Satellite DNA at the Centromere Is Dispensable for Segregation Fidelity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Line and Metaphase Spread Preparation
2.2. Drug Treatment and Cytokinesis-Blok
2.3. BAC Extraction and FISH
3. Results and Discussion
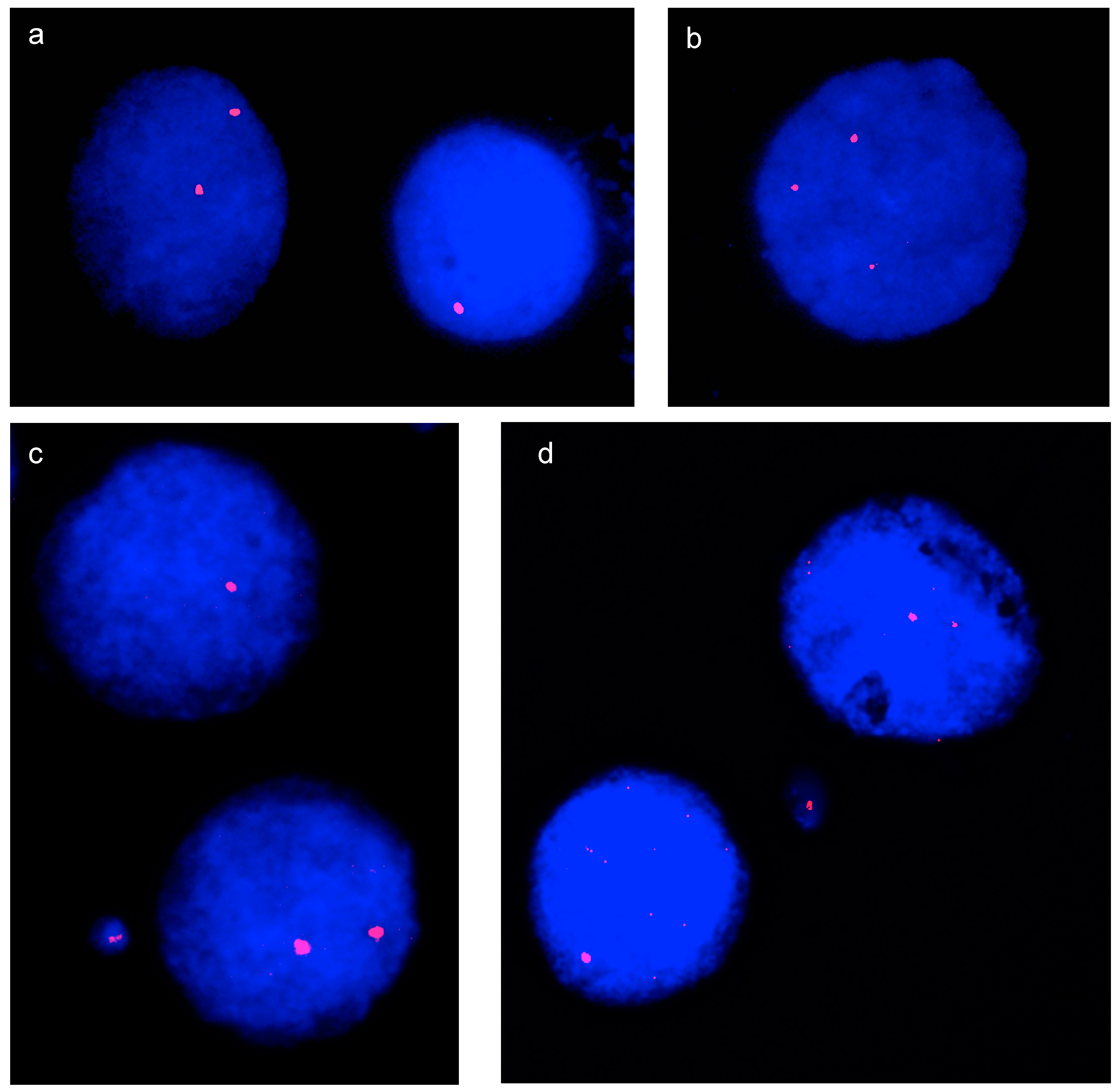
3.1. Interphase Aneuploidy Analysis
3.2. Micronucleus Assay and Cytokinesis-Block MicroNucleus (CBMN) Assay
3.3. Chromatid Cohesion Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plohl, M.; Luchetti, A.; Mestrovic, N.; Mantovani, B. Satellite DNAs between selfishness and functionality: Structure, genomics and evolution of tandem repeats in centromeric (hetero) chromatin. Gene 2008, 409, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Plohl, M.; Mestrovic, N.; Mravinac, B. Centromere identity from the DNA point of view. Chromosoma 2014, 123, 313–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voullaire, L.E.; Slater, H.R.; Petrovic, V.; Choo, K.H. A functional marker centromere with no detectable alpha-satellite, satellite III, or CENP-B protein: Activation of a latent centromere? Am. J. Hum. Genet. 1993, 52, 1153–1163. [Google Scholar] [PubMed]
- Choo, K.H. Centromere DNA dynamics: Latent centromeres and neocentromere formation. Am. J. Hum. Genet. 1997, 61, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Marshall, O.J.; Chueh, A.C.; Wong, L.H.; Choo, K.H. Neocentromeres: New insights into centromere structure, disease development, and karyotype evolution. Am. J. Hum. Genet. 2008, 82, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Wade, C.M.; Giulotto, E.; Sigurdsson, S.; Zoli, M.; Gnerre, S.; Imsland, F.; Lear, T.L.; Adelson, D.L.; Bailey, E.; Bellone, R.R.; et al. Genome sequence, comparative analysis, and population genetics of the domestic horse. Science 2009, 326, 865–867. [Google Scholar] [CrossRef]
- Shang, W.H.; Hori, T.; Toyoda, A.; Kato, J.; Popendorf, K.; Sakakibara, Y.; Fujiyama, A.; Fukagawa, T. Chickens possess centromeres with both extended tandem repeats and short non-tandem-repetitive sequences. Genome Res. 2010, 20, 1219–1228. [Google Scholar] [CrossRef] [Green Version]
- Locke, D.P.; Hillier, L.W.; Warren, W.C.; Worley, K.C.; Nazareth, L.V.; Muzny, D.M.; Yang, S.P.; Wang, Z.; Chinwalla, A.T.; Minx, P.; et al. Comparative and demographic analysis of orang-utan genomes. Nature 2011, 469, 529–533. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.; Wu, Y.; Koblizkova, A.; Torres, G.A.; Wang, K.; Iovene, M.; Neumann, P.; Zhang, W.; Novak, P.; Buell, C.R.; et al. Repeatless and repeat-based centromeres in potato: Implications for centromere evolution. Plant Cell 2012, 24, 3559–3574. [Google Scholar] [CrossRef]
