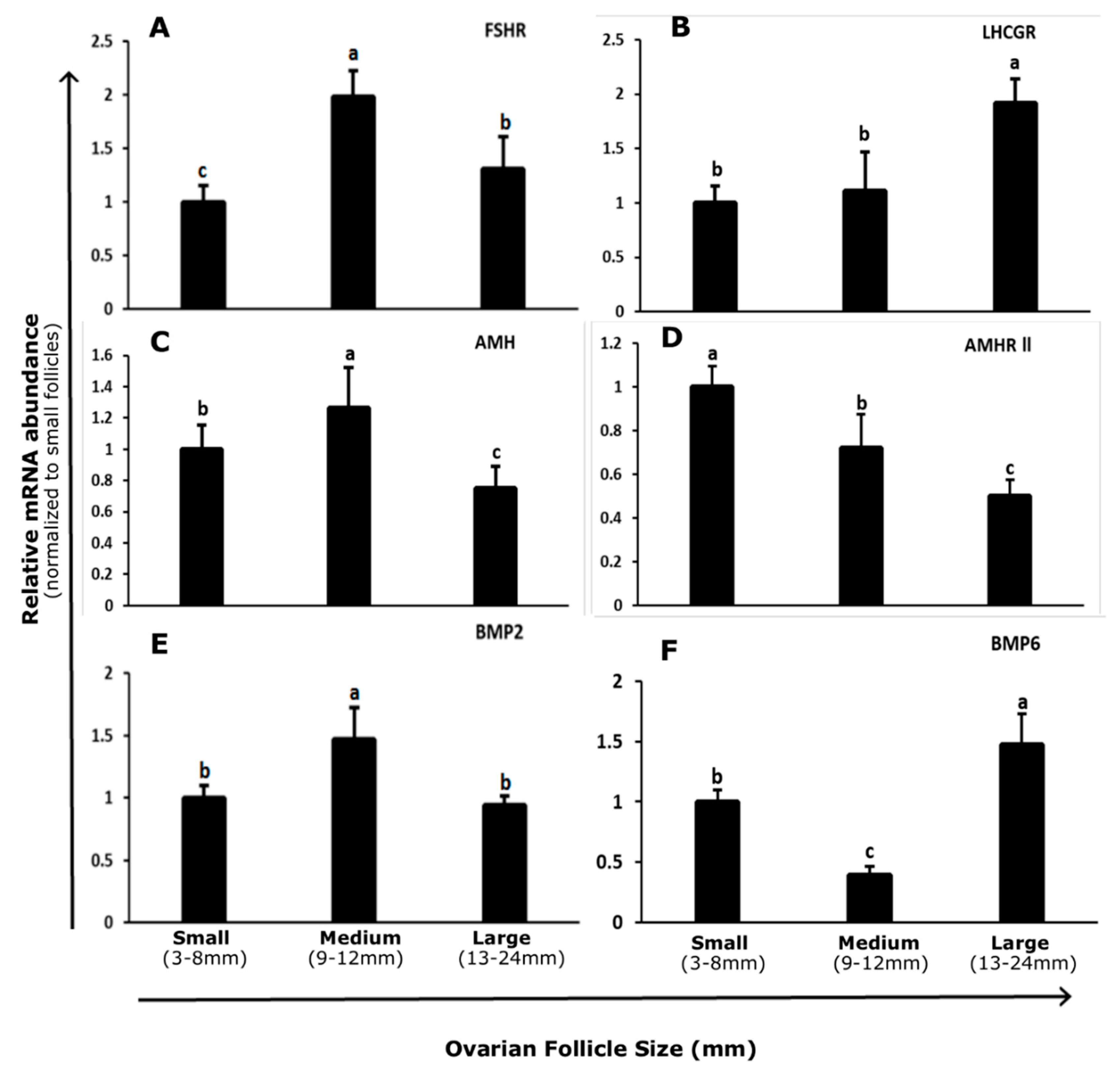
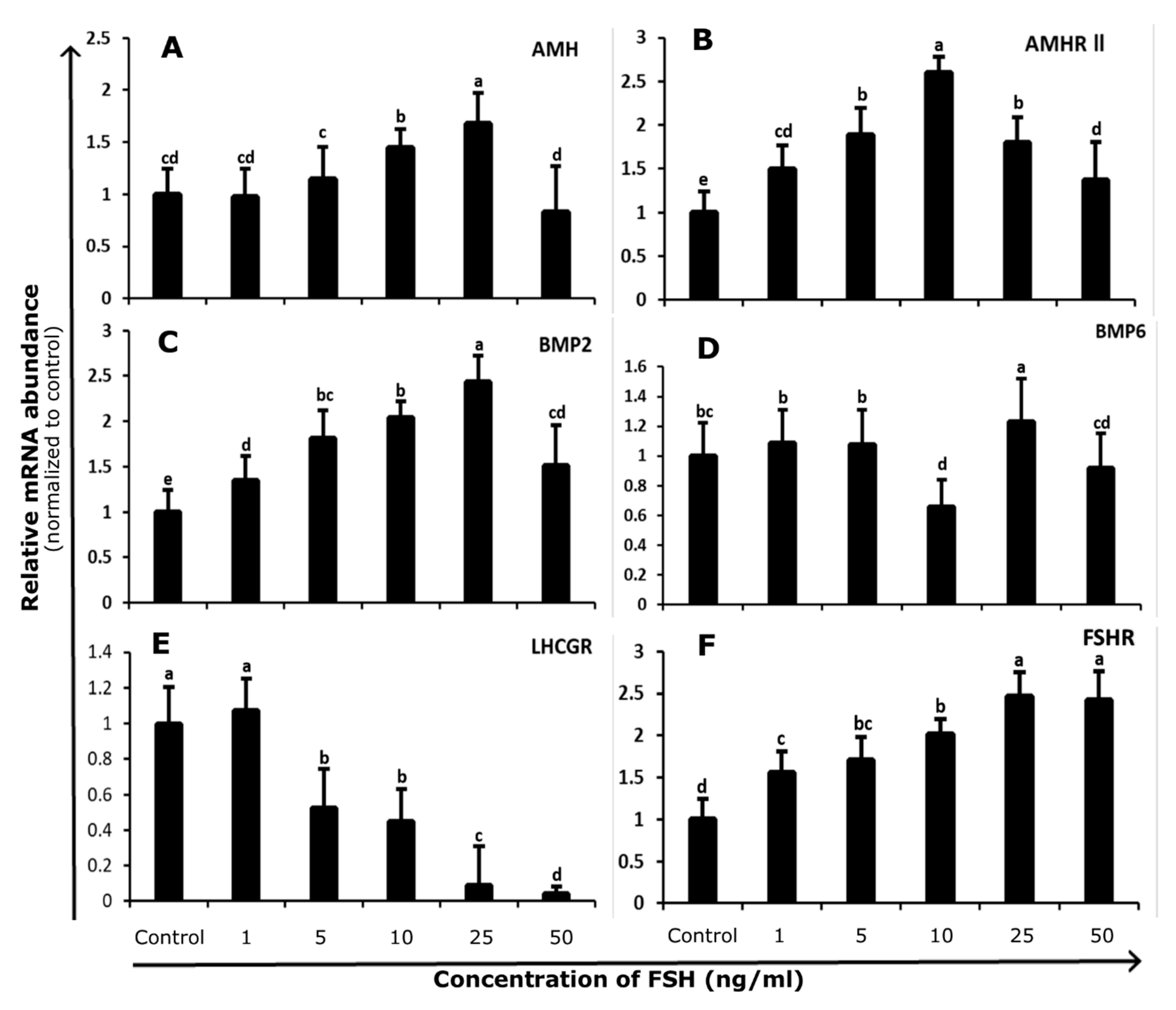
3.1. Abundance and Regulation of FSHR and LHCGR in Bovine GCs
3.2. Abundance and Regulation of AMH and AMHR-II in Bovine GCs
3.3. Abundance and Regulation of BMP2 and BMP6 in Bovine GCs
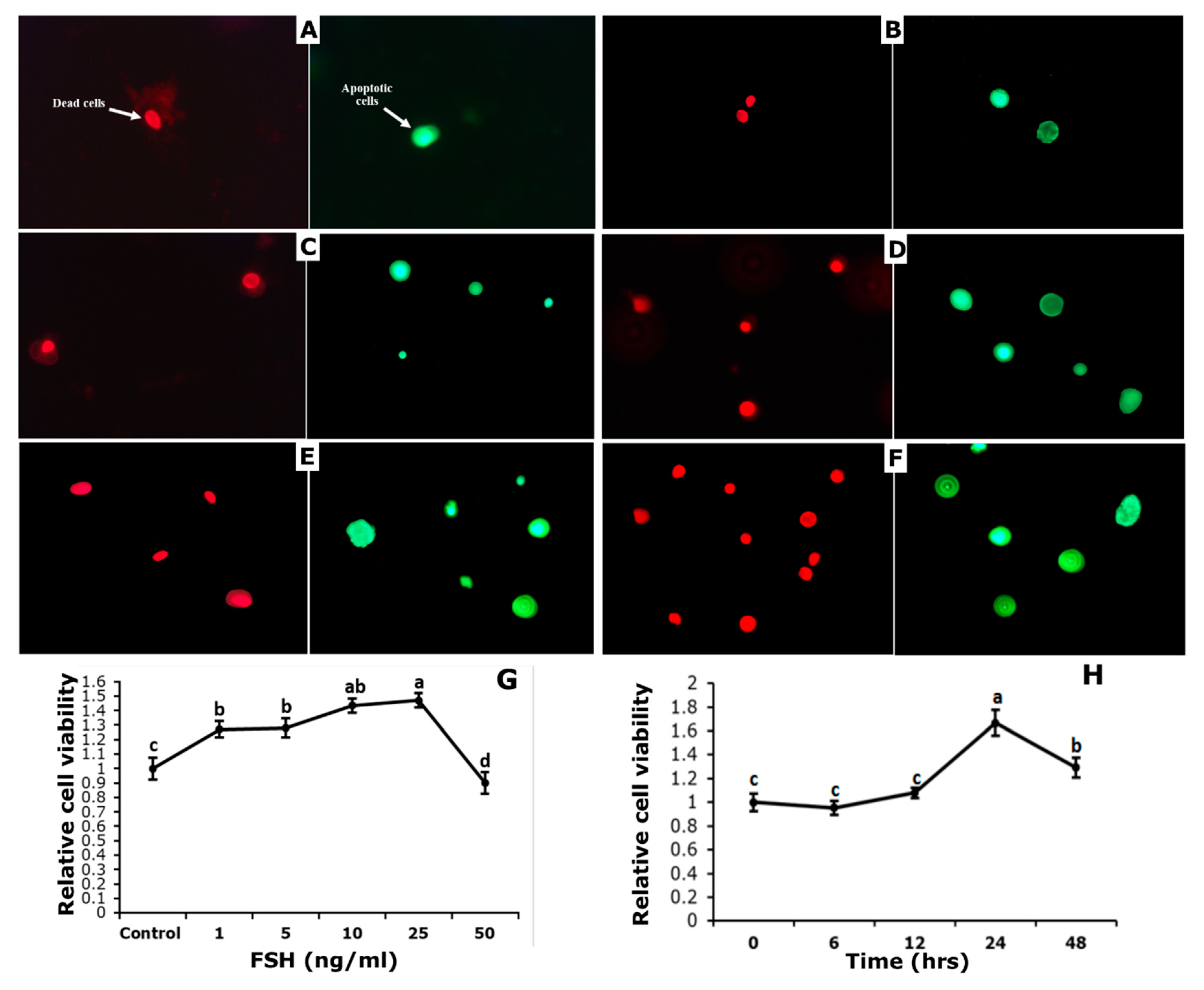
3.4. Effect of FSH on Survival and Apoptosis of GCs
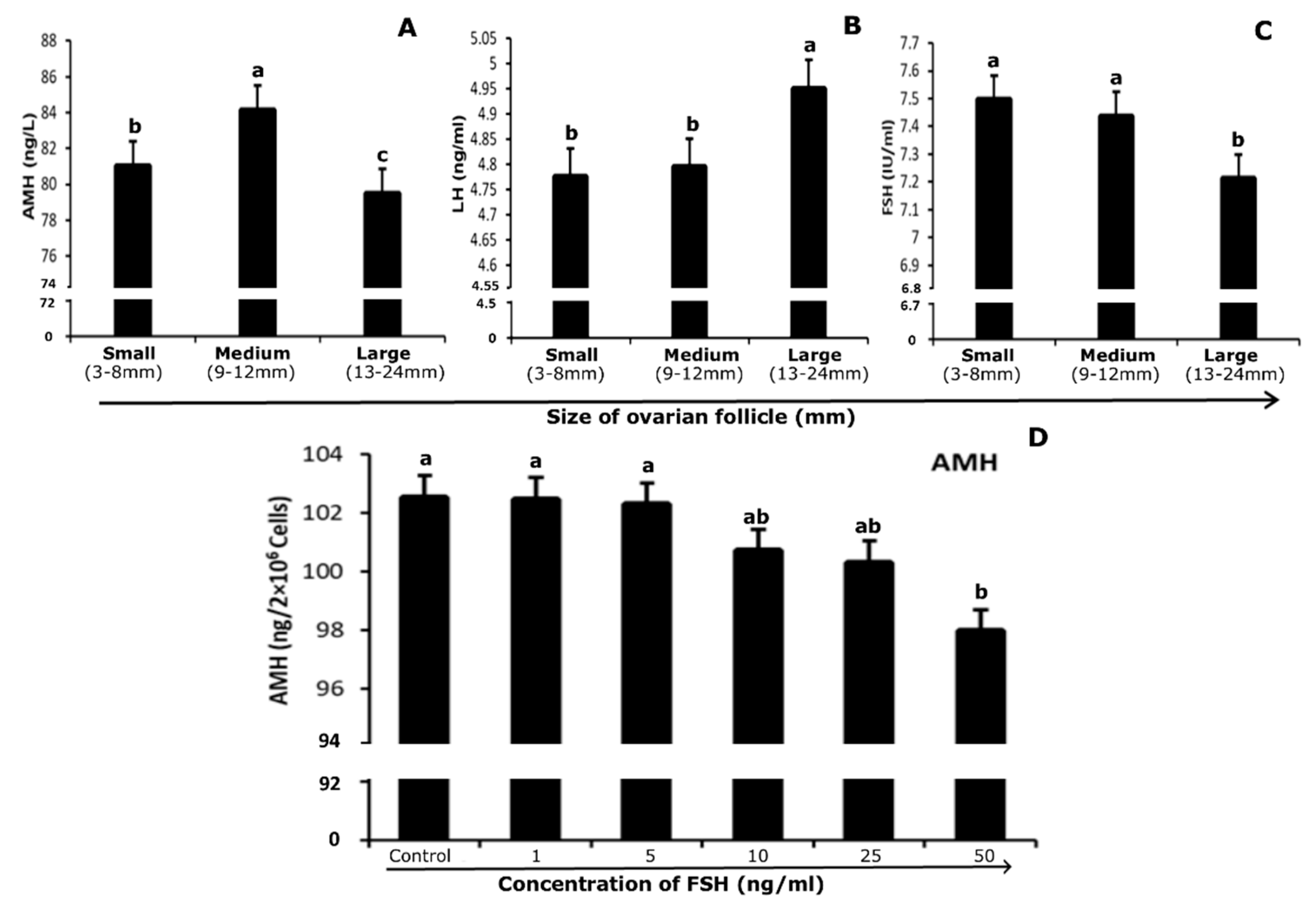
3.5. Quantification of Hormones in Follicular Fluid and Culture Medium
3.6. Association Between mRNA Abundance and Hormone (Protein) Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Humblot, P.; Le Bourhis, D.; Fritz, S.; Colleau, J.J.; Gonzalez, C.; Guyader Joly, C.; Malafosse, A.; Heyman, Y.; Amigues, Y.; Tissier, M.; et al. Reproductive technologies and genomic selection in cattle. Vet. Med. Int. 2010, 2010, 192787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Ruiz, A.; Cole, J.B.; VanRaden, P.M.; Wiggans, G.R.; Ruiz-López, F.J.; Van Tassell, C.P. Changes in genetic selection differentials and generation intervals in US Holstein dairy cattle as a result of genomic selection. Proc. Natl. Acad. Sci. USA 2016, 113, E3995–E4004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapletoft, R.J.; Steward, K.B.; Adams, G.P. Recent advances in the superovulation in cattle. Reprod. Nutr. Dev. 2002, 42, 601–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kligman, I.; Rosenwaks, Z. Differentiating clinical profiles: Predicting good responders, poor responders, and hyperresponders. Fertil. Steril. 2001, 76, 1185–1190. [Google Scholar] [CrossRef]
- Ginther, O.J. How ultrasound technologies have expanded and revolutionized research in reproduction in large animals. Theriogenology 2014, 81, 112–125. [Google Scholar] [CrossRef]
- Driancourt, M.A. Regulation of ovarian follicular dynamics in farm animals. Implications for manipulation of reproduction. Theriogenology 2001, 55, 1211–1239. [Google Scholar] [CrossRef]
- Mapletoft, R.J.; Bó, G.A. The evolution of improved and simplified superovulation protocols in cattle. Reprod. Fertil. Dev. 2012, 24, 278–283. [Google Scholar] [CrossRef]
- Knight, P.G.; Glister, C. Potential local regulatory functions of inhibins, activins and follistatin in the ovary. Reproduction 2001, 121, 503–512. [Google Scholar] [CrossRef] [Green Version]
- La Marca, A.; Volpe, A. Anti-Müllerian hormone (AMH) in female reproduction: Is measurement of circulating AMH a useful tool? Clin. Endocrinol. 2006, 64, 603–610. [Google Scholar] [CrossRef]
