The Role of the HOXA Gene Family in Acute Myeloid Leukemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. ONCOMINE Database Analysis
2.3. The Gene Expression Profiling Interactive Analysis (GEPIA) Dataset
2.4. The LinkedOmics Dataset
2.5. TCGA Data and the cBioPortal
2.6. Functional Enrichment and Bioinformatics Analysis
3. Results
3.1. The Transcriptional Expression of HOXA Genes in Leukemia Patients
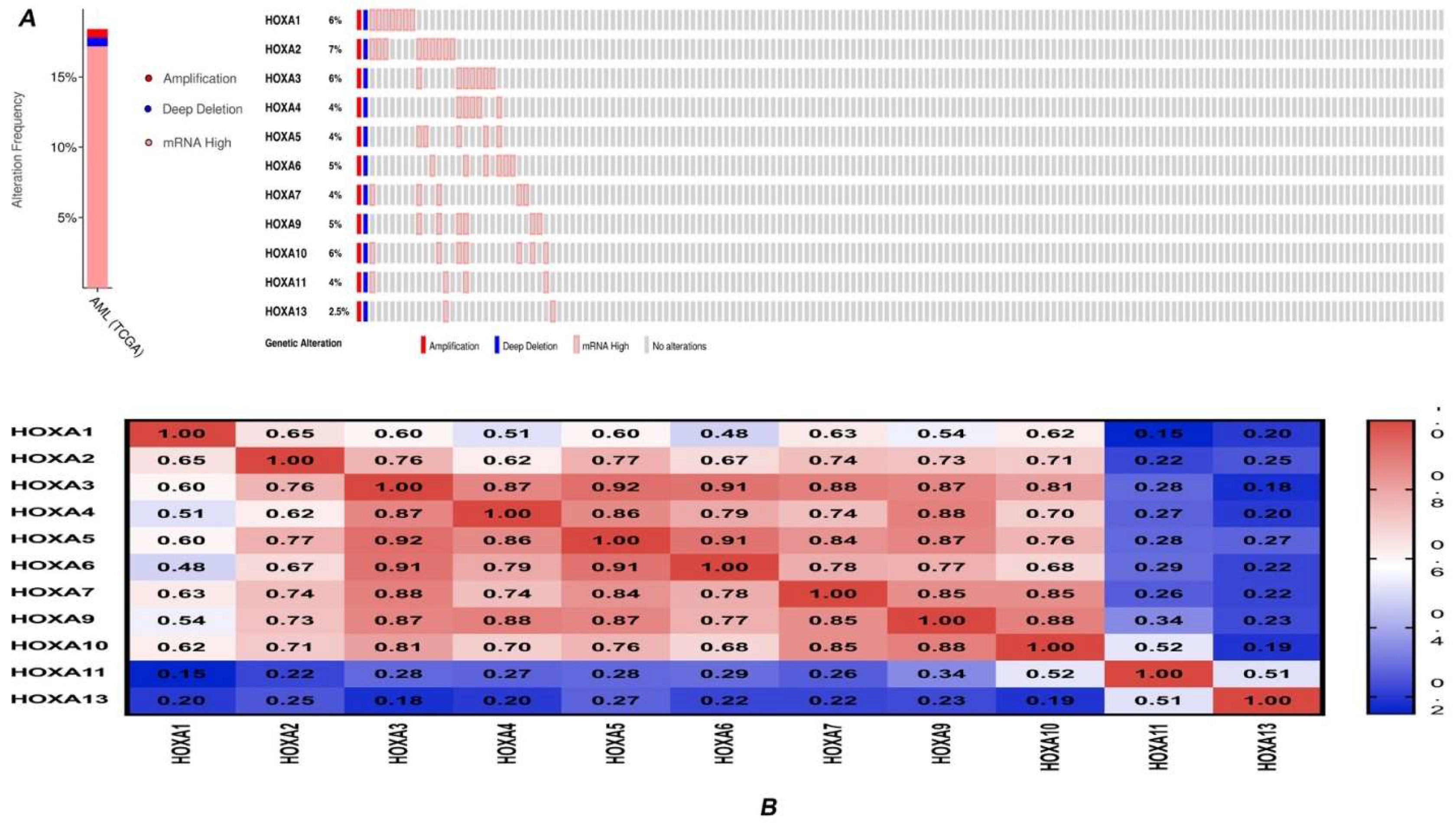
3.2. Genetic Alteration and Correlations of HOXA Genes in AML
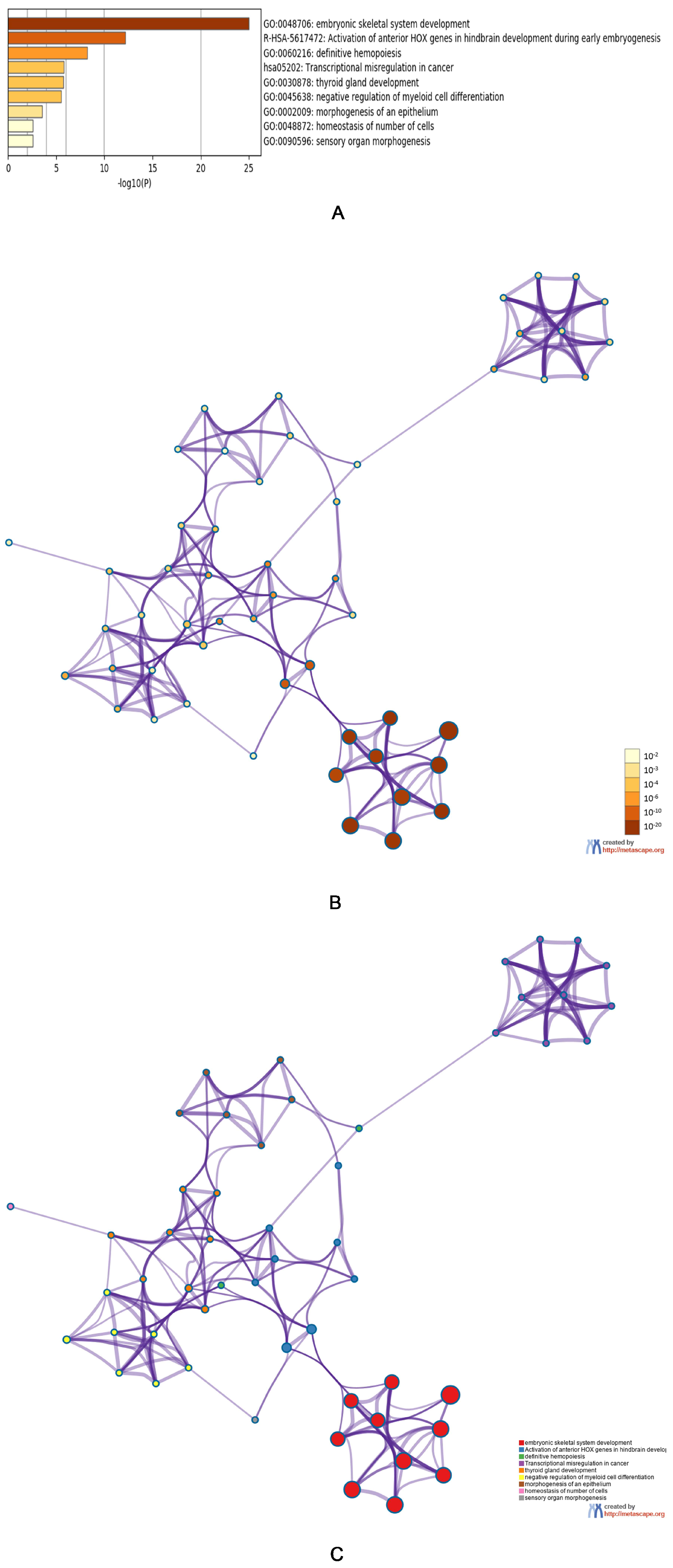
3.3. Predicted Functions and Pathway Enrichment Analysis of HOXA Genes in AML Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tallman, M.S.; Wang, E.S.; Altman, J.K.; Appelbaum, F.R.; Bhatt, V.R.; Bixby, D.; Coutre, S.E.; De Lima, M.; Fathi, A.T.; Fiorella, M.; et al. Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 721–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Pulte, D.; Redaniel, M.T.; Jansen, L.; Brenner, H.; Jeffreys, M. Recent trends in survival of adult patients with acute leukemia: Overall improvements, but persistent and partly increasing disparity in survival of patients from minority groups. Haematologica 2013, 98, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Howlader, A.M.N.; Krapcho, D.; Miller, A.; Brest, M.; Yu, J.; Ruhl, Z.; Tatalovich, A.; Mariotto, D.R.; Lewis, H.S.; Chen, E.J.; et al. SEER Cancer Statistics Review, 1975–2016; National Cancer Institute: Bethesda, MD, USA, 2019. Available online: https://seer.cancer.gov/csr/1975_2016 (accessed on 16 July 2019).
