Cytological Evaluations of Advanced Generations of Interspecific Hybrids between Allium cepa and Allium fistulosum Showing Resistance to Stemphylium vesicarium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chromosome Preparations
2.3. Analysis of Fertility
2.4. Genomic DNA Isolation and Probes Preparation
2.5. GISH
2.6. Microscopy, Image Analysis, and Karyotyping
2.7. Cytoplasmic Evaluations
2.8. Stemphyllium Screening
3. Results
3.1. Stemphylium Leaf Blight Evaluations
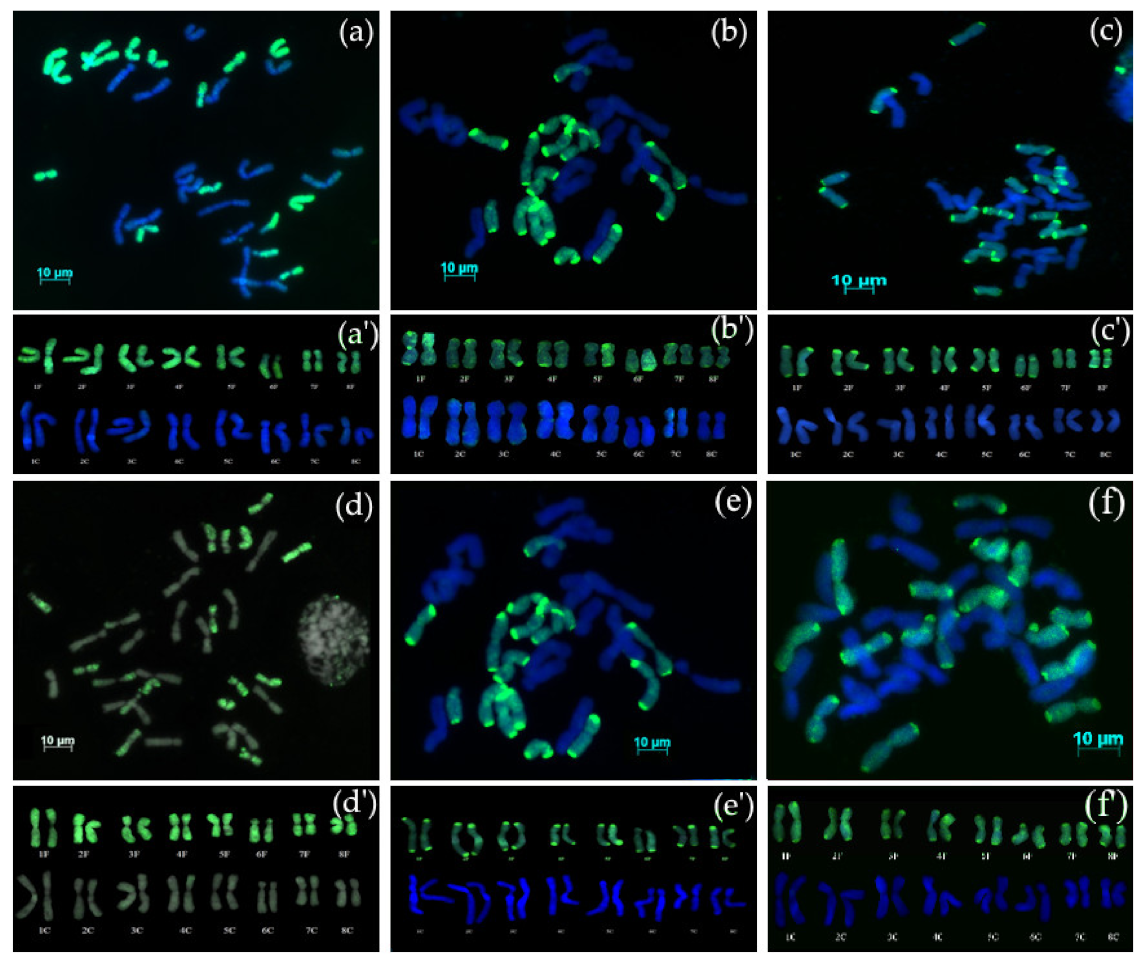
3.2. GISH Analysis
3.2.1. Chromosome Complement of Amphidiploid S5 Generation
3.2.2. Chromosome Complement of Amphidiploid Backcross Progenies
3.2.3. Backcross Progenies with Missing Chromosomes
3.2.4. Backcross Progenies with Recombinant Chromosomes
3.3. Pollen Fertility
3.4. Cytoplasms of Plants Derived from Interspecific Crosses between A. cepa and A. fistulosum
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singh, D.; Dhiman, J.S.; Sidhu, A.S.; Hari Singh, D.; Singh, H. Current status of onions in India: Strategies for disease resistance breeding for sustained production. Onion Newslett. Trop. 1992, 4, 43–44. [Google Scholar]
- Polat, Z.; Besirli, G.; Sönmez, I.; Yavuz, B. First report of Stemphylium leaf blight of garlic (Allium sativum) caused by Stemphylium vesicarium in Turkey. New Dis. Rep. 2012, 25, 29. [Google Scholar] [CrossRef]
- Johnson, D.A.; Lunden, J.D. First report of purple spot (Stemphylium vesicarium) of asparagus in Washington. Plant Dis. 1984, 68, 1099. [Google Scholar] [CrossRef]
- Arzanlou, M.; Khodaei, S.; Babai-Ahari, A. Helianthus annuus as a natural host for Stemphylium vesicarium in Iran. Australas. Plant Dis. Notes 2012, 7, 167–170. [Google Scholar] [CrossRef]
- Llorente, I.; Montesinos, E. Effect of relative humidity and interrupted wetness periods on brown spot severity of pear caused by Stemphylium vesicarium. Phytopathology 2002, 92, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Belisario, A.; Vitale, S.; Luongo, L.; Nardi, S.; Talevi, S.; Corvi, F. First report of Stemphylium vesicarium as causal agent of wilting and root rotting of radish sprouts in Italy. Plant Dis. 2008, 92, 651. [Google Scholar] [CrossRef] [PubMed]
- Behare, J.; Laterrot, H.; Sarfatti, M.; Zamir, D. Restriction fragment length polymorphism mapping of the Stemphylium resistance gene in tomato. Mol. Plant Microbe Interact. 1991, 4, 489–492. [Google Scholar] [CrossRef]
- Pathak, C.S.; Black, L.L.; Cheng, S.J.; Wang, T.C.; Ko, S.S. Breeding onions for Stemphylium leaf blight resistance. Acta Hortic. 2001, 555, 77–81. [Google Scholar] [CrossRef]