- Rico, C.; Fabre, S.; Médigue, C.; di Clemente, N.; Clément, F.; Bontoux, M.; Touzé, J.-L.; Dupont, M.; Briant, E.; Rémy, B. Anti-Müllerian hormone is an endocrine marker of ovarian gonadotropin-responsive follicles and can help to predict superovulatory responses in the cow. Biol. Reprod. 2009, 80, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Monniaux, D.; Drouilhet, L.; Rico, C.; Estienne, A.; Jarrier, P.; Touzé, J.-L.; Sapa, J.; Phocas, F.; Dupont, J.; Dalbiès-Tran, R. Regulation of anti-Müllerian hormone production in domestic animals. Reprod. Fertil. Dev. 2012, 25, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, F.; Lawson, M.S.; Tkachenko, O.Y.; Ting, A.Y.; Kahl, C.A.; Park, B.S.; Stouffer, R.R.; Bishop, C.V. Anti-Müllerian hormone is a survival factor and promotes the growth of rhesus macaque preantral follicles during matrix-free culture†. Biol. Reprod. 2018, 98, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, M.W.; Kelley, R.L.; Sanders, R.L.; Woodcock, S.R.; Oorschot, D.E.; Batchelor, N.J. Anti-Müllerian hormone overexpression restricts preantral ovarian follicle survival. J. Endocrinol. 2018, 237, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piouka, A.; Farmakiotis, D.; Katsikis, I.; Macut, D.; Gerou, S.; Panidis, D. Anti-Müllerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: Relationship with increased luteinizing hormone levels. Am. J. Physiol.-Endocrinol. Metab. 2009, 296, E238–E243. [Google Scholar] [CrossRef] [Green Version]
- Eldar-Geva, T.; Ben-Chetrit, A.; Spitz, I.M.; Rabinowitz, R.; Markowitz, E.; Mimoni, T.; Gal, M.; Zylber-Haran, E.; Margalioth, E.J. Dynamic assays of inhibin B, anti-Mullerian hormone and estradiol following FSH stimulation and ovarian ultrasonography as predictors of IVF outcome. Hum. Reprod. 2005, 20, 3178–3183. [Google Scholar] [CrossRef] [Green Version]
- Rico, C.; Drouilhet, L.; Salvetti, P.; Dalbìs-Tran, R.; Jarrier, P.; Touź, J.L.; Pillet, E.; Ponsart, C.; Fabre, S.; Monniaux, D. Determination of anti-Mllerian hormone concentrations in blood as a tool to select Holstein donor cows for embryo production: From the laboratory to the farm. Reprod. Fertil. Dev. 2012, 24, 932–944. [Google Scholar] [CrossRef]
- Umer, S.; Zhao, S.J.; Sammad, A.; Sahlu, B.W.; Yunwei, P. AMH: Could It Be Used as A Biomarker for Fertility and Superovulation in Domestic Animals? Genes 2019, 10, 1009. [Google Scholar] [CrossRef] [Green Version]
- Baarends, W.M.; Van Helmond, M.J.; Post, M.; van Der Schoot, P.J.; Hoogerbrugge, J.W.; de Winter, J.P.; Uilenbroek, J.T.; Karels, B.; Wilming, L.G.; Meijers, J.H. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the mullerian duct. Development 1994, 120, 189–197. [Google Scholar]
