Abstract
This study aimed to establish a pure single-cell Theileria annulata-infected B cell line for the assessment of cytokine production in transformed and lipopolysaccharide (LPS)-stimulated cells. Several studies have aimed to identify cell surface markers in T. annulata-transformed cells; however, no information on cytokine production in these cells is available. To investigate the potential of the transformed cells to produce cytokines and their potential responses to antigen-stimulation, we purified mature B cells (CD21) from the whole blood of cattle experimentally infected with the T. annulata Kashi strain by magnetic separation. The purity and specificity of the established cell line was assessed by the identification of specific cell surface markers (CD21, IgM, and WC4) by flow cytometry analysis. The transcript levels of the cytokines IL1A, IL1B, IL2, IL4, IL6, IL8, IL10, IL16, LTA, TGFB1, TNFA, IFNA, and IFNB in transformed, buparvaquone (BW720c)-treated cells, and antigen-stimulated cells were analyzed by quantitative polymerase chain reaction (qPCR) using cDNA from these cells. A T. annulata-infected bovine B cell line was successfully established with a purity of ~98.8% (CD21). IL4 and IL12A were significantly (p < 0.01) upregulated in the transformed cells. In BW720c-treated transformed cells, IL12B, TGFB1, and IFNB were significantly (p < 0.01) upregulated. Notably, no significant (p > 0.05) upregulation of cytokines was observed in LPS-stimulated transformed cells. Moreover, IL1A, IL1B, IL8, and IL16 were significantly (p < 0.01) upregulated in LPS-stimulated B cells. Our data signify the potential use of this cell line for cytokine production, observance of immunoglobulins, and production of an attenuated vaccine against tropical theileriosis.
1. Introduction
Theileria annulata, an obligate intracellular protozoan parasite that belongs to the phylum Apicomplexa, is economically important for domestic and wild bovids [1,2]. Infection by intracellular pathogens, such as T. annulata, causes bovine tropical theileriosis, which is especially fatal in calves. In chronic cases, this infection can assume a subclinical form, leading to productive losses, as previously described [2]. The infection rate depends on the dose and virulence of the parasite [3]. Studies have demonstrated that follicular dendritic cells (FDCs) capture, retain, and transfer unprocessed Ag to naïve B cells for the production of an immune (antibodies) response, and signaling types of dendritic cells (DCs) can secrete immunoglobulin IgG isotypes [4,5,6]. T. annulata macroschizonts transform macrophages, B cells, and DCs [7,8]. Theileria parva was previously shown to transform T lymphocytes, but B lymphocytes were observed at a low frequency due to the pathogenicity of these parasites [8]. The host immune response to T. annulata is complex due to different life stages of the parasite in the host and antigenic heterogeneity. The parasite displays diverse surface antigens that necessitate a specific immune response at each life stage rather than an immune response being generated for only one stage [9]. For this reason, T. annulata sporozoites escape the immune response and invade and transform leukocytes while macroschizonts are able to induce host cell transformation without integrating parasite DNA into the host genome [10]. The transformation rate of B cells was previously demonstrated to be 1:6897, indicating that only one out of 6897 cells is transformable [11]. These transformed cells endlessly proliferate without the supplementation of cytokines or growth promoters [12], and this process that can be maintained indefinitely by producing the next subsequent generation of cells in fresh, complete culture medium [13]. The association of the host cell mitotic apparatus with the parasite macroschizonts enables their simultaneous division, thus ensuring the transfer of parasites to daughter cells. The exposure of infected cells to buparvaquone (BW720c) leads to parasite macroschizonts death followed by the termination of proliferation and provokes the apoptosis of host cells within a few days [14]. Immunoglobulin M (IgM), cluster differentiation 21 (CD21), and CD19-like (WC4) are typical surface markers on B cells [15,16]. However, surface marker expression was shown to be downregulated in T. annulata-transformed cells in several previous studies [8,10].
B cells are engaged during innate, adaptive, and humoral immune responses against exposed antigens. The host immune response consists of two main types of immune cells, T and B lymphocytes that work in concert for the production of an adaptive immune response to eliminate or destroy invading pathogens. While naïve B lymphocytes are unable to illicit an immune response, naïve T cells produce multiple cytokines (interleukins: IL7, IL4, IL6, and IL10 and interferons: IFNA, IFNB, and IFNG) upon activation. These cytokines induce the activation and differentiation of B cells, resulting in the production of their own cytokines and immunoglobulin isotypes to regulate the immune response, which plays an important role in the survival, development, and proliferation of these cells [17]. The B cell responses to both T cell-independent (TI) and T cell-dependent (TD) antigens play an integral role in the generation of protective immune responses to various pathogens.
For immunological studies, lipopolysaccharide (LPS) is widely used as an antigen stimulator of cells allow observance of their immune response in the context of cytokine and immunoglobulin production [18]. Cytokines are soluble proteins with low molecular weights (5–70 kDa). Each type of immune cell produces and releases the same or a specific type of cytokine against the antigen [18,19]. These cytokines aid in the transmittance of activation messages, chemoattraction, inhibition, and apoptosis of infected cells. Cytokines are multifunctional molecules that mediate a wide range of physiological responses and play a fundamental role in immune responses, especially in normal T cell-mediated immunity, autoimmunity, cancer, inflammatory responses, and allergies [19]. Under diverse physiologically relevant conditions, normal B cells (nBCs) exhibit distinct effector cytokine expression patterns [20]. Previous studies have reported that viral and CD40-mediated B cell stimulation results in the secretion of anti-inflammatory IL10, proinflammatory IL1B and IL4, anti- and proinflammatory IL6, tumor necrosis factors (TNFs), lymphotoxin alpha (LTA), and transforming growth factor beta1 (TGFB1) [20,21], while T. annulata-transformed mononuclear cells (mostly focused on macrophages) secrete IL1A, IL1B, IL2, IL6, IL10, IL12, TNFA, and IFNA [3,22]. Moreover, IL1, TNFA, IFNA, and IFNB are reportedly secreted from B cells that act on T lymphocytes and macrophages [23]. Similarly, IL8 and IL16 are secreted from lymphocytes [24]. Cytokine production in B cells infected with viruses [21] or stimulated with antigens [19,20] and in macrophages infected with T. annulata [3] in vitro have been investigated; however, no studies on T. annulata-transformed B cells (TaBCs) have been reported, and the current project was thus designed on this basis. Collectively, the IL1A, IL1B, IL2, IL4, IL6, IL8, IL10, IL12A, IL12B, IL16, TNFA, IFNA, IFNB, LTA, and TGFB1 genes were considered for this study.
The present study provides an important framework for a comprehensive overview of cytokine production in transformed cells. Additionally, this study provides insight into the identification of B cell antigen recognition receptors and the in vitro application of T lymphocyte recombinant cytokines to transformed cells to further observe their immune response.
The current study aimed to establish a T. annulata sporozoite-infected bovine B cell line. Surface markers for cell purity and specificity were analyzed by flow cytometry. Additionally, we tested the hypothesis that transformed and antigen-stimulated cells upregulate B cell-specific cytokine production. The expression levels of the IL1A, IL1B, IL2, IL4, IL6, IL8, IL10, IL12A, IL12B, IL16, TNFA, IFNA, IFNB, LTA, and TGFB1 cytokines were analyzed in this study using an in house developed and validated quantitative polymerase chain reaction (qPCR) assay. This tool was used to study the expression levels of the selected cytokines in established and BW720c-treated B cell lines as well as in LPS-stimulated normal and transformed cells.
2. Materials and Methods
2.1. Reagents and Antibodies
The mouse anti-bovine CD21 (MCA1424PE), mouse anti-bovine IgM (AAI19F), mouse anti-bovine WC4 (MCA1648G), and negative control mouse IgG1 (MCA928F) antibodies were purchased from Bio-Rad (Hercules, CA, USA). The PrimeScript™ RT reagent kit with gDNA Eraser (cat. no. RR047A) and SYBR® Premix Ex Tq™ II (Tli RNaseH Plus) (cat. no. RR820A) were obtained from Takara, Co., Ltd. (Dalian, China). TRIzol (cat. no. 15596-026; Invitrogen, Carlsbad, CA, USA) and the pGEM T Easy vector (cat. no. A137A) were purchased from Promega (Madison, WI, USA). The anti-FITC microbeads (130-048-701), anti-PE microbeads (130-048-801), and LS column (cat. no. 130-042-401) were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). Culture medium (RPMI 1640, lot: 1930005) and fetal bovine serum (FBS, lot: 1828728) were purchased from Gibco, Life Technologies (Carlsbad, CA, USA) and LPS (cat. no. L2630) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Experimental Animals
T. annulata-free cattle (Chinese yellow cattle) <1 year of age were maintained at the animal experimental unit of the Chinese Academy of Agricultural Sciences (CAAS) at Lanzhou Veterinary Research Institute (LVRI), Lanzhou, Gansu, P.R. China in accordance with the instructions and guidelines of the animal ethics committee (permit no. LVRIAEC-2018-001), which were approved by the People’s Republic of China. For seven days, the cattle were exposed to nearly 100 adult Hyalomma anatolicum ticks carrying pure T. annulata Kashi strain pathogens that were maintained in the abovementioned laboratory. Infected cattle were kept under intensive care, and their health parameters (body temperature, lymph node swelling, confinement of ticks to the backbone by fixing a cloth bag, and thin blood smear examination) were regularly recorded on a daily basis for 13–15 days; finally, total blood was collected for merozoite isolation (used for laboratory project). After ten days of infection, blood from acutely infected cattle was collected for mature B cell (CD21) isolation, leading to the development of a transformed cell line. Furthermore, blood from pathogen-free cattle was processed for cell isolation and used as a control (∆Ct as calibrator for nBCs-LPS and TaBC), and nBCs were stimulated with LPS to induce cytokine production and compared with TaBCs. Piroplasm-free and piroplasm-infected cattle were confirmed by thin blood smear microscopic examination and PCR amplification of the target 18S rRNA gene by previously used and optimized genus-specific primers (primers details are shown in the Results section) [25].
2.3. Magnetic Cell Separation
Magnetic cell sorting is an effective method for the isolation of highly pure and viable cells [26]. Flow cytometry is a powerful technique for studying fluorescence-labeled surfaces as well as intracellular molecules [27]. CD21+ bovine mature B cells [28] were isolated by magnetic separation according to a previously described method [29,30] with minor modifications to obtain a highly pure population (>95%). Briefly, whole blood (from infected and normal cattle separately) was collected into 9 mL tubes containing K3EDTA by venipuncture of the jugular vein. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-PaqueTM plus (cat. no. 17-1440-02; GE Healthcare, Uppsala, Sweden) at a density of 1.078 g mL−1. The PBMCs were labeled with mouse anti-bovine antibodies (CD21) and anti-microbeads for magnetic separation via an LS column [31]. The percentage of specific cells (CD21+) in the PBMCs and the purity of the isolated cells were analyzed by flow cytometry. The remaining purified cells (infected and infection-free) were seeded in 24-well plates at a concentration of 2 × 106 cells well−1 and cultured in complete medium comprising Gibco RPMI 1640 medium (L-glutamine and 25 mM HEPES), 50 µg mL−1 gentamicin, 50 µM 2-mercaptoethanol, 25 µg mL−1 amphotericin B, and 10% heat inactivated FBS at 37 °C and 5% CO2 for further usage. During the cell isolation process, all of the solutions used were filtered through a syringe-supported Millex®GP 0.22 µm filter (lot: R5SA76494; Merck Millipore Ltd., Cork, Ireland) to avoid the risk of contamination.