- Giulotto, E.; Raimondi, E.; Sullivan, K.F. The Unique DNA Sequences Underlying Equine Centromeres. Prog. Mol. Subcell. Biol. 2017, 56, 337–354. [Google Scholar] [CrossRef]
- Quenet, D.; Dalal, Y. A long non-coding RNA is required for targeting centromeric protein A to the human centromere. Elife 2014, 3, e03254. [Google Scholar] [CrossRef] [PubMed]
- Rosic, S.; Kohler, F.; Erhardt, S. Repetitive centromeric satellite RNA is essential for kinetochore formation and cell division. J. Cell Biol. 2014, 207, 335–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biscotti, M.A.; Canapa, A.; Forconi, M.; Olmo, E.; Barucca, M. Transcription of tandemly repetitive DNA: Functional roles. Chromosome Res. 2015, 23, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, F.; Gamba, R.; Mazzagatti, A.; Piras, F.M.; Cappelletti, E.; Belloni, E.; Nergadze, S.G.; Raimondi, E.; Giulotto, E. The major horse satellite DNA family is associated with centromere competence. Mol. Cytogenet. 2016, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, H.; Masukata, H.; Muro, Y.; Nozaki, N.; Okazaki, T. A human centromere antigen (CENP-B) interacts with a short specific sequence in alphoid DNA, a human centromeric satellite. J. Cell Biol. 1989, 109, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Miga, K.H.; Newton, Y.; Jain, M.; Altemose, N.; Willard, H.F.; Kent, W.J. Centromere reference models for human chromosomes X and Y satellite arrays. Genome Res. 2014, 24, 697–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fachinetti, D.; Han, J.S.; McMahon, M.A.; Ly, P.; Abdullah, A.; Wong, A.J.; Cleveland, D.W. DNA Sequence-Specific Binding of CENP-B Enhances the Fidelity of Human Centromere Function. Dev. Cell 2015, 33, 314–327. [Google Scholar] [CrossRef] [Green Version]
- Drinnenberg, I.A.; Henikoff, S.; Malik, H.S. Evolutionary Turnover of Kinetochore Proteins: A Ship of Theseus? Trends Cell. Biol. 2016, 26, 498–510. [Google Scholar] [CrossRef] [Green Version]
- Farr, C.J.; Bayne, R.A.; Kipling, D.; Mills, W.; Critcher, R.; Cooke, H.J. Generation of a human X-derived minichromosome using telomere-associated chromosome fragmentation. Embo J. 1995, 14, 5444–5454. [Google Scholar] [CrossRef]