- Orvis, G.D.; Jamin, S.P.; Kwan, K.M.; Mishina, Y.; Kaartinen, V.M.; Huang, S.; Roberts, A.B.; Umans, L.; Huylebroeck, D.; Zwijsen, A.; et al. Functional Redundancy of TGF-beta Family Type I Receptors and Receptor-Smads in Mediating Anti-Müllerian Hormone-Induced Müllerian Duct Regression in the Mouse1. Biol. Reprod. 2008, 78, 994–1001. [Google Scholar] [CrossRef] [Green Version]
- Sèdes, L.; Leclerc, A.; Moindjie, H.; Cate, R.L.; Picard, J.Y.; Di Clemente, N.; Jamin, S.P. Anti-Müllerian hormone recruits BMPR-IA in immature granulosa cells. PLoS ONE 2013, 8, e81551. [Google Scholar] [CrossRef] [Green Version]
- Visser, J.A. The Serine/Threonine Transmembrane Receptor ALK2 Mediates Mullerian Inhibiting Substance Signaling. Mol. Endocrinol. 2001, 15, 936–945. [Google Scholar] [PubMed] [Green Version]
- Estienne, A.; Pierre, A.; Di Clemente, N.; Picard, J.Y.; Jarrier, P.; Mansanet, C.; Monniaux, D.; Fabre, S. Anti-müllerian hormone regulation by the bone morphogenetic proteins in the sheep ovary: Deciphering a direct regulatory pathway. Endocrinology 2015, 156, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierre, A.; Estienne, A.; Racine, C.; Picard, J.Y.; Fanchin, R.; Lahoz, B.; Alabart, J.L.; Folch, J.; Jarrier, P.; Fabre, S.; et al. The bone morphogenetic protein 15 up-regulates the anti-müllerian hormone receptor expression in granulosa cells. J. Clin. Endocrinol. Metab. 2016, 101, 2602–2611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glister, C.; Satchell, L.; Knight, P.G. Changes in expression of bone morphogenetic proteins (BMPs), their receptors and inhibin co-receptor betaglycan during bovine antral follicle development: Inhibin can antagonize the suppressive effect of BMPs on thecal androgen production. Reproduction 2010, 140, 699–712. [Google Scholar] [CrossRef] [Green Version]
- Hosoya, T.; Otsuka, F.; Nakamura, E.; Terasaka, T.; Inagaki, K.; Tsukamoto-Yamauchi, N.; Hara, T.; Toma, K.; Komatsubara, M.; Makino, H. Regulatory role of BMP-9 in steroidogenesis by rat ovarian granulosa cells. J. Steroid Biochem. Mol. Biol. 2015, 147, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, F. Multifunctional bone morphogenetic protein system in endocrinology. Acta Med. Okayama 2013, 62, 75–86. [Google Scholar]
- Shimasaki, S.; Zachow, R.J.; Danmei, L.I.; Kim, H.; Iemura, S.I.; Ueno, N.; Sampath, K.; Chang, R.J.; Erickson, G.F. A functional bone morphogenetic protein system in the ovary. Proc. Natl. Acad. Sci. USA 1999, 96, 7282–7287. [Google Scholar] [CrossRef] [Green Version]