2.4. Cell Line Establishment and Maintenance
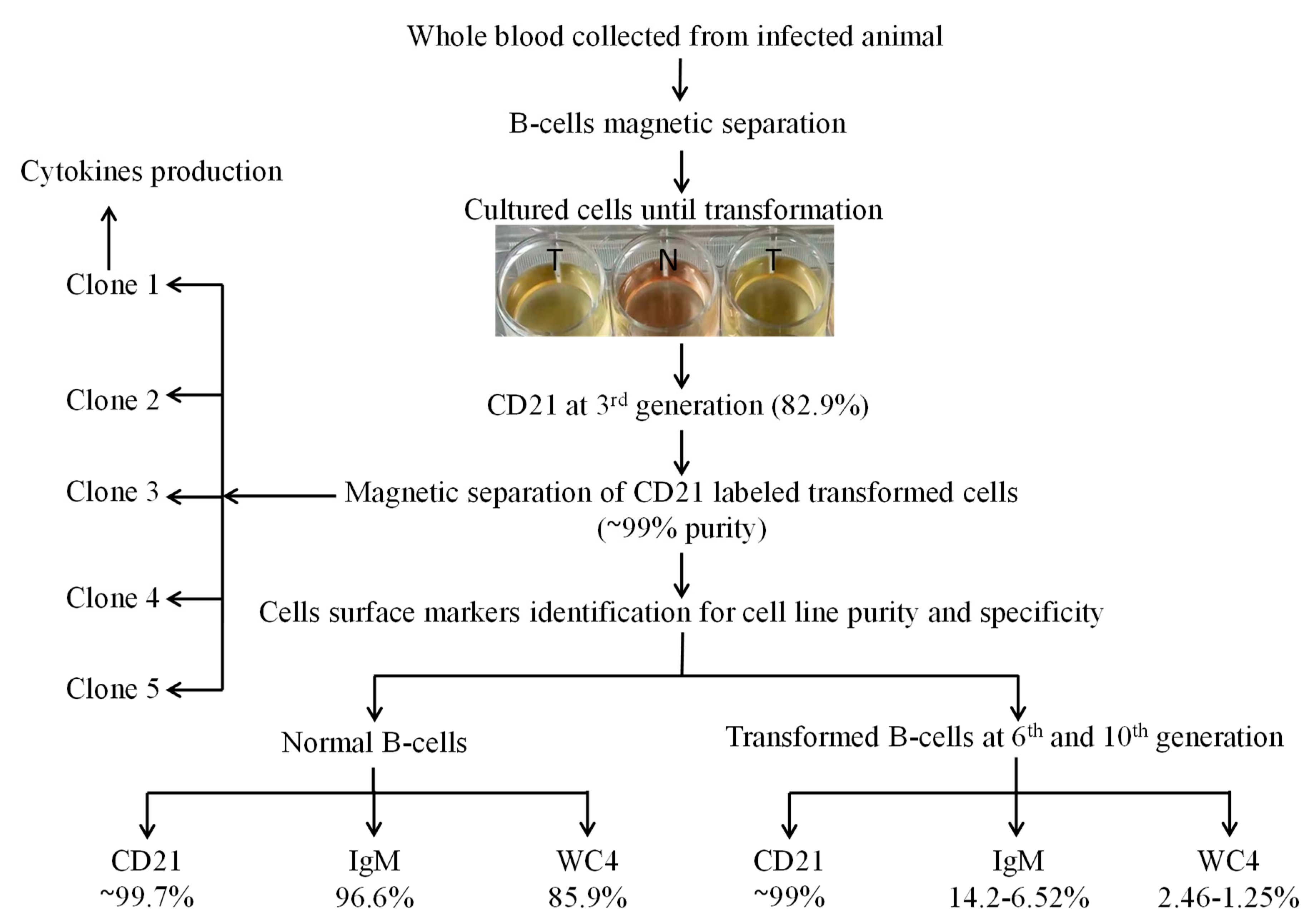
For cell line development, initially mature nBCs were isolated and co-cultured in vitro with sporozoites (grounded tick salivary glands) several times. Later, mature B cells were isolated from experimentally tropical theileriosis-infected cattle. These cells (2 × 106 cells well−1 in 24-well plates) were maintained in complete culture medium at 37 °C and 5% CO2, and the medium was replaced twice a week. Cells began to proliferate at 13 days of postisolation and proliferating cells were transferred into a 25 cm2 culture flask for bulk production on day 23 post-isolation. These transformed cells were labeled with CD21 antibodies at the 3rd passage to identify the surface marker. To obtain a pure cell line, transformed cells were again labeled with CD21 antibodies followed by magnetic separation at the 4th generation and allowed to grow after purity analysis. A serial dilution of purified transformed cells was performed in a 96-well plate to clone a cell for homogeneous cell line development [32]. The surface markers of five clones were analyzed, and appropriate clones were selected for development of a cell line originating from a single cell and cytokine production. To further investigate the cell specificity of the established cell line, transformed cells were labeled with CD21, IgM, and WC4 surface markers antibodies at the 6th, 10th (polyclone), and 15th (single-cell clones) generations (Figure 1), and then analyzed by flow cytometry.
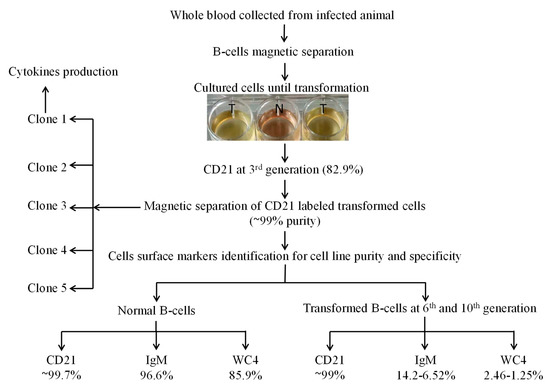
Figure 1.
Schematic diagram for establishment of the Theileria annulata-transformed bovine B cell line. Here, N represents normal B cell culture, and T represents the infected cell culture wells used for transformation and proliferation for establishment of cell line.
2.5. Cytotoxicity Assay
The MTT assay is the colorimetric assay for assessing the cell metabolic activity. This assay was used for optimization of LPS and BW720c concentrations to evaluate the immune response in antigen-stimulated and treated cells. For this assay, 10 µL (5 µg mL−1 PBS) of MTT solution was added to each well during the final 6 h of incubation. After this incubation period, the plates were centrifuged at 400 g for 8–10 min, and the supernatant was collected. DMSO (100 µL) was added to each well, and the plates were then shaken to ensure that all crystals were dissolved. The amount of MTT formazan produced during incubation was measured by an ELISA reader at a reference wavelength of 630 nm and a test wavelength of 550 nm (OD550) [19]. The simulation index (SI) was calculated as follows:
2.6. Antigenic Stimulation
The stimulation was performed to compare the efficacies of cytokine production in normal and transformed cells and the abilities of these cells to recognize antigens. The nBCs and TaBCs were plated in six-well culture plates at 5 × 106 cells well−1 and stimulated with 1 µg mL−1 LPS in 3 mL culture medium for 24 h at 37 °C and 5% CO2 to induce significant cytokine production, while control cells remained untreated [33]; the cells were then washed with PBS for mRNA extraction by the TRIzol method as described below in detail.
2.7. Theileriacidal Treatment
BW720c, a hydroxynaphthoquinone that likely acts as a ubiquinone analog [34], specifically blocks electron transport in parasites. The transformed cells were treated with the theileriacidal drug to evaluate the regulation of cytokine production in the absence of schizonts. TaBCs were incubated with 100 ng mL−1 BW720c for 48 h at 37 °C and 5% CO2 [33,35] and then washed prior to mRNA extraction according to the method described below. The mRNA was processed to quantify the quantification of the transcription levels of the various cytokines analyzed in this study.
2.8. Flow Cytometry Analysis
For cell purity and surface marker identification, antibody-labeled cells were washed three times with ice-cold PBS containing 0.2% Tween 20. The fluorescence intensities of surface markers (CD21+, IgM+, and WC4+) were analyzed on the BD Accuri C6 instrument (Becton Dickinson and Company 1, Becton Drive Franklin Lakes, NJ, USA), while untreated PBMCs and TaBCs were used as background fluorescence controls [36]. Labeled cells were gated by side and forward scatter characteristics. For each sample, 10,000 events were collected on the FACSCalibur instrument (Accuri C6) for flow cytometry analysis. Gating of the live target cells was performed by selecting the main cell populations in the forward and side scatter profiles. Normalized mean florescence was computed by subtracting of the geometric mean florescence of the cells that were not incubated with any florescence labels [37].
2.9. PCR and Sequencing Analysis
Bovine cytokines, consisting of IL1A, IL1B, IL2, IL4, IL6, IL8, IL10, IL12A, IL12B, IL16, TNFA, IFNA, IFNB, LTA, and TGFB1, reported by [17,20,21,23,24,38], were considered in this study. Reference sequences used for primer design were obtained from a public database (National Center for Biotechnology Information, NCBI). Primers used for the amplification of short cytokine gene segments were designed using the GenScript online browser [39] and NCBI [40], and melting temperatures (less than 2 °C difference) and self-dimers (less than 10 kcal/mole) were evaluated with an online browser [41]. The primers that met the criteria were selected and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The target genes were amplified, purified, and cloned into the pGEM TM-T Easy vector. Briefly, PCR products were separated on 4% agarose gels and the desired bands were purified using a gel/PCR extraction kit (Biomiga, Inc., San Diego, CA, USA). The purified PCR products were then ligated into the pGEMTM-T Easy vector and transformed into competent cells (DH5α) [42]. The bacterial culture samples were sent to Sangon Biotech Co., Ltd. for sequencing.
2.10. RNA Extraction and cDNA Synthesis
RNA was extracted from nBCs, TaBCs, BW720c-TaBCs and LPS-stimulated nBCs and TaBCs using TRIzol reagent. Briefly, each cell type was harvested in a separate 1.5 mL tube by centrifugation at 12,000 g for 2 min and then washed with 1 × PBS. For total RNA isolation, the TRIzol method was used as previously described [43]. The concentration and purity (260/280 and 26/230 nm ratios) of isolated RNA was determined on a NanoDrop spectrophotometer (Thermo Scientific 2000/2001, Wilmington, DE, USA). cDNA was synthesized from 1 µg of RNA using the PrimeScript™ RT reagent kit with gDNA Eraser according to the manufacturer’s instructions and stored at −20 °C until further experimental usage.
2.11. Quantitative PCR Analysis
qPCR is an extremely sensitive technique that allows the quantification of rare transcripts and the identification of slight changes in gene expression from a limited sample quantity [44]. qPCR was performed using SYBR® Premix Ex Tq™ II (Tli RNaseH Plus) according to the manufacturer’s instructions. Briefly, the 20 µL reaction mixture comprised 10 µL of SYBR® Premix Ex Taq II, 0.8 µL (16 µM) of each primer (forward and reverse), 0.4 µL of Rox reference dye II (50×), 2 µL of cDNA, and 6 µL of RNase-free water. The reactions were performed on the Mx3005P qPCR Systems™ (Agilent Mx3005P; Agilent Technologies, Santa Clara, CA, USA) in three repeating steps/segments as follow: denaturation at 95 °C for 30 s; 40 cycles of annealing at 95 °C for 5 s and 60 °C for 34 s, followed by extension at 95 °C for 15 s, held at 60 °C for 1 min and held at 95 °C for 0.15 s according to the SYBR® Premix Ex Tq™ II kit instruction. cDNA was serially diluted (1/10) prior to qPCR analysis to optimize the cytokine primers. The qPCR data were analyzed by regression (known cDNA quantities) based on the threshold cycle (Ct) for each cytokine to calculate R2 values [45]. All reactions were run in triplicate, and three samples of each gene were run in each qPCR assay to analyze the gene expression levels in transformed, treated and stimulated cells. The Ct values of β-actin were used for the calculation of ∆Ct, and the ∆Ct of BCs (normal and transformed) were used for the calculation of ∆∆Ct, termed the normalizer and calibrator, respectively. The fold change values were calculated by the 2−∆∆Ct formula as previously described [46].
2.12. Data Analysis
Sequence data were analyzed by EditSeq, SeqMan, and MegAlign (DNA Star, Madison, WI, USA). Flow cytometry florescence data were analyzed by FlowJo X version, 10.0.7 (BD Biosciences, Franklin Lakes, NJ, USA). Fold change values were calculated by the 2^−∆∆Ct method using Microsoft Excel 2010 (Redmond, Washington, USA). One-way ANOVA, multiple comparisons (fold change in nBCs-LPS for TaBCs and TaBCs in TaBCs-BW720c and TaBCs-LPS as the reference to analyze significant values), and graphical analyses were performed using GraphPad Prism software version 7 (La Jolla, CA, USA). Significant values obtained are presented as * p < 0.05, ** p < 0.01, and *** p < 0.001; ns represents non-significance (p > 0.05).