- Heller, R.; Brown, K.E.; Burgtorf, C.; Brown, W.R. Mini-chromosomes derived from the human Y chromosome by telomere directed chromosome breakage. Proc. Natl. Acad. Sci. USA 1996, 93, 7125–7130. [Google Scholar] [CrossRef]
- Raimondi, E.; Balzaretti, M.; Moralli, D.; Vagnarelli, P.; Tredici, F.; Bensi, M.; De Carli, L. Gene targeting to the centromeric DNA of a human minichromosome. Hum. Gene Ther. 1996, 7, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.J.; Van Bokkelen, G.; Mays, R.W.; Gustashaw, K.; Willard, H.F. Formation of de novo centromeres and construction of first-generation human artificial microchromosomes. Nat. Genet. 1997, 15, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, H.; Nakano, M.; Ohzeki, J. The role of CENP-B and alpha-satellite DNA: De novo assembly and epigenetic maintenance of human centromeres. Chromosome Res. 2004, 12, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Henikoff, J.G.; Thakur, J.; Kasinathan, S.; Henikoff, S. A unique chromatin complex occupies young alpha-satellite arrays of human centromeres. Sci. Adv. 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Cardinale, S.; Noskov, V.N.; Gassmann, R.; Vagnarelli, P.; Kandels-Lewis, S.; Larionov, V.; Earnshaw, W.C.; Masumoto, H. Inactivation of a human kinetochore by specific targeting of chromatin modifiers. Dev. Cell 2008, 14, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Ohzeki, J.; Bergmann, J.H.; Kouprina, N.; Noskov, V.N.; Nakano, M.; Kimura, H.; Earnshaw, W.C.; Larionov, V.; Masumoto, H. Breaking the HAC Barrier: Histone H3K9 acetyl/methyl balance regulates CENP-A assembly. Embo J. 2012, 31, 2391–2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, O.; Vargiu, G.; Abad, M.A.; Zhiteneva, A.; Jeyaprakash, A.A.; Masumoto, H.; Kouprina, N.; Larionov, V.; Earnshaw, W.C. Epigenetic engineering reveals a balance between histone modifications and transcription in kinetochore maintenance. Nat. Commun. 2016, 7, 13334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, B.A.; Karpen, G.H. Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat. Struct. Mol. Biol. 2004, 11, 1076–1083. [Google Scholar] [CrossRef]
- Piras, F.M.; Nergadze, S.G.; Poletto, V.; Cerutti, F.; Ryder, O.A.; Leeb, T.; Raimondi, E.; Giulotto, E. Phylogeny of horse chromosome 5q in the genus Equus and centromere repositioning. Cytogenet. Genome Res. 2009, 126, 165–172. [Google Scholar] [CrossRef]