- Erickson, G.F.; Shimasaki, S. The spatiotemporal expression pattern of the bone morphogenetic protein family in rat ovary cell types during the estrous cycle. Reprod. Biol. Endocrinol. 2003, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, K.; Otsuka, F.; Miyoshi, T.; Yamashita, M.; Takahashi, M.; Goto, J.; Suzuki, J.; Makino, H. P38-mitogen-activated protein kinase stimulated steroidogenesis in granulosa cell-oocyte cocultures: Role of bone morphogenetic proteins 2 and 4. Endocrinology 2009, 150, 1921–1930. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, F.; McTavish, K.J.; Shimasaki, S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol. Reprod. Dev. 2011, 78, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Lerner, A.A.C.; Salamone, D.F.; Chiappe, M.E.; Barañao, J.L. Comparative studies between freshly isolated and spontaneously immortalized bovine granulosa cells: Protein secretion, steroid metabolism, and responsiveness to growth factors. J. Cell. Physiol. 1995, 164, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.; Ayres, H.; Chiaratti, M.R.; Ferraz, M.L.; Araújo, A.B.; Rodrigues, C.A.; Watanabe, Y.F.; Vireque, A.A.; Joaquim, D.C.; Smith, L.C.; et al. The low fertility of repeat-breeder cows during summer heat stress is related to a low oocyte competence to develop into blastocysts. J. Dairy Sci. 2011, 94, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Gasperin, B.G.; Ferreira, R.; Rovani, M.T.; Bordignon, V.; Duggavathi, R.; Buratini, J.; Oliveira, J.F.C.; Gonçalves, P.B.D. Expression of receptors for BMP15 is differentially regulated in dominant and subordinate follicles during follicle deviation in cattle. Anim. Reprod. Sci. 2014, 144, 72–78. [Google Scholar] [CrossRef]
- Gasperin, B.G.; Rovani, M.T.; Ferreira, R.; Ilha, G.F.; Bordignon, V.; Gonçalves, P.B.D.; Duggavathi, R. Functional status of STAT3 and MAPK3/1 signaling pathways in granulosa cells during bovine follicular deviation. Theriogenology 2015, 83, 353–359. [Google Scholar] [CrossRef]
- Rico, C.; Médigue, C.; Fabre, S.; Jarrier, P.; Bontoux, M.; Clément, F.; Monniaux, D. Regulation of Anti-Müllerian Hormone Production in the Cow: A Multiscale Study at Endocrine, Ovarian, Follicular, and Granulosa Cell Levels1. Biol. Reprod. 2011, 84, 560–571. [Google Scholar] [CrossRef]
- Ilha, G.F.; Rovani, M.T.; Gasperin, B.G.; Ferreira, R.; de Macedo, M.P.; Neto, O.A.; Duggavathi, R.; Bordignon, V.; Gonçalves, P.B.D. Regulation of Anti-Müllerian Hormone and Its Receptor Expression around Follicle Deviation in Cattle. Reprod. Domest. Anim. 2016, 51, 188–194. [Google Scholar] [CrossRef]