3. Results
3.1. Confirmation of Experimental Animals for Piroplasmosis
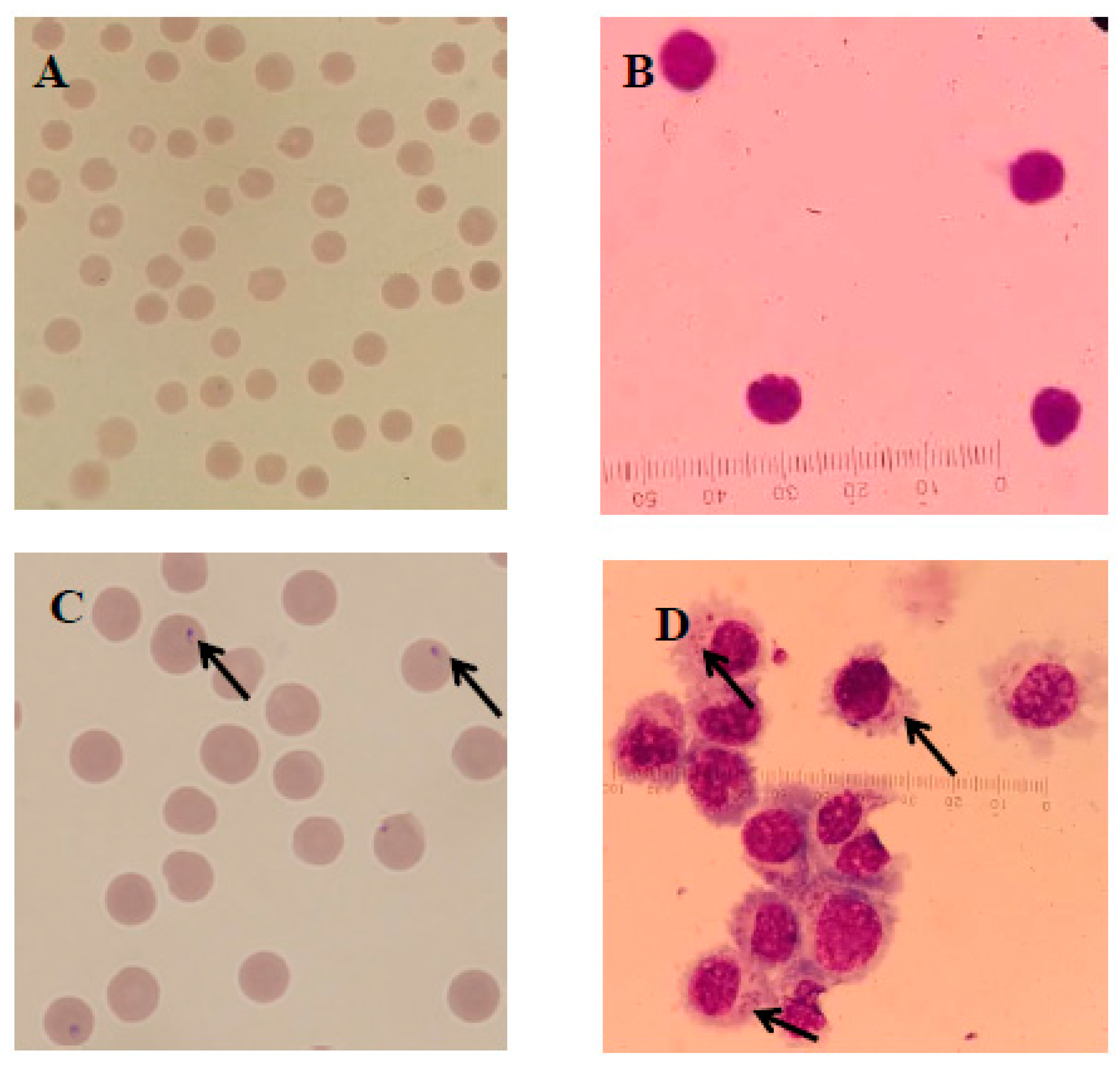
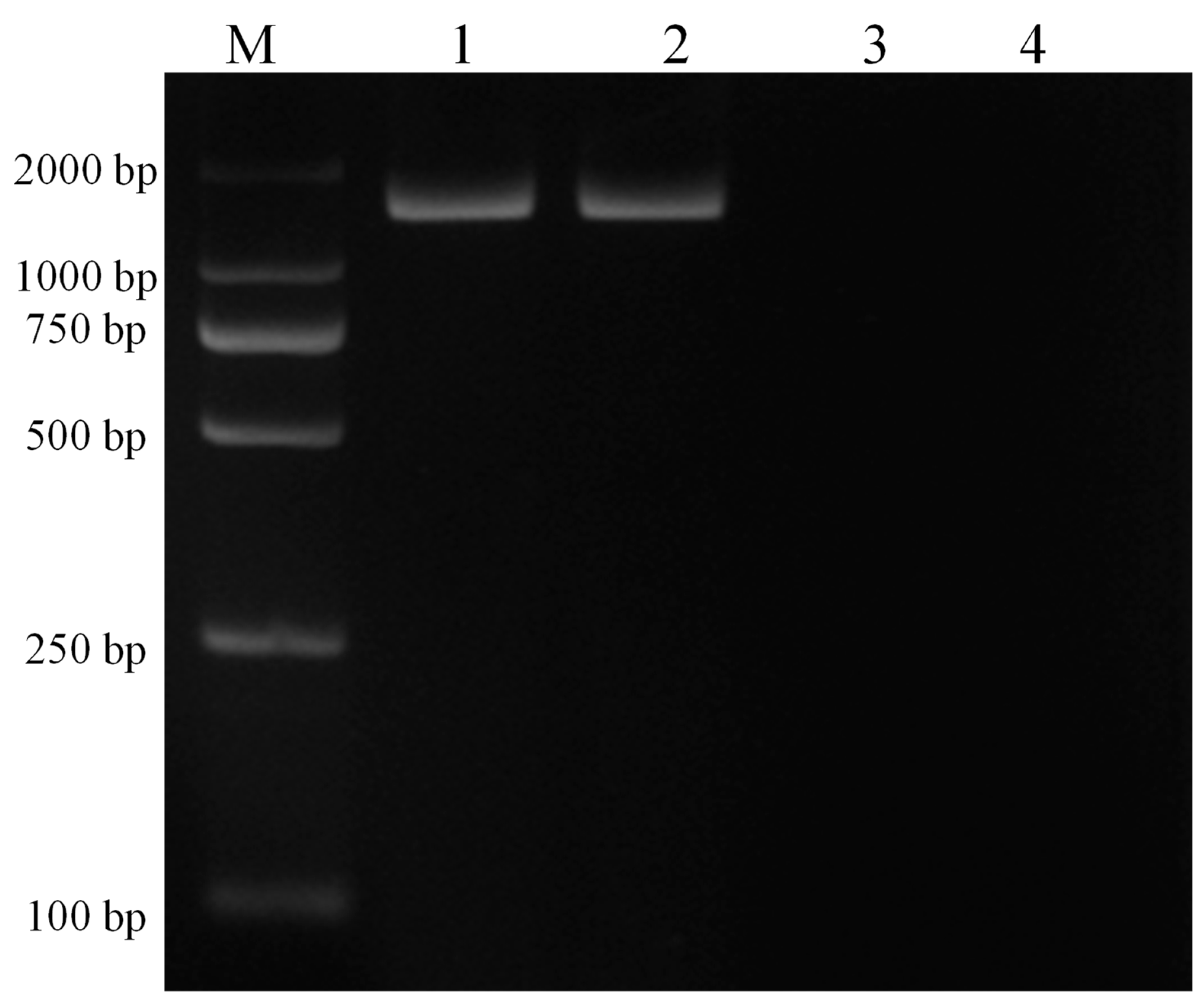
Infected and infection-free experimental animals as well as the established cell line were confirmed using thin blood smear microscopic examination (Figure 2) and a genus-specific pair of primers, (forward) 5′-AAGCCATGCATGTCTAAGTAGAAGCTTTT-3′ and (reverse) 5′-GAATAATTCACCGGATCACTCG-3′ which produced a 1586 bp product at an annealing temperature of 57 °C for 1.5 min and a final extension at 72 °C for 10 min (Figure 3). The sequence obtained was submitted to the NCBI database under accession number: MK415058. Cells isolated from infection-free cattle were used as controls, while cells isolated from infected cattle were processed for cell line development.
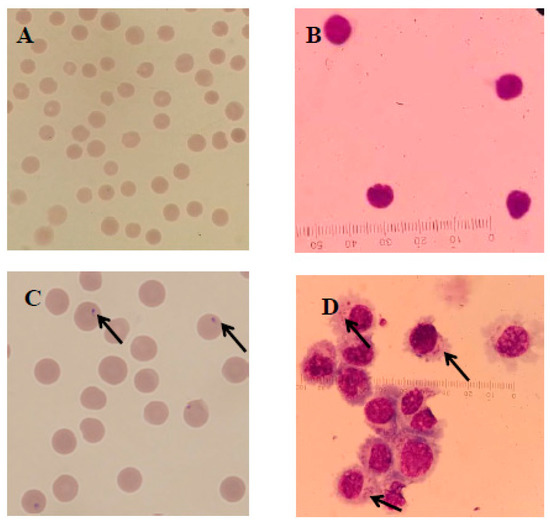
Figure 2.
Microscopic examination of T. annulata merozoites in red blood cells (RBCs) and macroschizonts in transformed B cells as well as normal cells stained with Giemsa observed under a compound microscope at 1000×. Here, (A,B) represent the normal RBCs and lymphocytes, respectively, while the arrows in (C,D) show the presence of merozoites in the RBCs of infected cattle and macroschizonts in the established cell line, respectively.
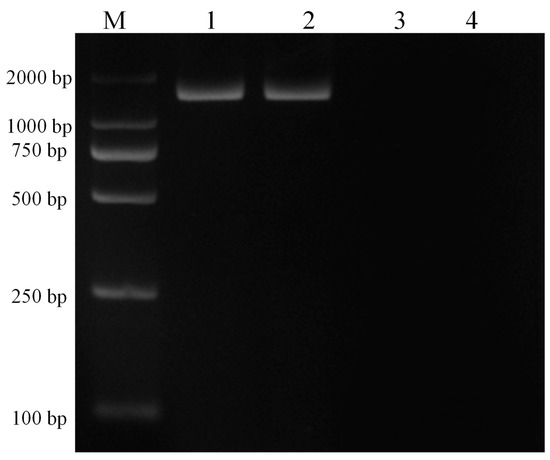
Figure 3.
PCR amplification of 18S RNA with genus-specific primers for confirmation of piroplasm infection in experimental animals and the established cell line. Here, M represents the DNA marker (DL2000), lane 1 depicts the experimentally infected cattle with sporozoites, lane 2 depicts the transformed cells, lane 3 depicts the piroplasm free cattle, and lane 4 depicts the negative control.
3.2. Established B-Cell Line
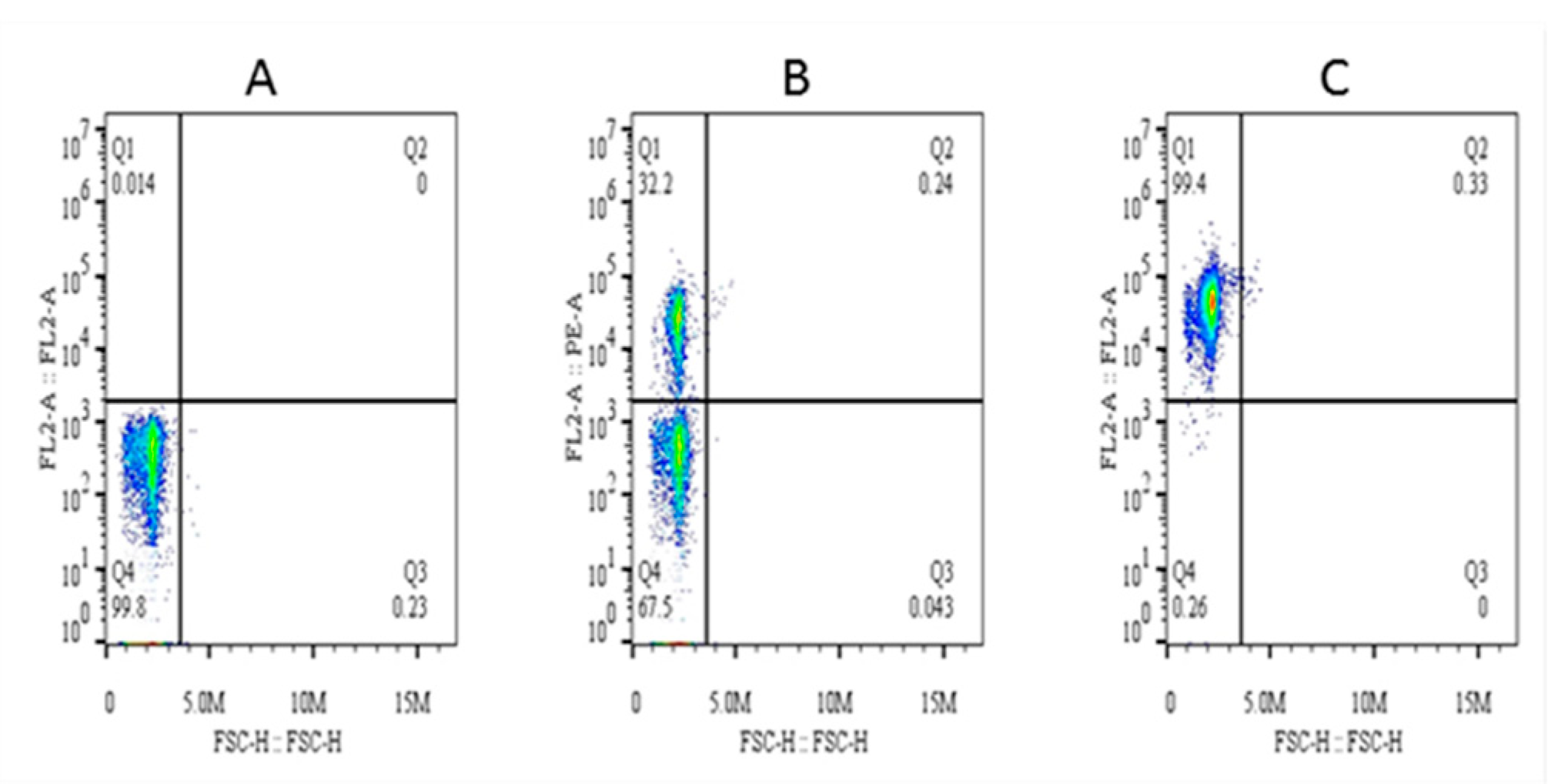
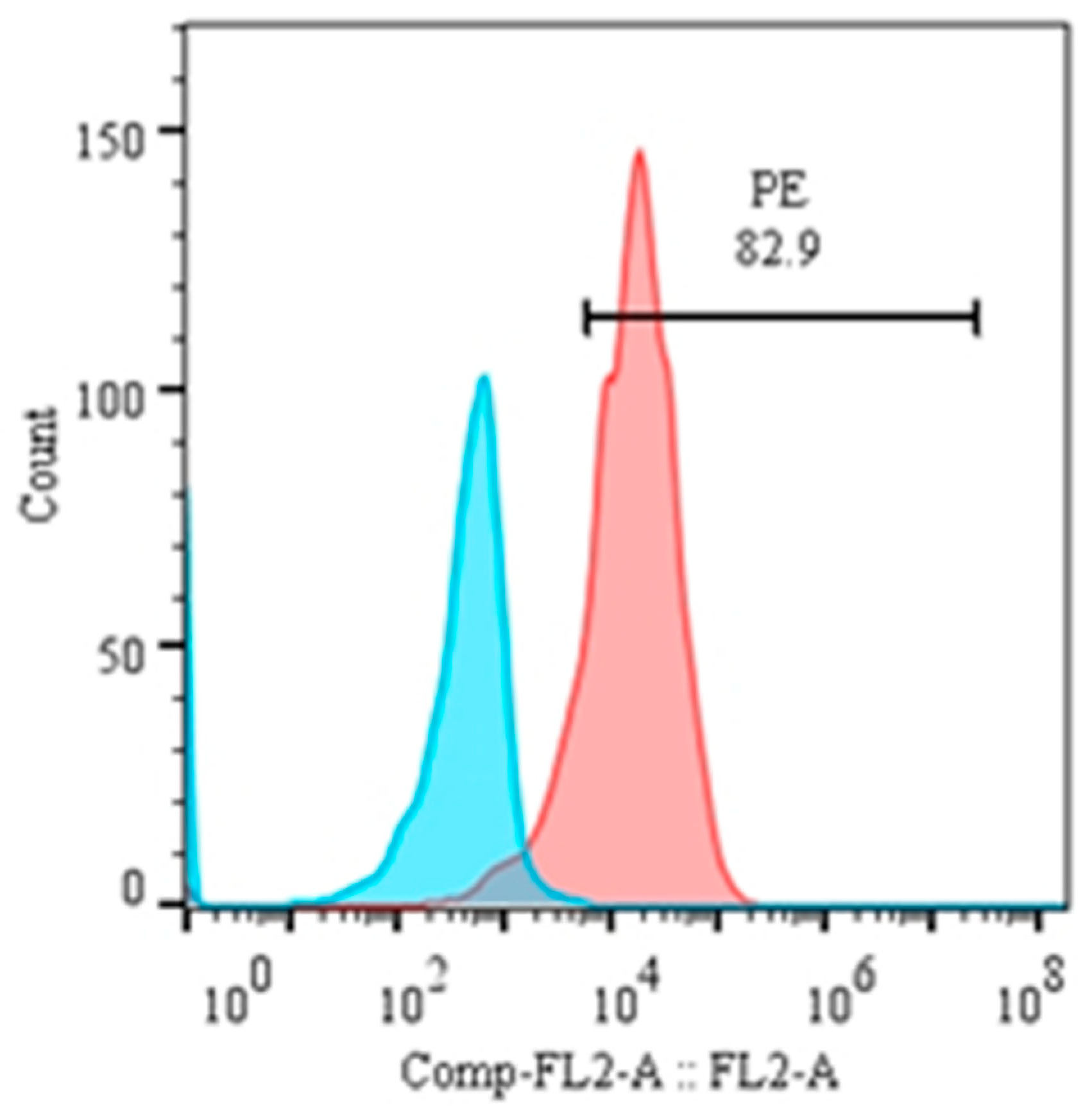
The purity of magnetically isolated B cells (CD21+) and the percentage of B cells in PBMCs were ~99 and ~32%, respectively (Figure 4). The cells purified from piroplasm-free cattle were unable to transform after in-vitro infection with sporozoites. Hence, B cells were isolated from experimentally infected cattle for culture until transformation and for cell line maintenance (culture conditions are described in the materials and methods section under the same subheading). Transformation of the infected cells began on the 13th day postisolation and incubation. CD21 surface markers were identified in 83% of these transformed cells at 3rd passage, and the remaining 17% were considered efficiently transformed but impure mononuclear cells (<1%) (Figure 5). To obtain a highly pure cell population, 4th generation transformed cells were labeled with CD21 antibodies for magnetic cell separation, and a purity of ~99% was achieved. From 5 