- Emsweller, S.L.; Jones, H.A. An interspecifc hybrid in Allium. Hilgardia 1935, 9, 265–273. [Google Scholar] [CrossRef]
- Hou, A.; Peffley, E.B. Recombinant chromosomes of advanced backcross plants between Allium cepa L. and A. fistulosum L. revealed by in situ hybridization. Theor. Appl. Genet. 2000, 100, 1190–1196. [Google Scholar] [CrossRef]
- Schwarzacher, T.; Leitch, A.R.; Bennett, M.D.; Heslop-Harrison, J.S. In situ localization of parental genomes in a wide hybrid. Ann. Bot. 1989, 64, 315–324. [Google Scholar] [CrossRef]
- Dong, F.; Novy, R.G.; Helgeson, J.P.; Jiang, J. Cytological characterization of potato-Solanum etuberosum somatic hybrids and their backcross progenies by genomic in situ hybridization. Genome 1999, 42, 987–992. [Google Scholar] [CrossRef]
- Khrustaleva, L.I.; Kik, C. Introgression of Allium fistulosum into A. cepa mediated by A. roylei. Theor. Appl. Genet. 2000, 100, 17–26. [Google Scholar] [CrossRef]
- Gonzalez, L.G.; Ford-Lloyd, B.V. Facilitation of Wide-Crossing Through Embryo Rescue and Pollen Storage in Interspecific Hybridization of Cultivated Allium species. Plant Breed. 1987, 98, 318–322. [Google Scholar] [CrossRef]
- Khrustaleva, L.I.; Kik, C. Cytogenetical studies in the bridge cross Allium cepa × (A. fistulosum × A. roylei). Theor. Appl. Genet. 1998, 96, 8–14. [Google Scholar] [CrossRef]
- Jakše, M.; Hirschegger, P.; Bohanec, B.; Havey, M.J. Evaluation of Gynogenic Responsiveness and Pollen Viability of Selfed Doubled Haploid Onion Lines and Chromosome Doubling via Somatic Regeneration. J. Am. Soc. Hort. Sci. 2010, 135, 67–73. [Google Scholar] [CrossRef]
- Rogers, S.O.; Bendich, A.J. Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol. Biol. 1985, 5, 69–76. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.N. Onion chromosome nomenclature and homoeology relationships—Workshop report. Euphytica 1990, 49, 1–3. [Google Scholar] [CrossRef]
- De Vries, J.N.; Jongerius, M.C. Interstitial C-bands on the chromosomes of Allium-species from section Cepa. In Proceedings of the 4th Eucarpia Allium Symposium, Wellesbourne, UK, 6–9 September 1988; pp. 71–78. [Google Scholar]
- Kohn, V.; Kiełkowska, C.A.; Havey, J.M. Sequencing and annotation of the chloroplast DNAs of normal (N) male-fertile and male-sterile (S) cytoplasms of onion and single nucleotide polymorphisms distinguishing these cytoplasms. Genome 2013, 56, 737–742. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2015; Available online: http://www.rstudio.com/ (accessed on 28 March 2011).