- Poole, D.H.; Ocón-Grove, O.M.; Johnson, A.L. Anti-Müllerian hormone (AMH) receptor type II expression and AMH activity in bovine granulosa cells. Theriogenology 2016, 86, 1353–1360. [Google Scholar] [CrossRef] [Green Version]
- Baarends, W.M.; Hoogerbrugge, J.W.; Post, M.; Visser, J.A.; Rooij, D.G.D.; Parvinen, M.; Themmen, A.P.N.; Grootegoed, J.A. Anti-müllerian hormone and anti-müllerian hormone type ii receptor messenger ribonucleic acid expression during postnatal testis development and in the adult testis of the rat. Endocrinology 1995, 136, 4951–4962. [Google Scholar] [CrossRef]
- Weenen, C.; Laven, J.S.E.; von Bergh, A.R.M.; Cranfield, M.; Groome, N.P.; Visser, J.A.; Kramer, P.; Fauser, B.C.J.M.; Themmen, A.P.N. Anti-Müllerian hormone expression pattern in the human ovary: Potential implications for initial and cyclic follicle recruitment. MHR Basic Sci. Reprod. Med. 2004, 10, 77–83. [Google Scholar] [CrossRef]
- Grøndahl, M.L.; Nielsen, M.E.; Dal Canto, M.B.; Fadini, R.; Rasmussen, I.A.; Westergaard, L.G.; Kristensen, S.G.; Yding Andersen, C. Anti-Müllerian hormone remains highly expressed in human cumulus cells during the final stages of folliculogenesis. Reprod. Biomed. Online 2011, 22, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeppesen, J.V.; Anderson, R.A.; Kelsey, T.W.; Christiansen, S.L.; Kristensen, S.G.; Jayaprakasan, K.; Raine-Fenning, N.; Campbell, B.K.; Yding Andersen, C. Which follicles make the most anti-Mü llerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol. Hum. Reprod. 2013, 19, 519–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheetz, D.M.; Folger, J.K.; Smith, G.W.; Ireland, J.J. Regulation of Anti-Mullerian Hormone (AMH) Production by Bovine Granulosal Cells. Biol. Reprod. 2009, 81, 533. [Google Scholar] [CrossRef]
- Scheetz, D.; Folger, J.K.; Smith, G.W.; Ireland, J.J. Granulosa cells are refractory to FSH action in individuals with a low antral follicle count. Reprod. Fertil. Dev. 2012, 24, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Ocón-Grove, O.M.; Poole, D.H.; Johnson, A.L. Bone morphogenetic protein 6 promotes FSH receptor and anti-Müllerian hormone mRNA expression in granulosa cells from hen prehierarchal follicles. Reproduction 2012, 143, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Dumesic, D.A.; Lesnick, T.G.; Stassart, J.P.; Ball, G.D.; Wong, A.; Abbott, D.H. Intrafollicular antimüllerian hormone levels predict follicle responsiveness to follicle-stimulating hormone (FSH) in normoandrogenic ovulatory women undergoing gonadotropin releasing-hormone analog/recombinant human FSH therapy for in vitro fertilization and embryo transfer. Fertil. Steril. 2009, 92, 217–221. [Google Scholar]