clones, one expressing CD21 (98.8%), IgM (3.51%), and WC4 (0.51%) was selected for establishment of cell line from a single cell and cytokine production. These newly established B cell lines were maintained in complete culture medium at 37 °C and 5% CO2 and passaged twice a week according to their proliferation growth rate. These cell lines were preserved at the 8th (polyclonal) and 18th (single-cell clone) generations and stored at the Vector and Vector Borne Disease Laboratory, LVRI, Lanzhou, for further experimental purposes.
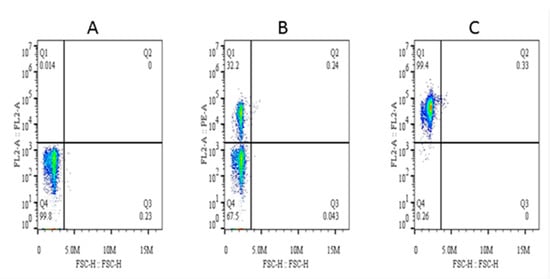
Figure 4.
Percentage of CD21+ cells in peripheral blood mononuclear cells (PBMCs) and analysis of their purity after magnetic separation. Here, (A) is the background control, (B) is the percentage of CD21+ cells in PBMCs, and (C) is the purity of magnetically isolated cells.
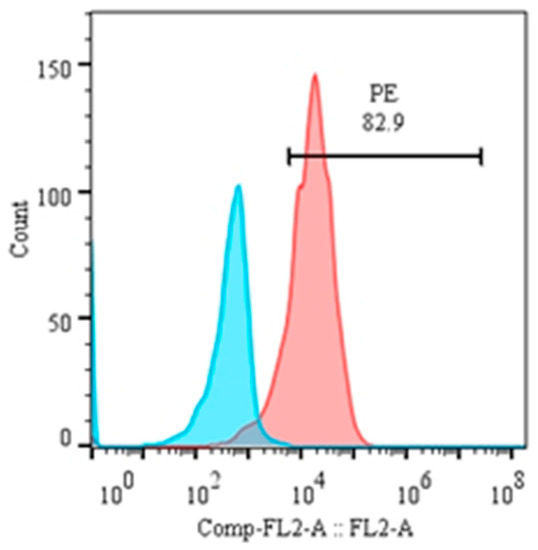
Figure 5.
Percentage of CD21 in transformed B cells at the 3rd passage.
3.3. Specificity Analysis of the B Cells Line
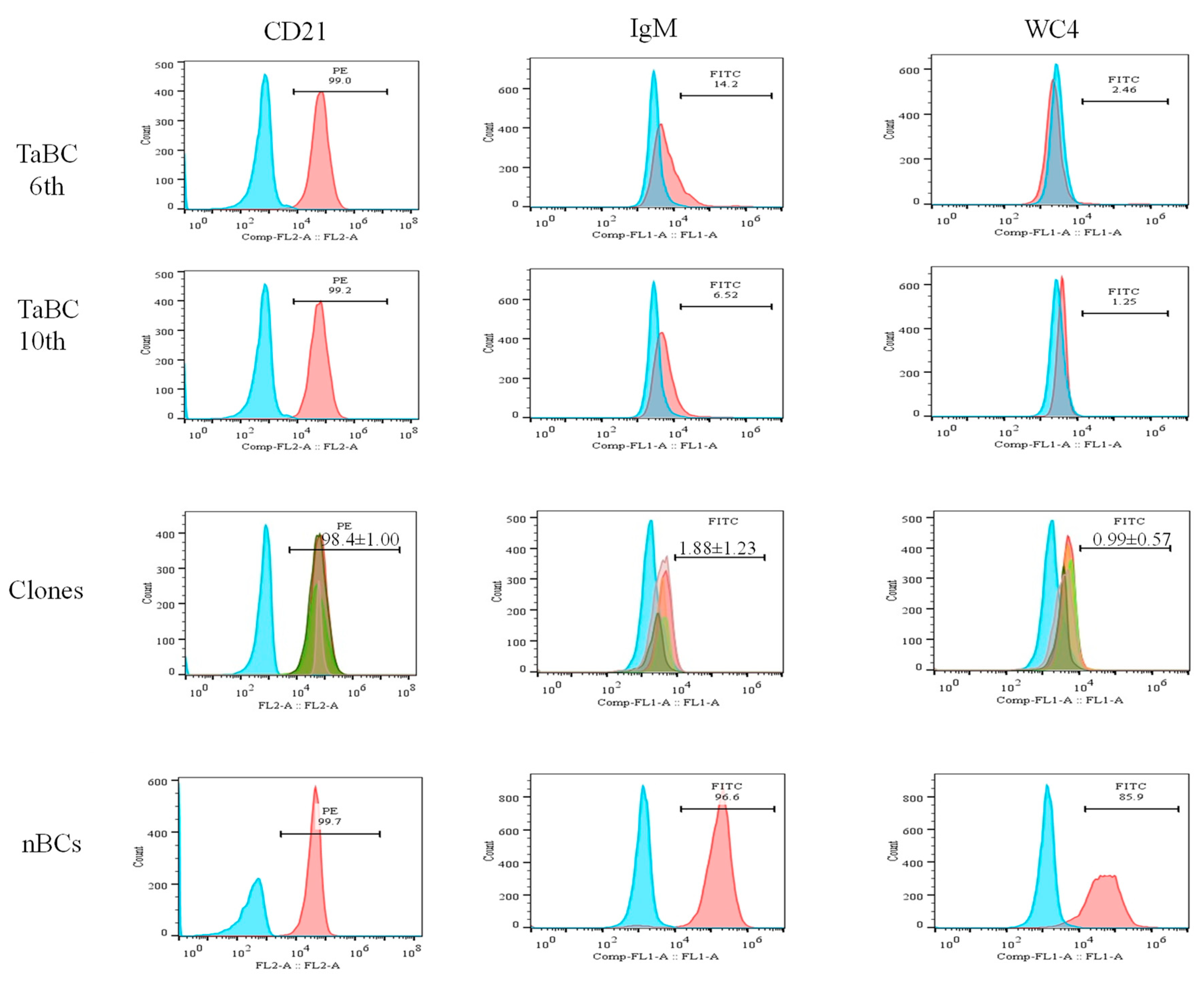
The percentages of the CD21, IgM and WC4 surface markers in 6th and 10th generation cells (polyclonal cell line) were ~99.0, 6.52–14.2 and 1.3–2.5%, respectively, whereas those of IgM and WC4 in normal isolated pure B cells were 96.6 and 85.9%, respectively. The percentages of CD21 (98.8, 99.7, 98, 98.5, and 97%; mean ± SD of 98.4 ± 1.00), IgM (3.51, 0.64, 1.42, 0.99, and 2.83%; mean ± SD of 1.88 ± 1.23) and WC4 (0.51, 1.96, 0.71, 0.80, and 0.97%; mean ± SD of 0.99 ± 0.57) were determined in the five clones, respectively. Of these, clone 1 was maintained, preserved, and used for the identification of cytokine production (Figure 6).
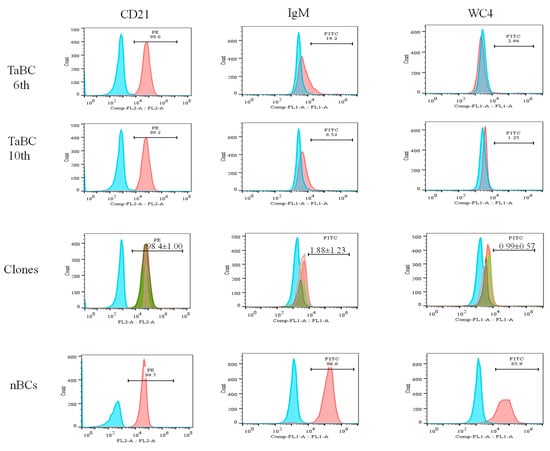
Figure 6.
Percentages of B cell-specific surface markers (CD21, IgM, and WC4) present in transformed cells (6th and 10th generations), single-cell line clones (approximately 15th generation), and normal purified cells. The percentages of CD21 (98.8, 99.7, 98.0, 98.5, and 97%; mean ± SD of 98.4 ± 1.00), IgM (3.51, 0.64, 1.42, 0.99, and 2.83%; mean ± SD of 1.88 ± 1.23) and WC4 (0.51, 1.96, 0.71, 0.80, and 0.97%; mean ± SD of 0.99 ± 0.57) in five clones were determined. CD21 was present in mono- and polyclonal cell lines, while negligible percentage of IgM and WC4 were present.
3.4. Optimized Conditions for BW720c and LPS
Cells in a wide number of ranges were cultured with various concentrations of LPS and BW720c for 24 and 48 h, respectively. As determined by MTT assay, the optimum concentrations of LPS and BW70c for the maximum viability of 5 × 106 cells well−1 were 1 µg mL−1 and 100 ng mL−1, respectively, and these concentrations were used in later experiments to examine cytokine production in BW720c-treated and LPS-stimulated cells.