- Ulloa, G.M.; Corgan, J.N.; Dunford, M. Evidence for nuclear-cytoplasmic incompatibility between Allium fistulosum and A. cepa. Theor. Appl. Genet. 1995, 90, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Irifune, K.; Hirai, K.; Zheng, J.; Tanaka, R.; Morikawa, H. Nucleotide sequences of a highly repeated DNA sequences and its chromosomal localization in Allium fistulosum. Theor. Appl. Genet. 1995, 90, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Pich, U.; Fritsch, R.; Shubert, I. Closely related Allium species (Alliaceae) share a very similar satellite sequence. Plant Syst. Evol. 1996, 202, 255–264. [Google Scholar] [CrossRef]
- Vitte, C.; Estep, M.C.; Leebens-Mack, J.; Bennetzen, J.L. Young, intact and nested retrotransposons are abundant in the onion and asparagus genomes. Ann. Bot. 2013, 112, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Levan, A. The cytology of the species hybrid Allium cepa × fistulosum and its polyploidy derivates. Hereditas 1941, 27, 253–272. [Google Scholar] [CrossRef]
- Budylin, M.V.; Kan, L.Yu; Romanov, V.S.; Khrustaleva, L.I. GISH Study of Advanced Generation of the Interspecific Hybrids between Allium cepa L. and Allium fistulosum L. with Relative Resistance to Downy Mildew. Russ. J. Genet. 2014, 50, 387–394. [Google Scholar] [CrossRef]
- Crusz, A.L.; Sigel, E.M.; Witherup, C. Homoeologous chromosome pairing across the eukaryote phylogeny. Mol. Phylogenet. Evol. 2017, 117, 83–94. [Google Scholar] [CrossRef]
- Stevenson, M.; Armstrong, S.J.; Ford Lloyd, B.V.; Jones, G.H. Comparative analysis of crossover exchanges and chiasmata in Allium cepa × fistulosum after genomic in situ hybridization (GISH). Chromosome Res. 1998, 6, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, R.T.; Pires, J.C. Homoeologous recombination in allopolyploids: The polyploidy ratchet. New Phytol. 2010, 186, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Hizume, M. Allodiploid nature of Allium wakegi Araki revealed by genomic in situ hybridization and localization of 5S and 18S rDNAs. Jpn. J. Genet. 1994, 69, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Shibata, F.; Hizume, M. Evolution of 5S rDNA units and their chromosomal localization in Allium cepa and Allium schoenoprasum revealed by microdissection and FISH. Theor. Appl. Genet. 2002, 105, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Son, J.H.; Park, K.C.; Lee, S.I.; Jeon, E.J.; Kim, H.H.; Kim, N.S. Sequence variation and comparison of the 5S rRNA sequences in Allium species and their chromosomal distribution in four Allium species. J. Plant Biol. 2012, 55, 15–25. [Google Scholar] [CrossRef]
- Van der Valk, P.; Kik, C.; Verstappen, F.; Everink, J.T.; de Vries, J.N. Independent segregation of two isozyme markers and inter-plant differences in nuclear DNA content in the interspecific backcross (Allium fistulosum L. × A. cepa L.) × A. cepa L. Euphytica 1991, 55, 151–156. [Google Scholar] [CrossRef]
- Poulsen, N. Chives (Allium schoenoprasum). In Onions and Allied Crops; Brewster, J., Rabinowitch, H., Eds.; CRC Press: Boca Raton, FL, USA, 1990; Volume 3, pp. 231–250. [Google Scholar]
- Kojima, A.; Nagato, Y. Pseudogamous embryo genesis and the degree of parthenogenesis in Allium tuberosum. Sex. Plant Reprod. 1992, 5, 79–85. [Google Scholar] [CrossRef]