- Patel, J.P.; Gonen, M.; Figueroa, M.E.; Fernandez, H.; Sun, Z.; Racevskis, J.; Van Vlierberghe, P.; Dolgalev, I.; Thomas, S.; Aminova, O.; et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N. Engl. J. Med. 2012, 366, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Rezsohazy, R.; Saurin, A.J.; Maurel-Zaffran, C.; Graba, Y. Cellular and molecular insights into HOX protein action. Development 2015, 142, 1212–1227. [Google Scholar] [CrossRef] [PubMed]
- Dunwell, T.L.; Holland, P.W. Diversity of human and mouse homeobox gene expression in development and adult tissues. BMC Dev. Biol. 2016, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Rux, D.R.; Wellik, D.M. HOX genes in the adult skeleton: Novel functions beyond embryonic development. Dev. Dyn. 2017, 246, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, R.A.; Pandha, H.S.; Simpson, G.R.; Pettengell, R.; Poterlowicz, K.; Thompson, A.; Harrington, K.; El-Tanani, M.; Morgan, R. Inhibition of HOX/PBX dimer formation leads to necroptosis in acute myeloid leukemia cells. Oncotarget 2017, 8, 89566–89579. [Google Scholar] [CrossRef]
- Kachgal, S.; Mace, K.A.; Boudreau, N.J. The dual roles of homeobox genes in vascularization and wound healing. Cell Adhes. Migr. 2012, 6, 457–470. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Taylor, H.S. The Role of HOX Genes in Female Reproductive Tract Development, Adult Function, and Fertility. Cold Spring Harb. Perspect. Med. 2015, 6, a023002. [Google Scholar]
- Ernst, P.; Mabon, M.; Davidson, A.J.; Zon, L.I.; Korsmeyer, S.J. An Mll-dependent HOX program drives hematopoietic progenitor expansion. Curr. Biol. 2004, 14, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Milne, T.A.; Martin, M.E.; Brock, H.W.; Slany, R.K.; Hess, J.L. Leukemogenic MLL fusion proteins bind across a broad region of the HOX a9 locus, promoting transcription and multiple histone modifications. Cancer Res. 2005, 65, 11367–11374. [Google Scholar] [CrossRef] [PubMed]
- Dorrance, A.M.; Liu, S.; Yuan, W.; Becknell, B.; Arnoczky, K.J.; Guimond, M.; Strout, M.P.; Feng, L.; Nakamura, T.; Yu, L.; et al. Mll partial tandem duplication induces aberrant HOX expression in vivo via specific epigenetic alterations. J. Clin. Investig. 2006, 116, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Andreeff, M.; Ruvolo, V.; Gadgil, S.; Zeng, C.; Coombes, K.; Chen, W.; Kornblau, S.; Baron, A.E.; Drabkin, H.A. HOX expression patterns identify a common signature for favorable AML. Leukemia 2008, 22, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Quinn, M.F.; Grimwade, D.; O’Neill, C.M.; Ahmed, M.R.; Grimes, S.; McMullin, M.F.; Cotter, F.; Lappin, T.R. Global down-regulation of HOX gene expression in PML-RARalpha acute promyelocytic leukemia identified by small-array real-time PCR. Blood 2003, 101, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.; Schneider, F.; Metzeler, K.H.; Hoster, E.; Schneider, S.; Zellmeier, E.; Benthaus, T.; Sauerland, M.C.; Berdel, W.E.; Buchner, T.; et al. Acute myeloid leukemia with biallelic CEBPA gene mutations and normal karyotype represents a distinct genetic entity associated with a favorable clinical outcome. J. Clin. Oncol. 2010, 28, 570–577. [Google Scholar] [CrossRef]