- Piras, F.M.; Nergadze, S.G.; Magnani, E.; Bertoni, L.; Attolini, C.; Khoriauli, L.; Raimondi, E.; Giulotto, E. Uncoupling of satellite DNA and centromeric function in the genus Equus. PLoS Genet. 2010, 6, e1000845. [Google Scholar] [CrossRef]
- Raimondi, E.; Piras, F.M.; Nergadze, S.G.; Di Meo, G.P.; Ruiz-Herrera, A.; Ponsa, M.; Ianuzzi, L.; Giulotto, E. Polymorphic organization of constitutive heterochromatin in Equus asinus (2n = 62) chromosome 1. Hereditas 2011, 148, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Nergadze, S.G.; Belloni, E.; Piras, F.M.; Khoriauli, L.; Mazzagatti, A.; Vella, F.; Bensi, M.; Vitelli, V.; Giulotto, E.; Raimondi, E. Discovery and comparative analysis of a novel satellite, EC137, in horses and other equids. Cytogenet. Genome Res. 2014, 144, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Purgato, S.; Belloni, E.; Piras, F.M.; Zoli, M.; Badiale, C.; Cerutti, F.; Mazzagatti, A.; Perini, G.; Della Valle, G.; Nergadze, S.G.; et al. Centromere sliding on a mammalian chromosome. Chromosoma 2015, 124, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Nergadze, S.G.; Piras, F.M.; Gamba, R.; Corbo, M.; Cerutti, F.; McCarter, J.G.W.; Cappelletti, E.; Gozzo, F.; Harman, R.M.; Antczak, D.F.; et al. Birth, evolution, and transmission of satellite-free mammalian centromeric domains. Genome Res. 2018, 28, 789–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montefalcone, G.; Tempesta, S.; Rocchi, M.; Archidiacono, N. Centromere repositioning. Genome Res. 1999, 9, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Nergadze, S.G.; Magnani, E.; Misceo, D.; Francesca Cardone, M.; Roberto, R.; Bertoni, L.; Attolini, C.; Francesca Piras, M.; de Jong, P.; et al. Evolutionary movement of centromeres in horse, donkey, and zebra. Genomics 2006, 87, 777–782. [Google Scholar] [CrossRef] [Green Version]
- Faas, B.H.; Cirigliano, V.; Bui, T.H. Rapid methods for targeted prenatal diagnosis of common chromosome aneuploidies. Semin. Fetal Neonatal Med. 2011, 16, 81–87. [Google Scholar] [CrossRef]
- Leeb, T.; Vogl, C.; Zhu, B.; de Jong, P.J.; Binns, M.M.; Chowdhary, B.P.; Scharfe, M.; Jarek, M.; Nordsiek, G.; Schrader, F.; et al. A human-horse comparative map based on equine BAC end sequences. Genomics 2006, 87, 772–776. [Google Scholar] [CrossRef]