- Durlinger, A.L.L.; Kramer, P.; Karels, B.; De Jong, F.H.; Uilenbroek, J.T.J.; Anton Grootegoed, J.; Themmen, A.P.N. Control of primordial follicle recruitment by anti-mullerian hormone in the mouse ovary. Endocrinology 1999, 140, 5789–5796. [Google Scholar] [CrossRef]
- Durlinger, A.L.L.; Gruijters, M.J.G.; Kramer, P.; Karels, B.; Kumar, T.R.; Matzuk, M.M.; Rose, U.M.; de Jong, F.H.; Uilenbroek, J.T.J.; Grootegoed, J.A. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 2001, 142, 4891–4899. [Google Scholar] [CrossRef]
- Durlinger, A.L.L.; Gruijters, M.J.G.; Kramer, P.; Karels, B.; Ingraham, H.A.; Nachtigal, M.W.; Uilenbroek, J.T.J.; Anton Grootegoed, J.; Themmen, A.P.N. Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 2002, 143, 1076–1084. [Google Scholar] [CrossRef]
- Chang, H.M.; Klausen, C.; Leung, P.C.K. Antimüllerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil. Steril. 2013, 100, 585–592. [Google Scholar] [CrossRef]
- Ogura-Nose, S.; Yoshino, O.; Osuga, Y.; Shi, J.; Hiroi, H.; Yano, T.; Taketani, Y. Anti-Mullerian hormone (AMH) is induced by bone morphogenetic protein (BMP) cytokines in human granulosa cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 164, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yoshino, O.; Osuga, Y.; Koga, K.; Hirota, Y.; Hirata, T.; Yano, T.; Nishii, O.; Taketani, Y. Bone morphogenetic protein-6 stimulates gene expression of follicle-stimulating hormone receptor, inhibin/activin β subunits, and anti-Müllerian hormone in human granulosa cells. Fertil. Steril. 2009, 92, 1794–1798. [Google Scholar] [CrossRef]
- Rossi, R.O.D.S.; Costa, J.J.N.; Silva, A.W.B.; Saraiva, M.V.A.; Van Den Hurk, R.; Silva, J.R.V. The bone morphogenetic protein system and the regulation of ovarian follicle development in mammals. Zygote 2016, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.U.; Hein, G.J.; Belotti, E.M.; Rodríguez, F.M.; Rey, F.; Amweg, A.N.; Matiller, V.; Baravalle, M.E.; Ortega, H.H.; Salvetti, N.R. BMP2, 4 and 6 and BMPR1B are altered from early stages of bovine cystic ovarian disease development. Reproduction 2016, 152, 333–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glister, C.; Satchell, L.; Knight, P.G. Granulosal and thecal expression of bone morphogenetic protein- and activin-binding protein mRNA transcripts during bovine follicle development and factors modulating their expression in vitro. Reproduction 2011, 142, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G. Bone morphogenetic proteins (BMP) 2, 4, 6 and 7 affect ovarian follicular development through regulation of follicle-stimulating hormone receptor (FSHR) and luteinizing hormone receptor (LHR) expression in goat granulosa cells. J. Cell Biol. Genet. 