3.5. Cytokine Primer Optimization
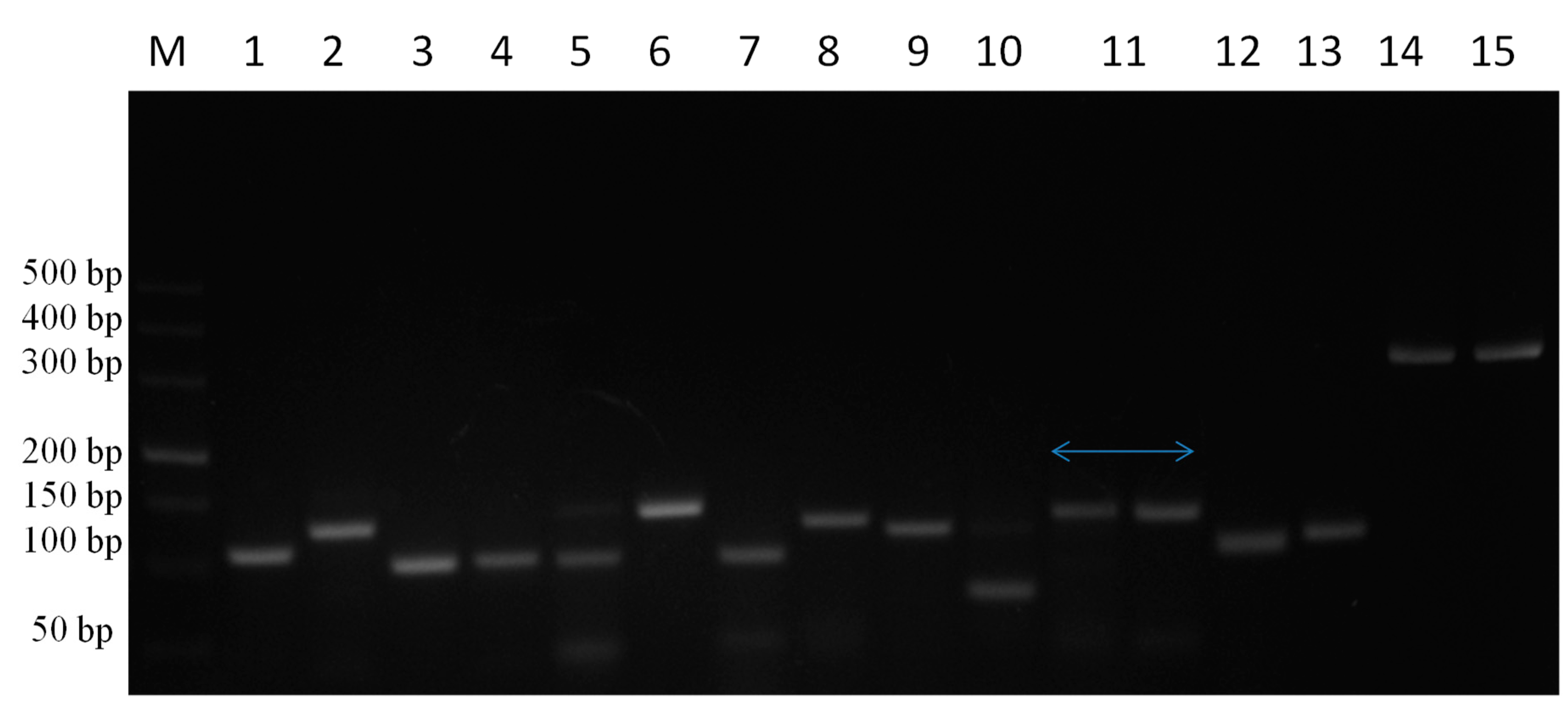
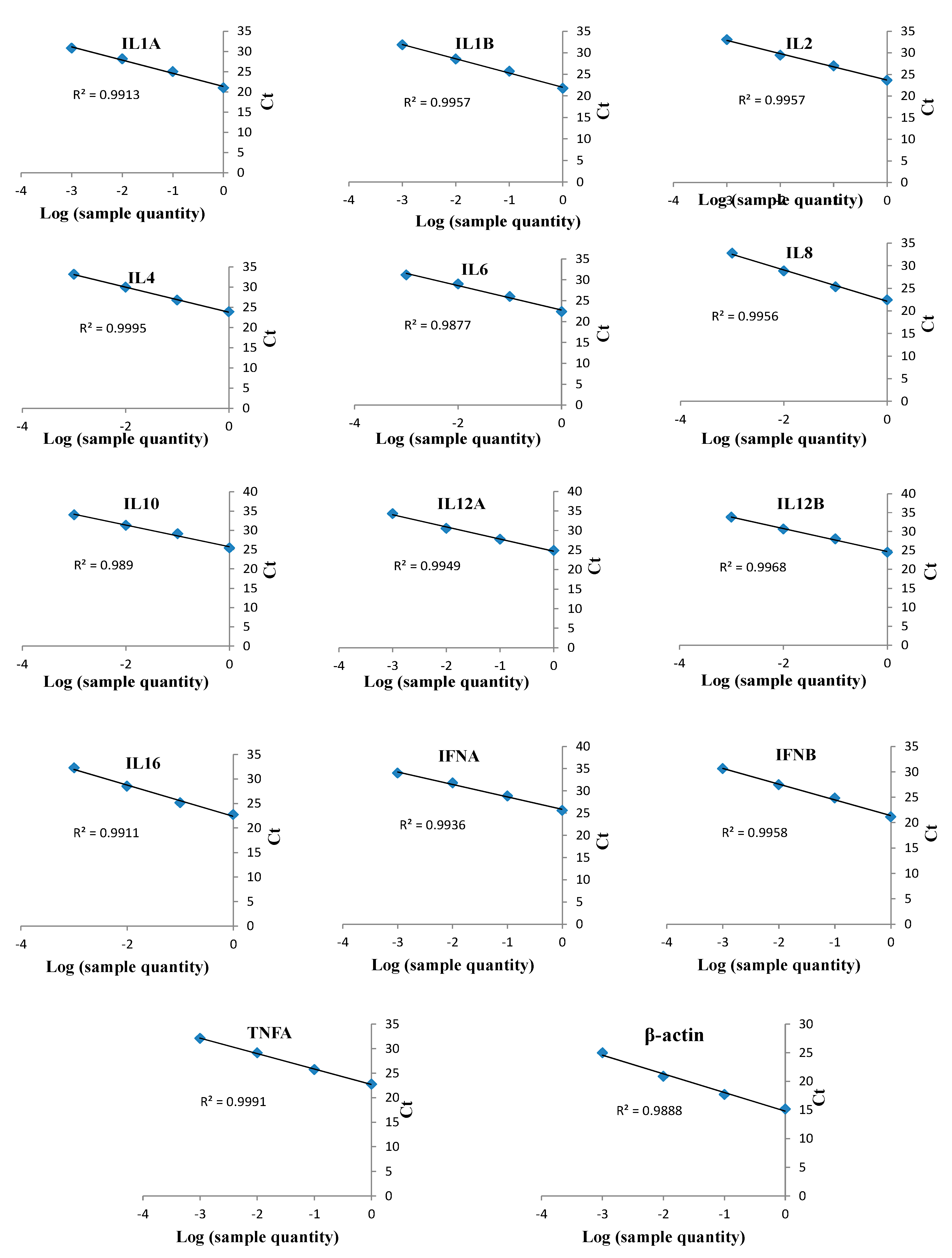
Target genes were amplified from DNA and cDNA extracted from TaBC with specific primers (Figure 7), purified, ligated into the pGEM T Easy vector, and transformed into DH5α competent cells. These genes were finally confirmed by sequencing analysis for each cytokine evaluated in this study. The optimized primers with correctly sequenced upon amplification and their R2 values (Table 1; Table 2; Figure 8) were used for qPCR to evaluate the transcript levels of the abovementioned genes in transformed and theileriacidal-treated TaBCs as well as in LPS-stimulated nBCs and TaBCs. Additionally, the primer sequences for IL8, IL16, LTA and TGFB1 were acquired from previously published literature [21,45,47].
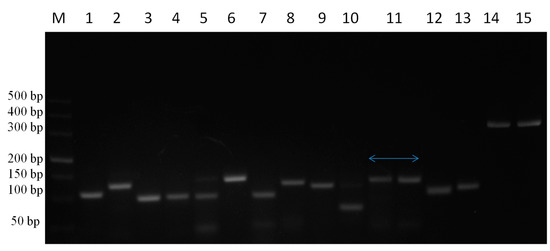
Figure 7.
PCR amplification of cytokine genes from TaBCs DNA and cDNA using specific primer pairs. Here, M represents the DNA marker (DL500), while lines 1–15 indicate IL1A, IL1B, IL2, IL4, IL6, IL8, IL10, IL12A, IL12B, IL16, TNFA, IFNB, IFNA, LTA, and TGFB1, respectively, amplified from DNA and cDNA harvested from of transformed B cells.

Table 1.
Details regarding the qPCR primers used for the quantification of cytokine production in this study.

Table 2.
Linearity measurements from regression analysis, mean fold changes, and standard deviation (SD) values of cytokines produced in T. annulata-transformed (TaBCs), BW720c-treated transformed cells, and LPS-stimulated normal B cells (nBCs) and TaBCs.
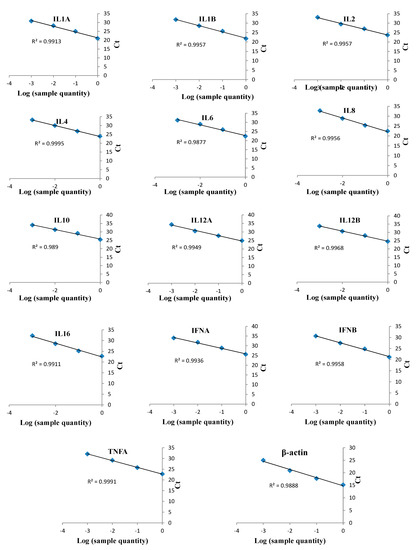
Figure 8.
qPCR linearity measurements of bovine cytokines in cDNA from T. annulata-transformed B cells (TaBCs) and LPS-stimulated peripheral blood mononuclear cells (PBMCs). qPCR was performed on serially diluted (1/10) cDNA to detect and optimize the efficacies of various cytokine primers. These optimized primers were used to evaluate the transcript levels of cytokine in TaBCs, BW720c-treated transformed B cells, and LPS-stimulated normal B cells (nBCs) and TaBCs. The qPCR data were analyzed by regression analysis based on log values of sample quantity and threshold cycles (Ct) for each cytokine. The monitored cytokines and R2 values of each regression line are shown.
3.6. Interleukins
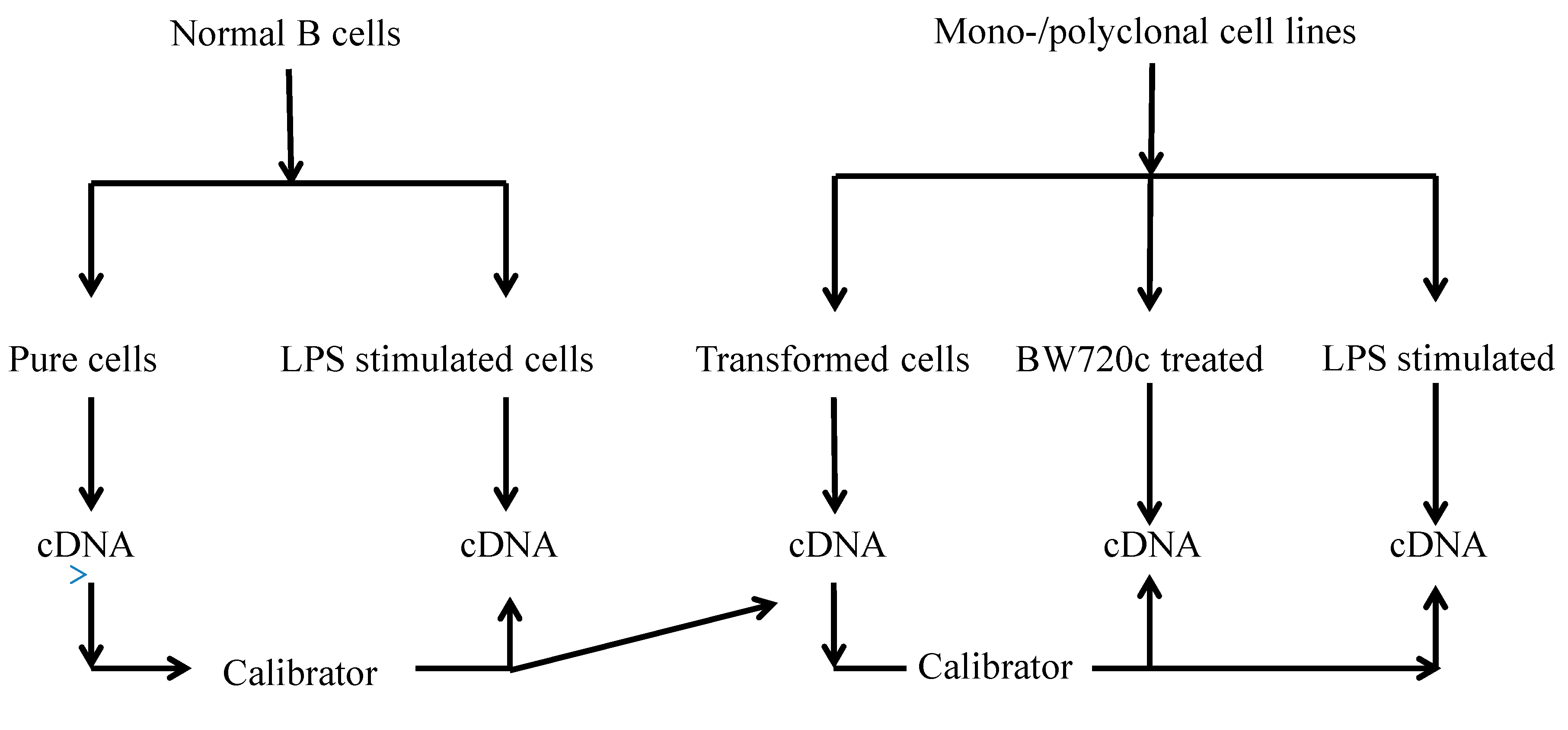
To quantify the cytokine levels, samples from TaBCs, BW720c-treated transformed B cells, and LPS-stimulated nBCs and TaBCs were acquired at the 16–18th generations for cDNA synthesis (Figure 9).