- Kirsch-Volders, M.; Elhajouji, A.; Cundari, E.; Van Hummelen, P. The in vitro micronucleus test: A multi-endpoint assay to detect simultaneously mitotic delay, apoptosis, chromosome breakage, chromosome loss and non-disjunction. Mutat. Res. 1997, 392, 19–30. [Google Scholar] [CrossRef]
- Fenech, M. The in vitro micronucleus technique. Mutat. Res. 2000, 455, 81–95. [Google Scholar] [CrossRef]
- Rudd, N.L.; Hoar, D.I.; Williams, S.E.; Hennig, U.G. Genotype and the cryopreservation process affect the levels of aneuploidy and chromosome breakage in cultured human fibroblasts. Genome 1989, 32, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Preuss, U.; Roser, M.; Weichenthal, M.; Rudiger, H.W. Elevated frequencies of micronuclei in cultured fibroblasts after freezing and thawing. Mutat. Res. 1990, 241, 279–282. [Google Scholar] [CrossRef]
- Kirsch-Volders, M.; Sofuni, T.; Aardema, M.; Albertini, S.; Eastmond, D.; Fenech, M.; Ishidate, M.; Kirchner, S.; Lorge, E.; Morita, T.; et al. Report from the in vitro micronucleus assay working group. Mutat. Res. 2003, 540, 153–163. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Vader, G.; Vromans, M.J.; Lampson, M.A.; Lens, S.M. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science 2009, 323, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Bassett, E.A.; Wood, S.; Salimian, K.J.; Ajith, S.; Foltz, D.R.; Black, B.E. Epigenetic centromere specification directs aurora B accumulation but is insufficient to efficiently correct mitotic errors. J. Cell Biol. 2010, 190, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Shang, W.H.; Hori, T.; Martins, N.M.; Toyoda, A.; Misu, S.; Monma, N.; Hiratani, I.; Maeshima, K.; Ikeo, K.; Fujiyama, A.; et al. Chromosome engineering allows the efficient isolation of vertebrate neocentromeres. Dev. Cell 2013, 24, 635–648. [Google Scholar] [CrossRef]
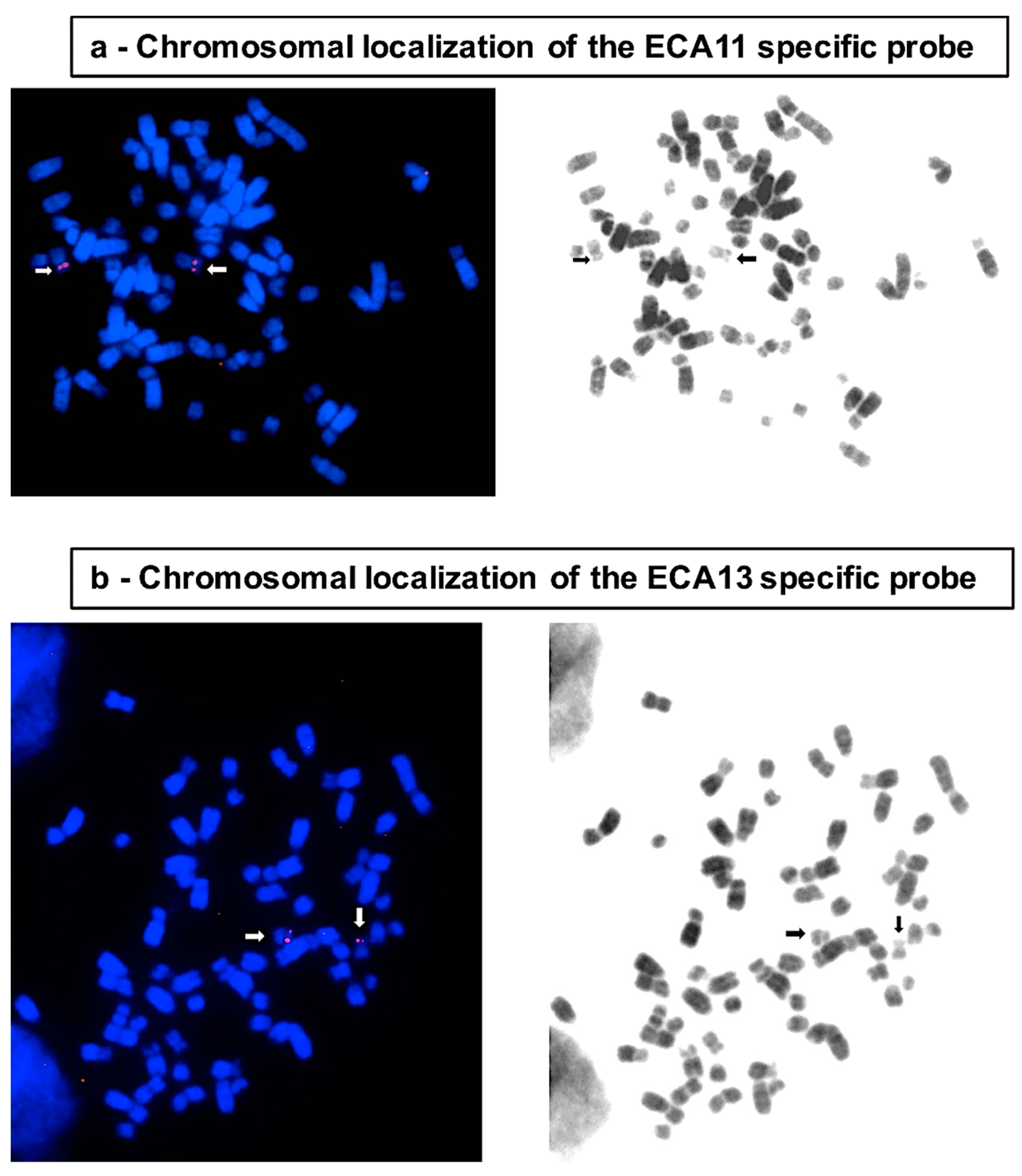
| Chromosome | Total Number of Nuclei | Diploid Nuclei (%) | Aneuploid Nuclei (% ± SE) | Type of Aneuploidy | Number (%) |
|---|---|---|---|---|---|
| ECA11 | 4000 | 3924 (98,10) | 76 (1.90 ± 0.196) | nullisomy | 5 (0,12) |
| monosomy | 30 (0,75) | ||||
| trisomy | 41 (1,02) | ||||
| ECA13 | 4000 | 3921 (98,03) | 79 (1.98 ± 0.329) | nullisomy | 8 (0,20) |
| monosomy | 32 (0,80) | ||||
| trisomy | 39 (0,98) |
| Chromosome | Total Number of Nuclei | Diploid Nuclei (%) | Aneuploid Nuclei (% ± SE) | Type of Aneuploidy | Number (%) |
|---|---|---|---|---|---|
| Control | |||||