2013, 3, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Faure, M.O.; Nicol, L.; Fabre, S.; Fontaine, J.; Mohoric, N.; McNeilly, A.; Taragnat, C. BMP-4 inhibits follicle-stimulating hormone secretion in ewe pituitary. J. Endocrinol. 2005, 186, 109–121. [Google Scholar] [CrossRef]
- Shi, X.; Qiu, S.; Zhuang, W.; Wang, C.; Zhang, S.; Yuan, N.; Yuan, F.; Qiao, Y. Follicle-stimulating hormone inhibits cervical cancer via NF-κb pathway. Onco Targets Ther. 2018, 11, 8107–8115. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Jiang, Y.; Guan, Z.; Cao, Y.; Sun, S.C.; Liu, H. FSH protects mouse granulosa cells from oxidative damage by repressing mitophagy. Sci. Rep. 2016, 6, 38090. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Shi, W.; Zhao, C.; Zhang, D.; Liang, P.; Wang, G.; Lu, L. Triptolide sensitizes human breast cancer cells to tumor necrosis factor-α-induced apoptosis by inhibiting activation of the nuclear factor-κB pathway. Mol. Med. Rep. 2016, 13, 3257–3264. [Google Scholar] [CrossRef]
- Gobikrushanth, M.; Purfield, D.C.; Colazo, M.G.; Butler, S.T.; Wang, Z.; Ambrose, D.J. The relationship between serum anti-Müllerian hormone concentrations and fertility, and genome-wide associations for anti-Müllerian hormone in Holstein cows. J. Dairy Sci. 2018, 101, 7563–7574. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene Symbol | Gene ID. | Oligo | Primer Sequence (5′–3′) |
|---|---|---|---|
| AMH | NM_173890 | Forward Reverse | CAGGGAAGAAGTCTTCAGCA AAGGTGGTCAAGTCACTCAG |
| AMHR ll | NM_001205328 | Forward Reverse | AGCTGTGCTTCTCCCAGGTCATA AAAGCGGACAATGTGGTCATGCTG |
| BMP2 | NM_001099141 | Forward Reverse | TGTCCAAACTCTGGTCAACTC CTCGACAACCATGTCCTGATAG |
| BMP6 | XM_005192966 | Forward Reverse | CGCCAGCGACACCACAAAGAGT CCCAGCAGAAACAGGTCCGAGTC |
| CYP19A1 | NM_174305.1 | Forward Reverse | GGCTATGTGGACGTGTTGACC TGAGAAGGAGAGCTTGCCATG |
| FSHR | NM_174061 | Forward Reverse | AGGCAAACGTGTTCTCCAAC CGGAGGTTGGGAAGGTTCTG |
| LHCGR | NM_174381 | Forward Reverse | TGAACTGAGTGGCTGGGATT AGGACAGTCACATTTCCCGT |
| BAX | NM_173894 | Forward Reverse | AACATGGAGCTGCAGAGGAT CAGTTGAAGTTGCCGTCAGA |
| GAPDH | NM_001034034.1 | Forward Reverse | CACCCTCAAGATTGTCAGCA GGTCATAAGTCCCTCCACGA |
| ACTB | NM_173979.3 | Forward Reverse | GTGACATCAAGGAGAAGCTCTG TTGAAGGTAGTTTCGTGAATGC |
| mRNA Expression › | AMH | AMHR II | BMP2 | FSHR | LHCGR | BMP6 | |
|---|---|---|---|---|---|---|---|
| AMH (ng/mL) | r value | 0.22 | −0.21 | 0.5 | 0.44 | −0.03 | −0.12 |