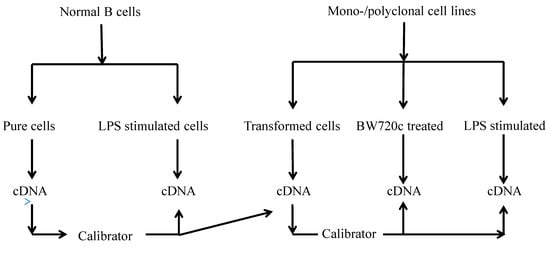
Figure 9.
Flow sheet diagram of samples collected from various cells types and the calibrators for cytokine production analysis.
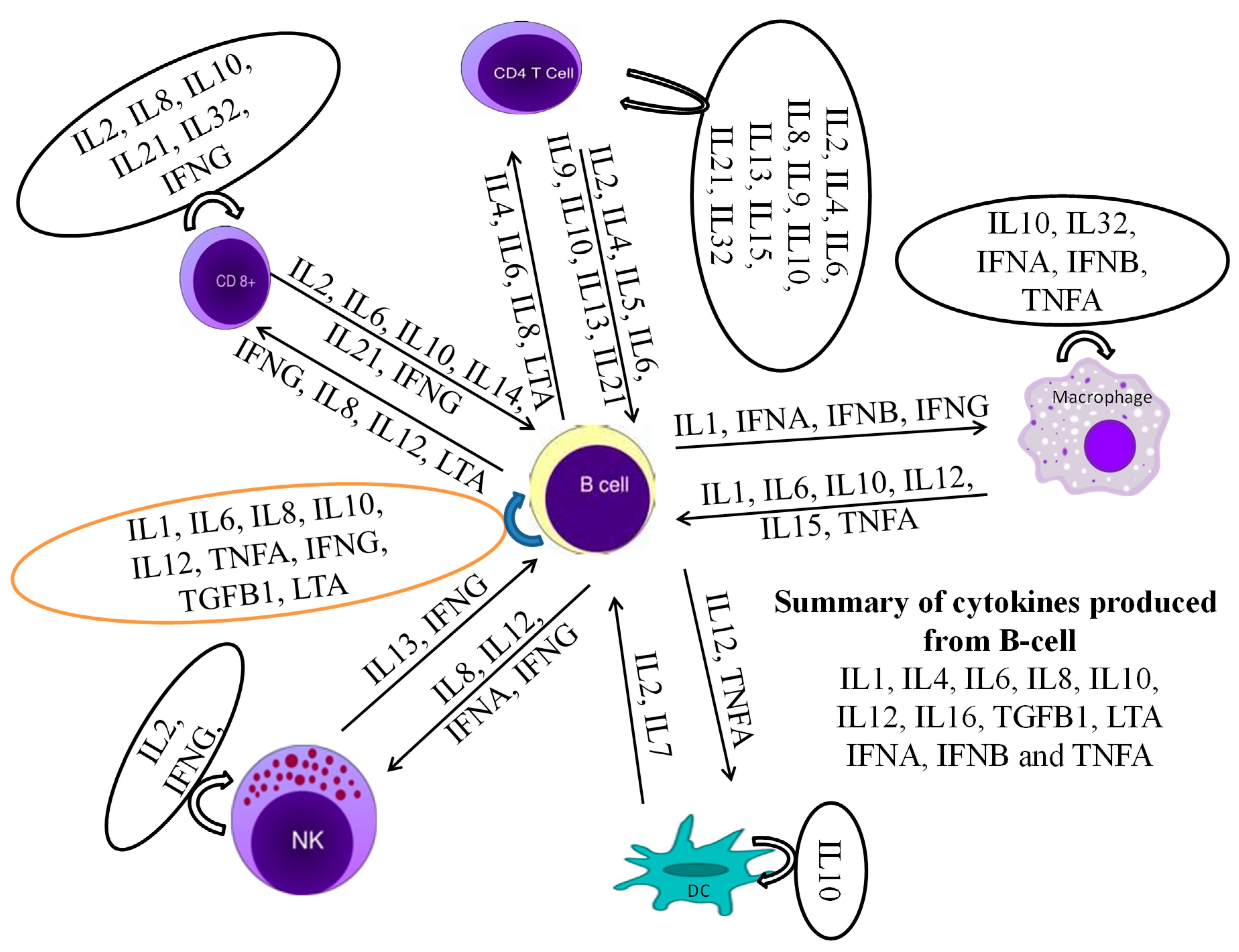
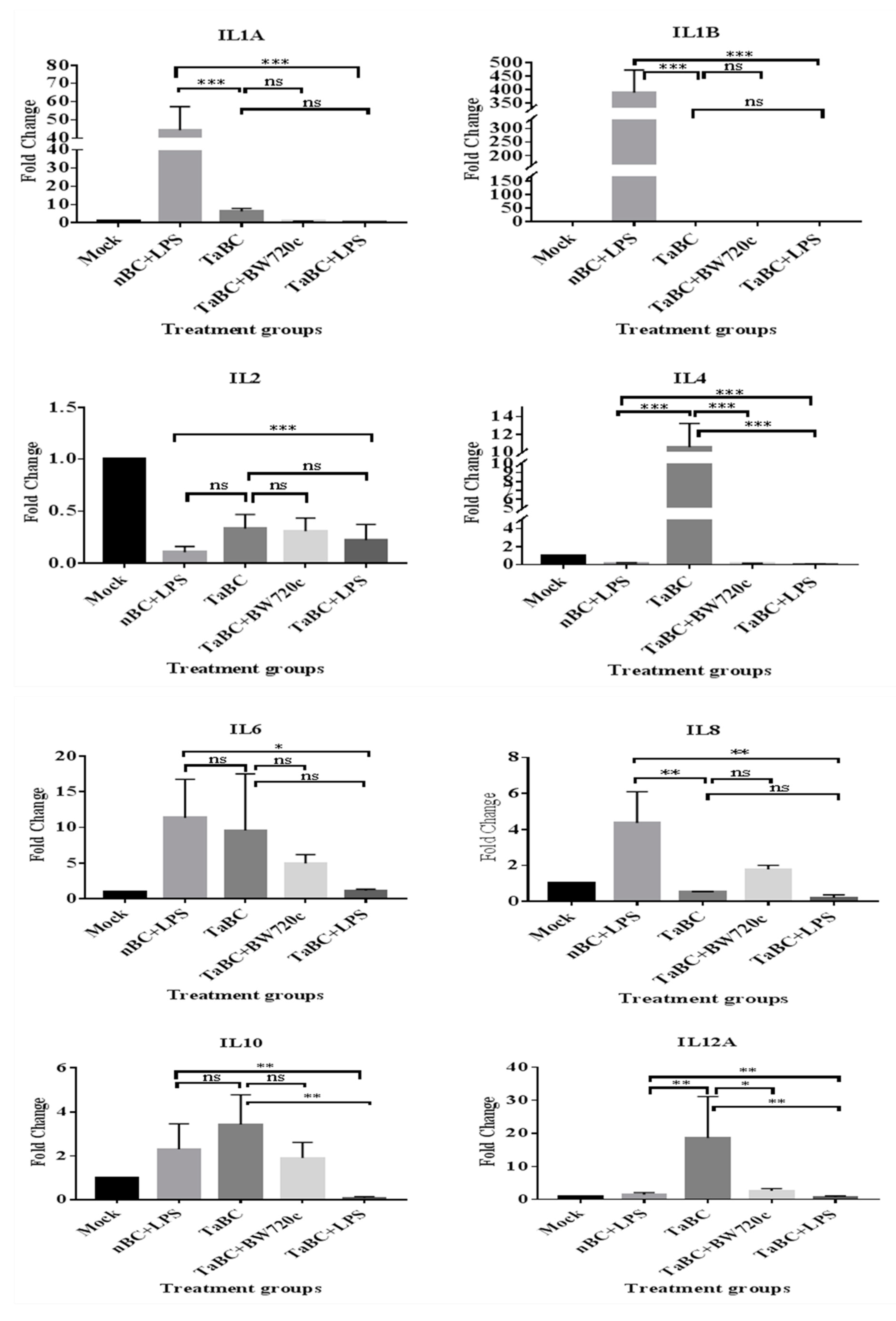
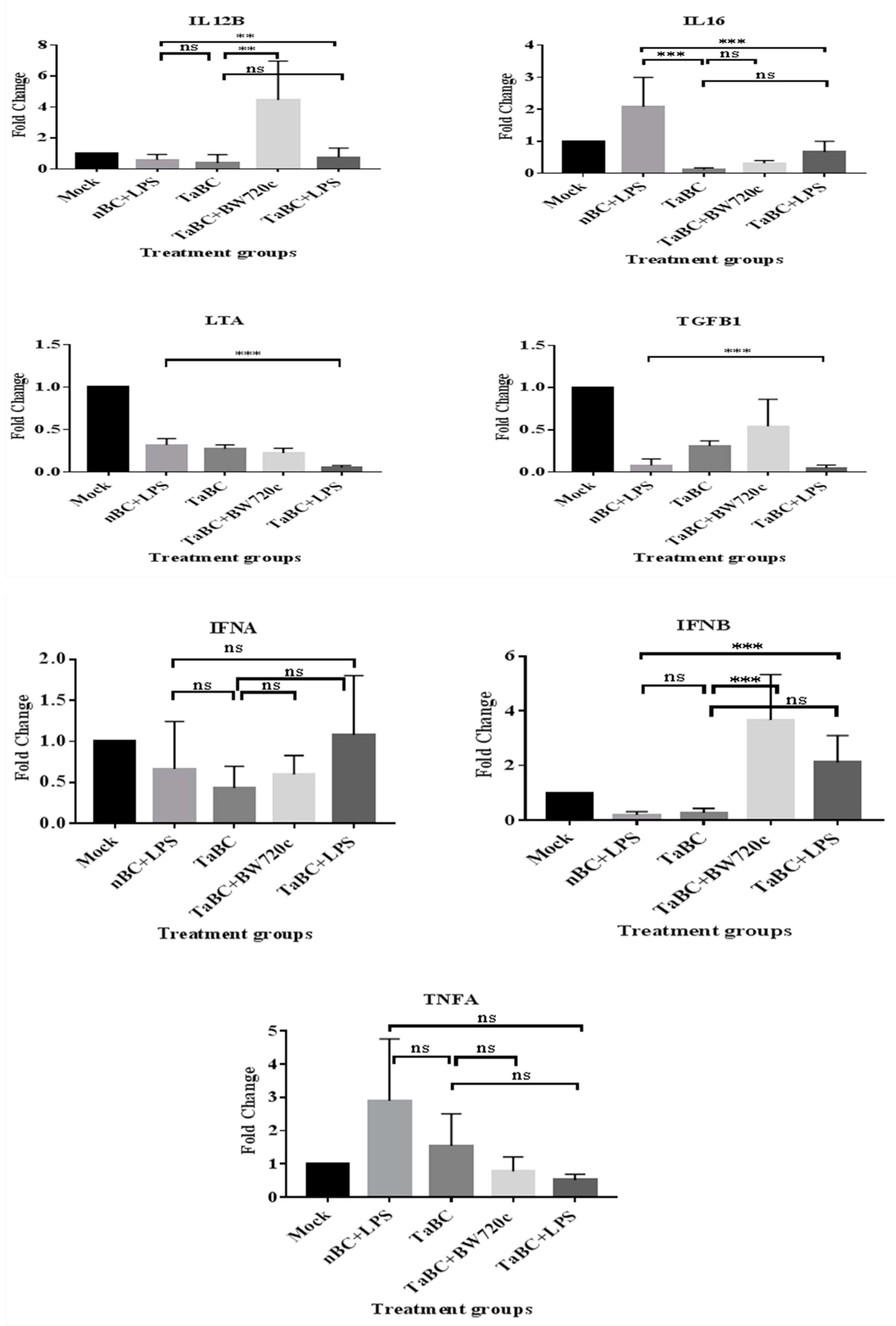
In this study, IL1A, IL1B, IL2, IL4, IL6, IL8, IL10, IL12A, IL12B, IL16, LTA, and TGFB1 [38] were considered to be the cytokines likely produced and to interact with bovine B cells (Figure 10). There was no significant difference in cytokine production between the mono—and polyclonal B cell lines of same passage, indicating that these derived from the same origin, which was confirmed by surface marker expression in these cell lines. Thus, only cytokines of monoclonal B cell line are mentioned here while polyclonal are provided in Supplementary Figure S1. In TaBCs (∆Ct of nBCs was used as the calibrator for the ∆∆Ct calculation), IL1A, IL1B, IL4, IL6, IL10, and IL12A were upregulated (fold change >1.0) on average by 6.33 ± 1.55-, 1.09 ± 0.51-, 10.57 ± 2.67-, 9.61 ± 7.91-, 3.44 ± 1.35-, and 18.62 ± 12.55-fold, respectively. In BW720c-treated transformed cells (∆Ct of TaBCs was used as the calibrator for the ∆∆Ct calculation), the cytokines IL6, IL8, IL10, IL12A, IL12B, and TGFB1 were upregulated on average by 4.96 ± 1.23-, 1.77 ± 0.22-, 1.92 ± 0.71-, 20.60 ± 0.72-, 4.45 ± 2.50-, and 0.54 ± 0.26-fold respectively, while the others were downregulated. In addition, the expression of all cytokines was downregulated in TaBCs stimulated with LPS (∆Ct of TaBCs was used as the calibrator for the ∆∆Ct calculation) except for IL6, which was upregulated by 1.15 ± 0.21-fold on average. However, in LPS-stimulated nBCs, cytokines IL1A, IL1B, IL6, IL8, IL10, IL12A, and IL16 were upregulated (∆Ct of nBCs was used as the calibrator for the ∆∆Ct calculation) on average by 44.41 ± 12.87-, 389.36 ± 83.73-, 11.38 ± 5.38-, 4.36 ± 1.73-, 2.30 ± 1.16-, and 2.08 ± 0.92-fold, respectively (Figure 11, Table 2).