- Rhodes, D.R.; Yu, J.; Shanker, K.; Deshpande, N.; Varambally, R.; Ghosh, D.; Barrette, T.; Pandey, A.; Chinnaiyan, A.M. Oncomine: A cancer microarray database and integrated data-mining platform. Neoplasia 2004, 6, 1–6. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. Gepia: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Valk, P.J.; Verhaak, R.G.; Beijen, M.A.; Erpelinck, C.A.; Barjesteh van Waalwijk van Doorn-Khosrovani, S.; Boer, J.M.; Beverloo, H.B.; Moorhouse, M.J.; van der Spek, P.J.; Lowenberg, B.; et al. Prognostically useful gene-expression profiles in acute myeloid leukemia. N. Engl. J. Med. 2004, 350, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.L.; Tsukasaki, K.; O’Neill, M.C.; Yamada, Y.; Onimaru, Y.; Matsumoto, K.; Ohashi, J.; Yamashita, Y.; Tsutsumi, S.; Kaneda, R.; et al. A genomic analysis of adult T-cell leukemia. Oncogene 2007, 26, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Haferlach, T.; Kohlmann, A.; Wieczorek, L.; Basso, G.; Kronnie, G.T.; Bene, M.C.; De Vos, J.; Hernandez, J.M.; Hofmann, W.K.; Mills, K.I.; et al. Clinical utility of microarray-based gene expression profiling in the diagnosis and subclassification of leukemia: Report from the International Microarray Innovations in Leukemia Study Group. J. Clin. Oncol. 2010, 28, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Ritz, C.; Lindgren, D.; Eden, P.; Lassen, C.; Heldrup, J.; Olofsson, T.; Rade, J.; Fontes, M.; Porwit-Macdonald, A.; et al. Microarray-based classification of a consecutive series of 121 childhood acute leukemias: Prediction of leukemic and genetic subtype as well as of minimal residual disease status. Leukemia 2007, 21, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Stegmaier, K.; Ross, K.N.; Colavito, S.A.; O’Malley, S.; Stockwell, B.R.; Golub, T.R. Gene expression-based high-throughput screening (GE-HTS) and application to leukemia differentiation. Nat. Genet. 2004, 36, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Argiropoulos, B.; Humphries, R.K. HOX genes in hematopoiesis and leukemogenesis. Oncogene 2007, 26, 6766–6776. [Google Scholar] [CrossRef] [PubMed]
- Kurscheid, S.; Bady, P.; Sciuscio, D.; Samarzija, I.; Shay, T.; Vassallo, I.; Criekinge, W.V.; Daniel, R.T.; van den Bent, M.J.; Marosi, C.; et al. Chromosome 7 gain and DNA hypermethylation at the HOXA10 locus are associated with expression of a stem cell related HOX-signature in glioblastoma. Genome Biol. 2015, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Quagliata, L.; Matter, M.S.; Piscuoglio, S.; Arabi, L.; Ruiz, C.; Procino, A.; Kovac, M.; Moretti, F.; Makowska, Z.; Boldanova, T.; et al. Long noncoding RNA HOTTIP/HOXA13 expression is associated with disease progression and predicts outcome in hepatocellular carcinoma patients. Hepatology 2014, 59, 911–923. [Google Scholar] [CrossRef]
- Luo, Z.; Rhie, S.K.; Lay, F.D.; Farnham, P.J. A Prostate Cancer Risk Element Functions as a Repressive Loop that Regulates HOXA13. Cell Rep. 2017, 21, 1411–1417. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.C.; Wang, S.S.W.; Liu, C.J.; Wuputra, K.; Kato, K.; Lee, Y.L.; Lin, Y.C.; Tsai, M.H.; Ku, C.C.; Lin, W.H.; et al. Reprogramming Antagonizes the Oncogenicity of HOXA13-Long Noncoding RNA HOTTIP Axis in Gastric Cancer Cells. Stem Cells 2017, 35, 2115–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, E.J.; Permuth-Wey, J.; Li, Y.; Chen, Y.A.; Coppola, D.; Reid, B.M.; Lin, H.Y.; Teer, J.K.; Berchuck, A.; Birrer, M.J.; et al. A functional variant in HOXA11-AS, a novel long non-coding RNA, inhibits the oncogenic phenotype of epithelial ovarian cancer. Oncotarget 2015, 6, 34745–34757. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Song, C.X.; Huang, H.; Frankenberger, C.A.; Sankarasharma, D.; Gomes, S.; Chen, P.; Chen, J.; Chada, K.K.; He, C.; et al. HMGA2/TET1/HOXA9 signaling pathway regulates breast cancer growth and metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 9920–9925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shima, Y.; Yumoto, M.; Katsumoto, T.; Kitabayashi, I. MLL is essential for NUP98-HOXA9-induced leukemia. Leukemia 2017, 31, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Spencer, D.H.; Young, M.A.; Lamprecht, T.L.; Helton, N.M.; Fulton, R.; O’Laughlin, M.; Fronick, C.; Magrini, V.; Demeter, R.T.; Miller, C.A.; et al. Epigenomic analysis of the HOX gene loci reveals mechanisms that may control canonical expression patterns in AML and normal hematopoietic cells. Leukemia 2015, 29, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, H.; Li, Y.; Jiang, X.; Chen, P.; Arnovitz, S.; Radmacher, M.D.; Maharry, K.; Elkahloun, A.; Yang, X.; et al. Up-regulation of a HOXA-PBX3 homeobox-gene signature following down-regulation of miR-181 is associated with adverse prognosis in patients with cytogenetically abnormal AML. Blood 2012, 119, 2314–2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kok, C.H.; Brown, A.L.; Ekert, P.G.; D’Andrea, R.J. Gene expression analysis reveals HOX gene upregulation in trisomy 8 AML. Leukemia 2010, 24, 1239–1243. [Google Scholar] [CrossRef]
- Drabkin, H.A.; Parsy, C.; Ferguson, K.; Guilhot, F.; Lacotte, L.; Roy, L.; Zeng, C.; Baron, A.; Hunger, S.P.; Varella-Garcia, M.; et al. Quantitative HOX expression in chromosomally defined subsets of acute myelogenous leukemia. Leukemia 2002, 16, 186–195. [Google Scholar] [CrossRef]
- Grubach, L.; Juhl-Christensen, C.; Rethmeier, A.; Olesen, L.H.; Aggerholm, A.; Hokland, P.; Ostergaard, M. Gene expression profiling of Polycomb, HOX and Meis genes in patients with acute myeloid leukaemia. Eur. J. Haematol. 2008, 81, 112–122. [Google Scholar] [CrossRef]
- Xu, X.; Nagel, S.; Quentmeier, H.; Wang, Z.; Pommerenke, C.; Dirks, W.G.; Macleod, R.A.F.; Drexler, H.G.; Hu, Z. KDM3B shows tumor-suppressive activity and transcriptionally regulates HOXA1 through retinoic acid response elements in acute myeloid leukemia. Leuk. Lymphoma 2018, 59, 204–213. [Google Scholar] [CrossRef]
- Li, N.; Jia, X.; Wang, J.; Li, Y.; Xie, S. Knockdown of homeobox A5 by small hairpin RNA inhibits proliferation and enhances cytarabine chemosensitivity of acute myeloid leukemia cells. Mol. Med. Rep. 2015, 12, 6861–6866. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Jiang, Q.; Lemieux, M.; Jeannotte, L.; Su, L.; Zhang, Y. Leukaemic transformation by CALM-AF10 involves upregulation of Hoxa5 by hDOT1L. Nat. Cell Biol. 2006, 8, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Dickson, G.J.; Liberante, F.G.; Kettyle, L.M.; O’Hagan, K.A.; Finnegan, D.P.; Bullinger, L.; Geerts, D.; McMullin, M.F.; Lappin, T.R.; Mills, K.I.; et al. HOXA/PBX3 knockdown impairs growth and sensitizes cytogenetically normal acute myeloid leukemia cells to chemotherapy. Haematologica 2013, 98, 