- Kravets, E.A. Cytomixis, its nature, value and the cytological consequences. Tsitol. Genet. 2012, 3, 75–85. [Google Scholar]
- Cheng, K.; Nien, H.; Yang, C.; Wang, I.; Chou, I.; Chen, J. Light and electron microscopical observations on cytomixis and the study of its relation to variation and evolution. Acta Bot. Sin. 1975, 17, 60–69. [Google Scholar]
- Yang, J.; Yu, C.; Wang, X.; Zheng, G. Ultrastructural observation on the intra-and intercellular microtrabecular network of the pollen mother cells in onion (Allium cepa). Acta Bot. Sin. 2001, 43, 331–338. [Google Scholar]



| Accession | Pedigree | Generation |
|---|---|---|
| AVON 1275 | TA207 × AF468 | S5 |
| AVON 1278 | AVON 1275 × AC464 | S5BC1 |
| AVON 1284 | AVON 1275 × AC464 | S3BC1 |
| AVON 1282 | AVON 1275 × AC464 | S4BC2 |
| AVON 1283 | AVON 1275 × AC464 | S4BC2 |
| AVON 1290 | AVON 1275 × AC464 | S4BC2 |
| AVON 1291 | AVON 1275 × AC464 | S4BC2 |
| AVON 1501 | AVON 1275 × AC464 | S4BC2 |
| AVON 1502 | AVON 1275 × AC464 | S4BC2 |
| AVON 1503 | AVON 1275 × AC464 | S4BC2 |
| AVON 1504 | AVON 1275 × AC464 | S4BC2 |
| AF468 | Allium fistulosum ‘Tuwel’ | |
| AC464 | Allium cepa ‘Arka Niketan’ | |
| TA207 | Allium cepa ‘Rouge de Tana’ |
| Accession 1 | Cultivar | Mean DSR ± SD 2 |
|---|---|---|
| A. fistulosum | Green Banner | 1.3 ± 0.5 a |
| AVON 1282 | 1.3 ± 0.3 a | |
| AVON 1278 | 1.6 ± 0.5 ab | |
| AVON 1283 | 1.6 ± 0.6 ab | |
| AVON 1291 | 1.8 ± 0.5 ab | |
| AVON 1284 | 2.0 ± 0.4 ab | |
| AVON 1290 | 2.0 ± 0.7 ab | |
| AVON 1275 | 2.3 ± 0.9 abc | |
| A. cepa | 1620 | 2.8 ± 0.6 abc |
| A. cepa | 1606 | 3.1 ± 0.6 bcd |
| A. cepa | Granex 33 | 3.8 ± 0.9 cd |
| A. cepa | 1707 | 4.6 ± 0.5 d |
| Accession | Pollen Fertility, % |
|---|---|
| AVON 1275 (S5) | 94.1 |
| AVON 1290 (S4BC2) | 85.3 |
| AVON 1291 (S4BC2) | 92.9 |
| AVON 1501 (S4BC2) | 63.1 |
| AVON 1502 (S4BC2) | 94.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudryavtseva, N.; Havey, M.J.; Black, L.; Hanson, P.; Sokolov, P.; Odintsov, S.; Divashuk, M.; Khrustaleva, L. Cytological Evaluations of Advanced Generations of Interspecific Hybrids between Allium cepa and Allium fistulosum Showing Resistance to Stemphylium vesicarium. Genes 2019, 10, 195. https://doi.org/10.3390/genes10030195
Kudryavtseva N, Havey MJ, Black L, Hanson P, Sokolov P, Odintsov S, Divashuk M, Khrustaleva L. Cytological Evaluations of Advanced Generations of Interspecific Hybrids between Allium cepa and Allium fistulosum Showing Resistance to Stemphylium vesicarium. Genes. 2019; 10(3):195. https://doi.org/10.3390/genes10030195
Chicago/Turabian StyleKudryavtseva, Natalia, Michael J. Havey, Lowell Black, Peter Hanson, Pavel Sokolov, Sergey Odintsov, Mikhail Divashuk, and Ludmila Khrustaleva. 2019. "Cytological Evaluations of Advanced Generations of Interspecific Hybrids between Allium cepa and Allium fistulosum Showing Resistance to Stemphylium vesicarium" Genes 10, no. 3: 195. https://doi.org/10.3390/genes10030195
APA StyleKudryavtseva, N., Havey, M. J., Black, L., Hanson, P., Sokolov, P., Odintsov, S., Divashuk, M., & Khrustaleva, L. (2019). Cytological Evaluations of Advanced Generations of Interspecific Hybrids between Allium cepa and Allium fistulosum Showing Resistance to Stemphylium vesicarium. Genes, 10(3), 195. https://doi.org/10.3390/genes10030195