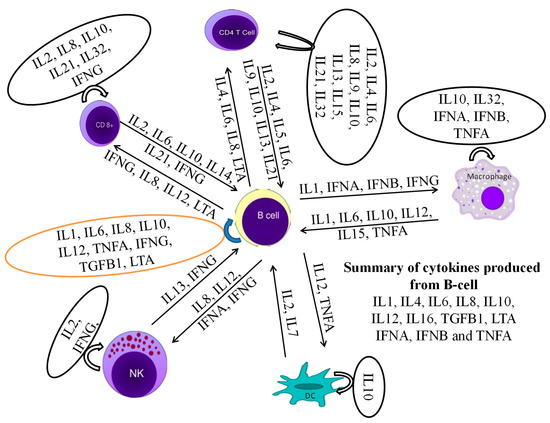
Figure 10.
A cytokine production network as well as interaction with themselves and other leukocytes is required for the stimulation of B cells to produce their own cytokines.
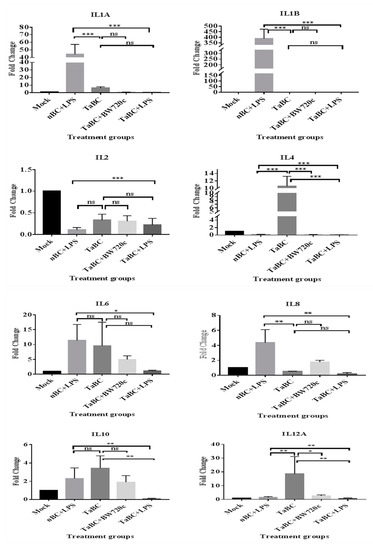
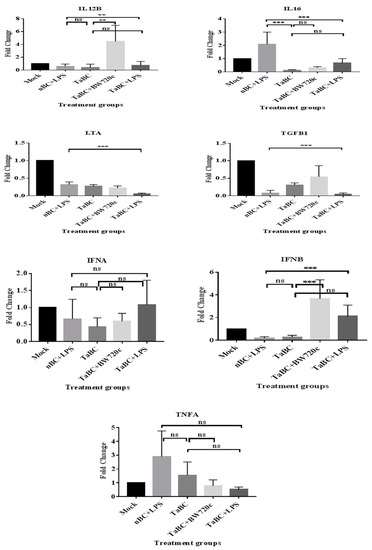
Figure 11.
Transcript levels of cytokines in monoclonal T. anuulata-transformed B cells (TaBCs), BW720c-treated transformed cells, and LPS-stimulated TaBCs and normal B cells (nBCs). Here, *** p < 0.001, ** p < 0.01, * p < 0.05, and ns is not significant.
3.7. Interferons
In transformed and LPS-stimulated B cells, the transcript level of IFNA and IFNB were not upregulated. Moreover, only IFNB was upregulated by 3.67 ± 1.66-fold on average in BW720c-treated transformed cells. Similarly, IFNA and IFNB were upregulated by 1.08 ± 0.72- and 2.12 ± 0.98-fold, respectively, in LPS-stimulated transformed cells (Figure 11, Table 2).
3.8. Tumor Necrosis Factor
TNFA is a cellular signaling protein that is involved in acute-phase reactions and systematic inflammation [49], and fever is a clinical symptom in infected organisms [50]. Schizont-infected cells also secrete TNFA for the stimulation of other leukocytes.
4. Discussion
Neonatal bovine B cells express CD5 [51], whereas mature B cells express the CD21 surface marker [28]. Previously, established B cell lines were developed from in vitro infection of these cells (CD5 and CD21) with T. annulata sporozoites for the identification of various cell surface markers [8,35]. However, in the current study, a single transformed cell was cloned to establish a cell line and quantify significant cytokine production. During this study, mature B cells (CD21) were unable to infect sporozoites after in vitro infection due to three possible reasons. First, lymphocytes are not directly exposed to antigens and antigens are instead presented by antigen-presenting cells (APCs), such as DCs and macrophages [6]. Second, elastin (hexapeptide) receptors, which are expressed in mainly immature monocytes, but not in B and T lymphocytes, are the potential ligands for sporozoite entry, which explains the previously observed low transformation rate (1:6897) of B cells [11]. Third, some animal breeds, such as those used as experimental animals in this study, may be less susceptible to tropical theileriosis [52]. Hence, B cells (CD21+) from in vivo T. annulata-infected cattle were isolated and cultured to develop a pure cell line. The purity and specificity of the established cell line was confirmed after the identification of three different but specific B cell surface makers on TaBCs and nBCs, ensuring that the established cell line originated purely from bovine B cells. However, there are no published data on the purity of this type of cell line after confirmation of its establishment other than the scarce information about the ability of T. annulata to transform B lymphocytes. Except for CD21, these surface markers were consistently downregulated in later passages/generations of the cell line. These findings are supported by previously reported studies in which the expression of transformed cell surface markers rapidly decreased [7,53], but coexpression of the CD21 marker was observed following infection [35].
The host immune response to any pathogen results from a complex network of various immune effector cells and their signals [17]. Cytokines constitute a considerable portion of this signaling cascade for stimulation, and the balance of various cytokines has an intense effect on the outcomes of infectious challenges. Interestingly, T. annulata hijacks the host immune response to invade and transform leukocytes [54]. Previous studies claimed that transformed cells (macroschizonts containing macrophages) secrete various types of cytokines [3], which is why a set of primers for each cytokine (mentioned above) was optimized for qPCR analysis to evaluate the cytokine profiles in the TaBCs, BW720c-treated cells and LPS-stimulated cells in this study. In transformed cells, the transcript levels of IL2, IL4, IL10, and IL12A were upregulated compared with those in LPS-stimulated nBCs, but only IL4 and IL12A were statistically significant (p < 0.01 and p = 0.01), which supports our hypothesis. Cytokines such as IL4 directly stimulate B cells for the production of IgG1 and IgE [55]. Moreover, IL4 also functions as a survival factor for B and T lymphocytes, and IL12 is responsible for the induction of Th1 cell differentiation. Likewise, significantly upregulated IL4 might lead to the secretion of immunoglobulin from transformed cells. These results are in consistent with previously reported findings that transformed T. annulata cells secrete IL1A, IL1B, IL6, IL10, IL12, TNFA, and IFNA [3,56]. Epstein Barr virus (EBV)-transformed human B cells constitutively secrete IL1, leading to T lymphocyte proliferation [57]. In this study, IL2 expression was downregulated in all cell types due to its origination from cells other than B cells. However, other studies have claimed that transformed cells secrete IL2 to serve as a growth factor and enhance cell proliferation [22]. Exposure of infected cells to BW720c induced to parasite death followed by cessation of host cell proliferation and apoptosis within a few days [14]. Furthermore, the transcript levels of IL8, IL12B, TGFB1 IFNA, and IFNB were upregulated in BW720c-treated TaBCs compared with those in untreated transformed cells, but only IL12B and IFNB were statistically significant. LTA and TGFB1 were significantly (p < 0.01) produced in nBCs and TaBC-BW720c treated cells, respectively. TNF, LTA, and TGFB1 reportedly play important roles in the differentiation and proliferation of B cells, and these cytokines may exert an autocrine effect on B cells or modulate their interaction with other cell types, such as helper T cells or FDCs [21]. Similarly, the expression levels of IL1A, IL1B, IL6, IL8, IL16, LTA, IFNA, and TNFA were upregulated in LPS-stimulated nBCs compared with those in TaBCs, and the upregulation of IL1A, IL1B, IL8, and IL16 was significant. These findings are in agreement with the reported findings that effector B cells significantly produce cytokines [51]. Interestingly, no statistically significant upregulation was found in LPS-stimulated TaBCs, which was opposite from our proposed hypothesis. Fundamentally, immune responses substantially relate to the recognition of exogenous antigens by Toll-like receptors (TLRs), B cell receptors (BCRs), and CD40, which detect different pathogen-associated molecular patterns [38]. After antigen recognition, immune cells generate a wide range of cellular responses, including the secretion of anti—and proinflammatory cytokines, chemokines, and type I interferons [18]. Evidence from the literature and our own findings indicate that transformed cells regress most of the surface receptors [7], which might include TLRs, BCRs, and CD40. This downregulation potentially accounts for why antigen (LPS) was not recognized and no immune response (cytokine production) was generated by these cells.
Upon activation, naïve B cells do not secrete any cytokines, while naïve T cells initiate cytokine production upon activation, which is the inherent difference between these cell types; B cells require additional activation signals to become cytokine producers. These signaling pathways are provided by immune cells in the surrounding and B cell-specific differentiation stages. Once B cells acquire the ability to produce cytokines, they become capable of cross-regulating responses through inhibition/polarization that can negatively regulate the entire host immune response system. LPS- and cytokine (IL5 and IL10)-stimulated B cells [38] are associated with the production of natural immunoglobulins (IgM and IgG) in response to antigens [58,59,60]. These associations served as the basis for using LPS to stimulate nBCs and TaBCs to compare their cytokines production abilities and further investigate immunological changes (immunoglobulin production) in these cell types. Other immune cell cytokines are key factors for switching the immunoglobulin and their isotype production from B lymphocytes [45,55]. Hence, this study provides a basis for future studies to observe the effects of various supplemental recombinant cytokines from other immune cells on these transformed cells. The effects of the direct exposure of other immune cells and their cytokines on these transformed cells to trigger their own significant production or the switching of immunoglobulins and their isotypes were not studied herein.
5. Conclusions
We successfully established Theileria annulata-infected poly- and monoclonal B cell lines. Significant cytokine production was demonstrated in this cell line, which can now be used in vaccines/therapy against pathogens. This established pure cell line can also be used in various types of in vitro immunological studies.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/10/5/329/s1, Figure S1: Transcript levels of cytokines in polyclonal T. anuulata-transformed B cells (TaBCs), BW720c-treated transformed cells, and LPS-stimulated TaBCs and normal B cells (nBCs). Here, *** p < 0.001, ** p < 0.01, * p < 0.05, and ns is not significant.
Author Contributions
M.R., G.G., J.L. (Junlong Liu), and M.I.R., conceived and designed the experiment; M.R. performed the experiment; J.L. (Junlong Liu), M.I.R., and M.A.H. analyzed the data; C.S., X.W., J.L. (Jianxun Luo), and H.Y. contributed reagents/materials/analysis tools. M.R. and M.U. wrote this paper. All authors read and approved the final version of the manuscript.
Funding
This study was financially supported by [973 Program] grant number [2015CB150300]; [National Key R&D Program of China] grant number [2017YFD050403]; [Central Public-interest Scientific Institution Basal Research Fund] grant number [1610312016009]; [NSFC] grant number [31402189]; [ASTIP] grant number [CAAS-ASTIP-2016-LVRI]; [NBCIS] grant number [CARS-37]; Jiangsu Co-innovation Center Program for Prevention and Control of Important Animal Infectious Disease and Zoonosis. The APC was funded by [2015CB150300].