| p value | 0.4295 | 0.4632 | 0.0569 | 0.0977 | 0.9184 | 0.6777 | |
| r2 value | 0.0486 | 0.0421 | 0.2514 | 0.1967 | 0.0008 | 0.0137 | |
| AdjR2 value | −0.0245 | −0.0316 | 0.1938 | 0.1349 | −0.0760 | −0.0621 | |
| LH (ng/mL) | r value | −0.21 | −0.05 | 0.05 | −0.04 | 0.14 | −0.63 * |
| p value | 0.4473 | 0.8619 | 0.8514 | 0.8844 | 0.6197 | 0.0121 | |
| r2 value | 0.0451 | 0.0024 | 0.0028 | 0.00168 | 0.0194 | 0.3948 | |
| AdjR2 value | −0.0283 | −0.0743 | −0.0739 | −0.0751 | −0.0559 | 0.3482 | |
| FSH (mIU/mL) | r value | 0.41 | 0.45 | 0.1 | −0.06 | −0.44 | −0.11 |
| p value | 0.1313 | 0.0932 | 0.7179 | 0.8401 | 0.1036 | 0.6839 | |
| r2 value | 0.1663 | 0.2015 | 0.0104 | 0.0032 | 0.1907 | 0.0132 | |
| AdjR2 value | 0.1022 | 0.1401 | −0.0657 | −0.0734 | 0.1285 | −0.0627 | |
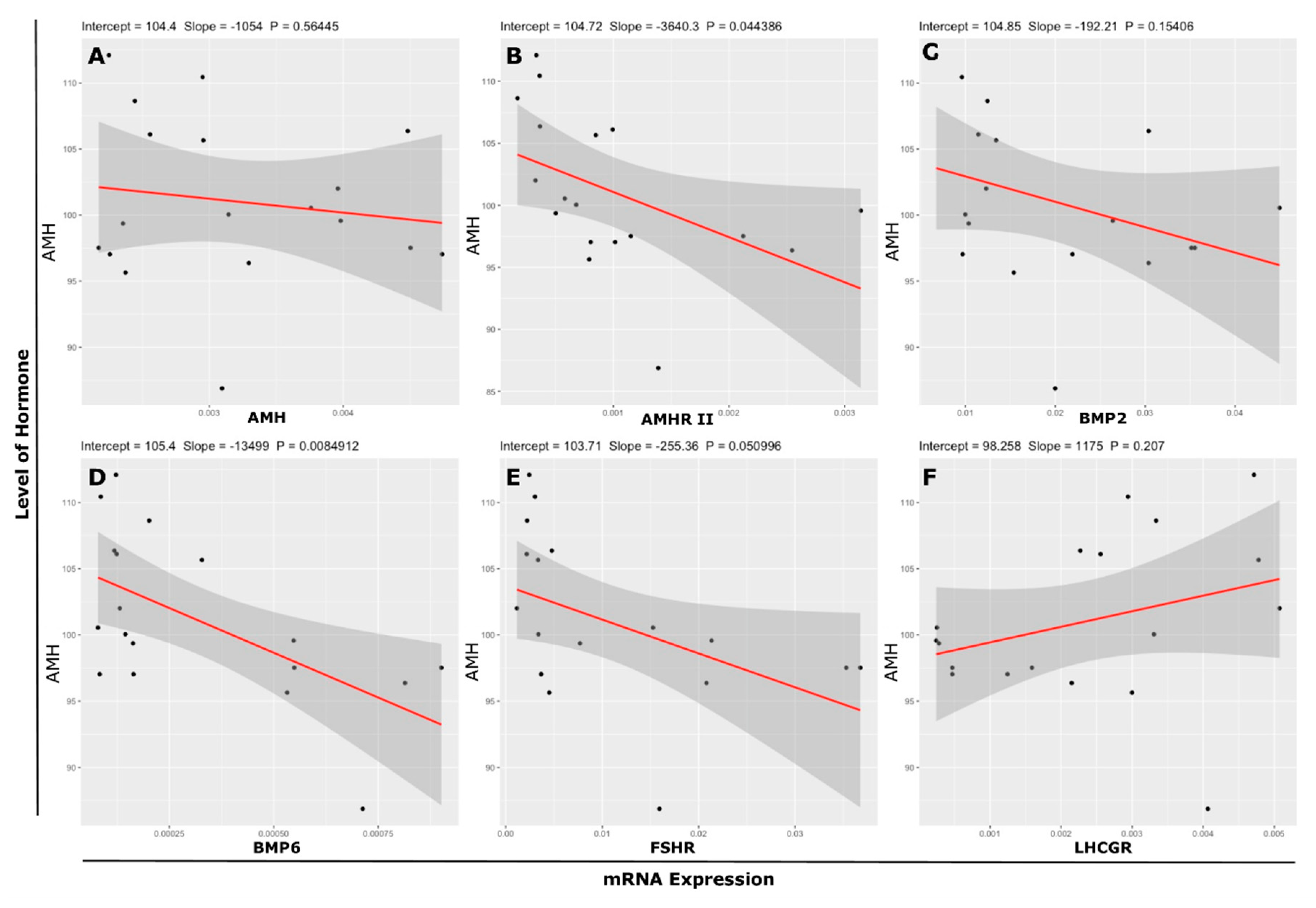
| mRNA Expression › | AMH | AMHR-II | FSHR | LHCGR | BMP2 | BMP6 | |
|---|---|---|---|---|---|---|---|
| AMH (ng/2 × 106 cells) | r value | −0.15 | −0.48 * | −0.47 | 0.31 | −0.35 | −0.60 ** |
| p value | 0.5645 | 0.0444 | 0.051 | 0.207 | 0.1541 | 0.0085 | |
| r2 value | 0.0212 | 0.2293 | 0.2176 | 0.0975 | 0.1227 | 0.3599 | |
| AdjR2value | −0.0399 | 0.1811 | 0.1687 | 0.0411 | 0.0679 | 0.3198 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umer, S.; Sammad, A.; Zou, H.; Khan, A.; Weldegebriall Sahlu, B.; Hao, H.; Zhao, X.; Wang, Y.; Zhao, S.; Zhu, H. Regulation of AMH, AMHR-II, and BMPs (2,6) Genes of Bovine Granulosa Cells Treated with Exogenous FSH and Their Association with Protein Hormones. Genes 2019, 10, 1038. https://doi.org/10.3390/genes10121038
Umer S, Sammad A, Zou H, Khan A, Weldegebriall Sahlu B, Hao H, Zhao X, Wang Y, Zhao S, Zhu H. Regulation of AMH, AMHR-II, and BMPs (2,6) Genes of Bovine Granulosa Cells Treated with Exogenous FSH and Their Association with Protein Hormones. Genes. 2019; 10(12):1038. https://doi.org/10.3390/genes10121038
Chicago/Turabian StyleUmer, Saqib, Abdul Sammad, Huiying Zou, Adnan Khan, Bahlibi Weldegebriall Sahlu, Haisheng Hao, Xueming Zhao, Yachun Wang, Shanjiang Zhao, and Huabin Zhu. 2019. "Regulation of AMH, AMHR-II, and BMPs (2,6) Genes of Bovine Granulosa Cells Treated with Exogenous FSH and Their Association with Protein Hormones" Genes 10, no. 12: 1038. https://doi.org/10.3390/genes10121038
APA StyleUmer, S., Sammad, A., Zou, H., Khan, A., Weldegebriall Sahlu, B., Hao, H., Zhao, X., Wang, Y., Zhao, S., & Zhu, H. (2019). Regulation of AMH, AMHR-II, and BMPs (2,6) Genes of Bovine Granulosa Cells Treated with Exogenous FSH and Their Association with Protein Hormones. Genes, 10(12), 1038. https://doi.org/10.3390/genes10121038