| ECA11 | 1107 | 1084 (97,92) | 23 (2.08 ± 0.014) | nullisomy | 0 |
| monosomy | 11 (0,99) | ||||
| trisomy | 12 (1,08) | ||||
| ECA13 | 1117 | 1098 (98,30) | 19 (1.70 ± 0.057) | nullisomy | 0 |
| monosomy | 11 (0,98) | ||||
| trisomy | 8 (0,72) | ||||
| Nocodazole 100 nM | |||||
| ECA11 | 1045 | 1000 (95,70) | 45 (4.31 ± 0.085) | nullisomy | 0 |
| monosomy | 13 (1,24) | ||||
| trisomy | 32 (3,01) | ||||
| ECA13 | 1023 | 976 (95,40) | 47 (4.60 ± 0.042) | nullisomy | 0 |
| monosomy | 12 (1,17) | ||||
| trisomy | 35 (3,42) | ||||
| Chromosome | Total Number of Nuclei | Total Number of Micronuclei (% ± SE) | Micronuclei Containing the Chromosome (% ± SE) | Micronuclei not Containing the Chromosome (%) |
|---|---|---|---|---|
| ECA11 | 4000 | 63 (1.6 ± 0.098) | 7 (11.1 ± 1.143) | 56 (88,9) |
| ECA13 | 4000 | 55 (1.4 ± 0.127) | 6 (10.9 ± 2.390) | 49 (89,1) |
| Chromosome | Total Number of BN Cells | Total Number of Micronuclei (% ± SE) | Micronuclei Containing the Chromosome (% ± SE) | Micronuclei not Containing the Chromosome (%) |
|---|---|---|---|---|
| Control | ||||
| ECA11 | 2000 | 31 (1.6 ± 0.002) | 6 (19.4 ± 0.019) | 25 (80,6) |
| ECA13 | 2000 | 32 (1.6 ± 0.000) | 6 (18.8 ± 0.000) | 26 (81,2) |
| Nocodazole 100 nM | ||||
| ECA11 | 2000 | 75 (3.8 ± 0.004) | 11 (14.7 ± 0.0004) | 64 (85,3) |
| ECA13 | 2000 | 65 (3.3 ± 0.009) | 10 (15.4 ± 0.0180) | 55 (84,6) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberti, A.; Bensi, M.; Mazzagatti, A.; Piras, F.M.; Nergadze, S.G.; Giulotto, E.; Raimondi, E. Satellite DNA at the Centromere Is Dispensable for Segregation Fidelity. Genes 2019, 10, 469. https://doi.org/10.3390/genes10060469
Roberti A, Bensi M, Mazzagatti A, Piras FM, Nergadze SG, Giulotto E, Raimondi E. Satellite DNA at the Centromere Is Dispensable for Segregation Fidelity. Genes. 2019; 10(6):469. https://doi.org/10.3390/genes10060469
Chicago/Turabian StyleRoberti, Annalisa, Mirella Bensi, Alice Mazzagatti, Francesca M. Piras, Solomon G. Nergadze, Elena Giulotto, and Elena Raimondi. 2019. "Satellite DNA at the Centromere Is Dispensable for Segregation Fidelity" Genes 10, no. 6: 469. https://doi.org/10.3390/genes10060469
APA StyleRoberti, A., Bensi, M., Mazzagatti, A., Piras, F. M., Nergadze, S. G., Giulotto, E., & Raimondi, E. (2019). Satellite DNA at the Centromere Is Dispensable for Segregation Fidelity. Genes, 10(6), 469. https://doi.org/10.3390/genes10060469