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| cDNA | Complementary DNA |
| IFN | Interferon |
| IL | Interleukin |
| LPS | Lipopolysaccharides |
| LVRI | Lanzhou Veterinary Research Institute |
| NCBI | National Center for Biological Information |
| nBC | Normal B cells |
| PBMCs | Peripheral blood mononuclear cells |
| PCR | Polymerase chain reaction |
| rRNA | Ribosomal RNA |
| qPCR | Quantitative PCR |
| TaBC | Theileria annulata transformed B cells |
| TLRs | Toll like receptors |
| TNF | Tumor necrosis factor |
| BW720c | Buparvaquone |
References
- Hayashida, K.; Hara, Y.; Abe, T.; Yamasaki, C.; Toyoda, A.; Kosuge, T.; Suzuki, Y.; Sato, Y.; Kawashima, S.; Katayama, T. Comparative genome analysis of three eukaryotic parasites with differing abilities to transform leukocytes reveals key mediators of Theileria-induced leukocyte transformation. MBio 2012, 3, e00204-12. [Google Scholar] [CrossRef]
- Rashid, M.; Akbar, H.; Rashid, I.; Saeed, K.; Ahmad, L.; Ahmad, A.S.; Shehzad, W.; Islam, S.; Farooqi, S. Economic significance of tropical theileriosis on a Holstein Friesian dairy farm in Pakistan. J. Parasitol. 2018, 104, 310–312. [Google Scholar] [CrossRef]
- Preston, P.; Hall, F.; Glass, E.; Campbell, J.; Darghouth, M.; Ahmed, J.; Shiels, B.; Spooner, R.; Jongejan, F.; Brown, C. Innate and adaptive immune responses co-operate to protect cattle against Theileria annulata. Parasitol. Today 1999, 15, 268–274. [Google Scholar] [CrossRef]
- Wykes, M.; Pombo, A.; Jenkins, C.; MacPherson, G.G. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 1998, 161, 1313–1319. [Google Scholar]
- Inaba, K.; Metlay, J.P.; Crowley, M.T.; Steinman, R.M. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J. Exp. Med. 1990, 172, 631–640. [Google Scholar] [CrossRef]
- Heesters, B.A.; van der Poel, C.E.; Das, A.; Carroll, M.C. Antigen presentation to B cells. Trends Immunol. 2016, 37, 844–854. [Google Scholar] [CrossRef]
- Stephens, S.; Howard, C. Infection and transformation of dendritic cells from bovine afferent lymph by Theileria annulata. Parasitology 2002, 124, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.-F.; Thibaud, J.-L.; Miled, L.B.; Chaussepied, M.; Baumgartner, M.; Davis, W.C.; Minoprio, P.; Langsley, G. Theileria annulata in CD5+ macrophages and B1 B cells. Infect. Immun. 1999, 67, 6678–6682. [Google Scholar] [PubMed]
- Ferrolho, J.; Domingos, A.; Campino, L. Cattle Specific Immune Mechanisms used against the Protozoan Theileria annulata. Int. Trends Immunity 2016, 4. [Google Scholar] [CrossRef]
- Spooner, R.; Innes, E.; Glass, E.; Brown, C. Theileria annulata and T. parva infect and transform different bovine mononuclear cells. Immunology 1989, 66, 284. [Google Scholar]
- Campbell, J.; Brown, D.; Glass, E.; Hall, F.; Spooner, R. Theileria annulata sporozoite targets. Parasite Immunol. 1994, 16, 501–505. [Google Scholar] [CrossRef]
- Dobbelaere, D.; Coquerelle, T.M.; Roditi, I.J.; Eichhorn, M.; Williams, R.O. Theileria parva infection induces autocrine growth of bovine lymphocytes. Proc. Natl. Acad. Sci. USA 1988, 85, 4730–4734. [Google Scholar] [CrossRef]
- Tretina, K.; Gotia, H.T.; Mann, D.J.; Silva, J.C. Theileria-transformed bovine leukocytes have cancer hallmarks. Trends Parasitol. 2015, 31, 306–314. [Google Scholar] [CrossRef]
- Guergnon, J.; Dessauge, F.; Langsley, G.; Garcia, A. Apoptosis of Theileria-infected lymphocytes induced upon parasite death involves activation of caspases 9 and 3. Biochimie 2003, 85, 771–776. [Google Scholar] [CrossRef]
- Naessens, J. Surface Ig on B lymphocytes from cattle and sheep. Int. Immunol. 1997, 9, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Ikebuchi, R.; Konnai, S.; Okagawa, T.; Nishimori, A.; Nakahara, A.; Murata, S.; Ohashi, K. Differences in cellular function and viral protein expression between IgMhigh and IgMlow B-cells in bovine leukemia virus-infected cattle. J. Gen. Virol. 2014, 95, 1832–1842. [Google Scholar] [CrossRef]
- Vazquez, M.I.; Catalan-Dibene, J.; Zlotnik, A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 2015, 74, 318–326. [Google Scholar] [CrossRef]
- Im, Y.B.; Jung, M.; Shin, M.-K.; Kim, S.; Yoo, H.S. Expression of cytokine and apoptosis-related genes in bovine peripheral blood mononuclear cells stimulated with Brucella abortus recombinant proteins. Vet. Res. 2016, 47, 30. [Google Scholar] [CrossRef]
- Norian, R.; Delirezh, N.; Azadmehr, A. Evaluation of proliferation and cytokines production by mitogen-stimulated bovine peripheral blood mononuclear cells. Vet. Res. Forum 2015, 6, 265–271. [Google Scholar] [PubMed]
- Duddy, M.E.; Alter, A.; Bar-Or, A. Distinct profiles of human B cell effector cytokines: A role in immune regulation? J. Immunol. 2004, 172, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- Amills, M.; Norimine, J.; Olmstead, C.A.; Lewin, H.A. Cytokine mRNA expression in B cells from bovine leukemia virus-infected cattle with persistent lymphocytosis. Cytokine 2004, 28, 25–28. [Google Scholar] [CrossRef]
- Ahmed, J.; Mehlhorn, H. The cellular basis of the immunity to and immunopathogenesis of tropical theileriosis. Parasitol. Res. 1999, 85, 539–549. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Akdis, M.; Burgler, S.; Crameri, R.; Eiwegger, T.; Fujita, H.; Gomez, E.; Klunker, S.; Meyer, N.; O’Mahony, L.; Palomares, O. Interleukins, from 1 to 37, and interferon-γ: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2011, 127, 701–721.e770. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Liu, J.; Rashid, M.; Iqbal, N.; Guan, G.; Yin, H.; Luo, J. Molecular survey of piroplasm species from selected areas of China and Pakistan. Parasites Vectors 2018, 11, 457. [Google Scholar] [CrossRef]
- Marek, R.; Caruso, M.; Rostami, A.; Grinspan, J.B.; Sarma, J.D. Magnetic cell sorting: A fast and effective method of concurrent isolation of high purity viable astrocytes and microglia from neonatal mouse brain tissue. J. Neurosci. Methods 2008, 175, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.A.; De Luca, P.M.; Bertho, Á.L.; Azeredo-Coutinho, R.B.; Coutinho, S.G. Detection of intracytoplasmic cytokines by flow cytometry. Memórias Do Inst. Oswaldo Cruz 2000, 95, 401–402. [Google Scholar] [CrossRef]
- Buchanan, R.; Popowych, Y.; Dagenais, C.; Arsic, N.; Mutwiri, G.K.; Potter, A.A.; Babiuk, L.A.; Griebel, P.J.; Wilson, H.L. Interferon-gamma and B-cell Activating Factor (BAFF) promote bovine B cell activation independent of TLR9 and T-cell signaling. Vet. Immunol. Immunopathol. 2012, 145, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Guan, G.; Liu, J.; Liu, A.; Li, Y.; Yin, H.; Luo, J. Screening and identification of host proteins interacting with Theileria annulata cysteine proteinase (TaCP) by yeast-two-hybrid system. Parasites Vectors 2017, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.; Lee, D.; Stucky, J.; Chiu, Y.-L.; Rubin, A.; Horton, H.; McElrath, M.J. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J. Immunol. Methods 2007, 322, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Obermaier, B.; Dauer, M.; Herten, J.; Schad, K.; Endres, S.; Eigler, A. Development of a new protocol for 2-day generation of mature dendritic cells from human monocytes. Biol. Proced. Online 2003, 5, 197. [Google Scholar] [CrossRef][Green Version]
- Ryan, J. Cell Cloning by Serial Dilution in 96 Well Plates; Corning: Lowell, MA, USA, 2008. [Google Scholar]
- Durrani, Z.; Weir, W.; Pillai, S.; Kinnaird, J.; Shiels, B. Modulation of activation-associated host cell gene expression by the apicomplexan parasite Theileria annulata. Cell. Microbiol. 2012, 14, 1434–1454. [Google Scholar] [CrossRef]
- Sharifiyazdi, H.; Namazi, F.; Oryan, A.; Shahriari, R.; Razavi, M. Point mutations in the Theileria annulata cytochrome b gene is associated with buparvaquone treatment failure. Vet. Parasitol. 2012, 187, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Sager, H.; Bertoni, G.; Jungi, T.W. Differences between B cell and macrophage transformation by the bovine parasite, Theileria annulata: A clonal approach. J. Immunol. 1998, 161, 335–341. [Google Scholar]
- Monrad, S.U.; Rea, K.; Thacker, S.; Kaplan, M.J. Myeloid dendritic cells display downregulation of C-type lectin receptors and aberrant lectin uptake in systemic lupus erythematosus. Arthritis Res. Ther. 2008, 10, R114. [Google Scholar] [CrossRef]
- Wong, N.K.; Shenoi, R.A.; Abbina, S.; Chafeeva, I.; Kizhakkedathu, J.N.; Khan, M.K. Nontransformed and cancer cells can utilize different endocytic pathways to internalize dendritic nanoparticle variants: Implications on nanocarrier design. Biomacromolecules 2017, 18, 2427–2438. [Google Scholar] [CrossRef]
- Bao, Y.; Cao, X. The immune potential and immunopathology of cytokine-producing B cell subsets: A comprehensive review. J. Autoimmun. 2014, 55, 10–23. [Google Scholar] [CrossRef]
- GeneScript. Available online: https://www.genscript.com/tools/real-time-pcr-tagman-primer-design-tool (accessed on 4 October 2018).
- NCBI. Available online: https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 8 October 2018).
- Integrated DNA Technologies. Available online: https://sg.idtdna.com/calc/analyzer (accessed on 10 October 2018).
- Hassan, M.A.; Liu, J.; Sajid, M.S.; Mahmood, A.; Zhao, S.Y.; Abbas, Q.; Guan, G.; Yin, H.; Luo, J. Molecular detection of Theileria annulata in cattle from different regions of Punjab, Pakistan using recombinase polymerase amplification and PCR. J. Parasitol. 2018, 104, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Rio, D.C.; Ares, M.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010. [Google Scholar] [CrossRef]
- Konnai, S.; Usui, T.; Ohashi, K.; Onuma, M. The rapid quantitative analysis of bovine cytokine genes by real-time RT-PCR. Vet. Microbiol. 2003, 94, 283–294. [Google Scholar] [CrossRef]
- Coussens, P.M.; Verman, N.; Coussens, M.A.; Elftman, M.D.; McNulty, A.M. Cytokine gene expression in peripheral blood mononuclear cells and tissues of cattle infected with Mycobacterium avium subsp. paratuberculosis: Evidence for an inherent proinflammatory gene expression pattern. Infect. Immun. 2004, 72, 1409–1422. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2–ΔΔCT method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71. [Google Scholar]
- Zaros, L.G.; Bricarello, P.A.; Amarante, A.F.T.; Coutinho, L.L. Quantification of bovine cytokine gene expression using real-time RT-PCR methodology. Genet. Mol. Biol. 2007, 30, 575–579. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.; Guan, G.; Liu, A.; Li, Y.; Yin, H.; Luo, J. Theileria annulata Cyclophilin1 (TaCyp1) Interacts With Host Cell MED21. Front. Microbiol. 2018, 9, 2973. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Glass, E.J.; Craigmile, S.C.; Springbett, A.; Preston, P.M.; Kirvar, E.; Wilkie, G.M.; Eckersall, P.D.; Hall, F.R.; Brown, C.D. The protozoan parasite, Theileria annulata, induces a distinct acute phase protein response in cattle that is associated with pathology. Int. J. Parasitol. 2003, 33, 1409–1418. [Google Scholar] [CrossRef]
- Lund, F.E. Cytokine-producing B lymphocytes—Key regulators of immunity. Curr. Opin. Immunol. 2008, 20, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Paxton, E.; Waddington, D.; Talbot, R.; Darghouth, M.A.; Glass, E.J. Differences in the transcriptional responses induced by Theileria annulata infection in bovine monocytes derived from resistant and susceptible cattle breeds. Int. J. Parasitol. 2008, 38, 313–325. [Google Scholar] [CrossRef]
- Jensen, K.; Makins, G.D.; Kaliszewska, A.; Hulme, M.J.; Paxton, E.; Glass, E.J. The protozoan parasite Theileria annulata alters the differentiation state of the infected macrophage and suppresses musculoaponeurotic fibrosarcoma oncogene (MAF) transcription factors. Int. J. Parasitol. 2009, 39, 1099–1108. [Google Scholar] [CrossRef][Green Version]
- Plattner, F.; Soldati-Favre, D. Hijacking of host cellular functions by the Apicomplexa. Annu. Rev. Microbiol. 2008, 62, 471–487. [Google Scholar] [CrossRef]
- Estes, D.M. Differentiation of B cells in the bovine. Role of cytokines in immunoglobulin isotype expression. Vet. Immunol. Immunopathol. 1996, 54, 61–67. [Google Scholar] [CrossRef]
- Ahmed, J.S.; Glass, E.J.; Salih, D.A.; Seitzer, U. Innate immunity to tropical theileriosis. Innate Immun. 2008, 14, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Procopio, A.; Abe, H.; Scala, G.; Ortaldo, J.; Oppenheim, J. Production of interleukin 1 activity by normal human peripheral blood B lymphocytes. J. Immunol. 1985, 135, 1132–1136. [Google Scholar] [PubMed]
- Haas, K.; Estes, D. Activation of bovine B cells via surface immunoglobulin M cross-linking or CD40 ligation results in different B-cell phenotypes. Immunology 2000, 99, 272–278. [Google Scholar] [CrossRef] [PubMed]
- De Grandmont, M.J.; Racine, C.; Roy, A.; Lemieux, R.; Néron, S. Intravenous immunoglobulins induce the in vitro differentiation of human B lymphocytes and the secretion of IgG. Blood 2003, 101, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Olobo, J.; Black, S. In vitro secretion of bovine immunoglobulins during pokeweed mitogen or pokeweed mitogen and antigen activation of lymphocytes. Vet. Res. Commun. 1989, 13, 193–197. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).