1216–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohr, S.; Doebele, C.; Comoglio, F.; Berg, T.; Beck, J.; Bohnenberger, H.; Alexe, G.; Corso, J.; Strobel, P.; Wachter, A.; et al. Hoxa9 and Meis1 Cooperatively Induce Addiction to Syk Signaling by Suppressing miR-146a in Acute Myeloid Leukemia. Cancer Cell 2017, 31, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.; Krivtsov, A.V.; Stubbs, M.C.; Wright, R.; Davis, T.N.; van den Heuvel-Eibrink, M.; Zwaan, C.M.; Kung, A.L.; Armstrong, S.A. HOXA9 is required for survival in human MLL-rearranged acute leukemias. Blood 2009, 113, 2375–2385. [Google Scholar] [CrossRef] [Green Version]
- Ogawara, Y.; Katsumoto, T.; Aikawa, Y.; Shima, Y.; Kagiyama, Y.; Soga, T.; Matsunaga, H.; Seki, T.; Araki, K.; Kitabayashi, I. IDH2 and NPM1 Mutations Cooperate to Activate Hoxa9/Meis1 and Hypoxia Pathways in Acute Myeloid Leukemia. Cancer Res. 2015, 75, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, Y.; Fujita, N.; Taki, T.; Hayashi, Y.; Hamamoto, K. Juvenile myelomonocytic leukemia with t(7;11)(p15;p15) and NUP98-HOXA11 fusion. Am. J. Hematol. 2009, 84, 295–297. [Google Scholar] [CrossRef]
- Taketani, T.; Taki, T.; Ono, R.; Kobayashi, Y.; Ida, K.; Hayashi, Y. The chromosome translocation t(7;11)(p15;p15) in acute myeloid leukemia results in fusion of the NUP98 gene with a HOXA cluster gene, HOXA13, but not HOXA9. Genes Chromosomes Cancer 2002, 34, 437–443. [Google Scholar] [CrossRef]
- Majeti, R. Monoclonal antibody therapy directed against human acute myeloid leukemia stem cells. Oncogene 2011, 30, 1009–1019. [Google Scholar] [CrossRef]
- Jung, N.; Dai, B.; Gentles, A.J.; Majeti, R.; Feinberg, A.P. An LSC epigenetic signature is largely mutation independent and implicates the HOXA cluster in AML pathogenesis. Nat. Commun. 2015, 6, 8489. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Ley, T.J.; Miller, C.; Ding, L.; Raphael, B.J.; Mungall, A.J.; Robertson, A.; Hoadley, K.; Triche, T.J., Jr.; Laird, P.W.; et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Gundry, M.C.; Sorcini, D.; Guzman, A.G.; Huang, Y.H.; Ramabadran, R.; Gionfriddo, I.; Mezzasoma, F.; Milano, F.; Nabet, B.; et al. Mutant NPM1 Maintains the Leukemic State through HOX Expression. Cancer Cell 2018, 34, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Gearhart, M.D.; Gery, S.; Shojaee, S.; Yang, H.; Sun, H.; Lin, D.C.; Bai, J.W.; Mead, M.; Zhao, Z.; et al. BCOR regulates myeloid cell proliferation and differentiation. Leukemia 2016, 30, 1155–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontro, M.; Kumar, A.; Majumder, M.M.; Eldfors, S.; Parsons, A.; Pemovska, T.; Saarela, J.; Yadav, B.; Malani, D.; Floisand, Y.; et al. HOX gene expression predicts response to BCL-2 inhibition in acute myeloid leukemia. Leukemia 2017, 31, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Jilg, S.; Reidel, V.; Muller-Thomas, C.; Konig, J.; Schauwecker, J.; Hockendorf, U.; Huberle, C.; Gorka, O.; Schmidt, B.; Burgkart, R.; et al. Blockade of BCL-2 proteins efficiently induces apoptosis in progenitor cells of high-risk myelodysplastic syndromes patients. Leukemia 2016, 30, 112–123. [Google Scholar] [CrossRef] [PubMed]



| Gene | Types of leukemia vs. Normal Samples | Fold Change | P Value | t-Test | Reference |
|---|---|---|---|---|---|
| HOXA1 | Acute Myeloid Leukemia vs. Normal | 3.451 | 1.95E-07 | 9.046 | Valk [23] |
| Acute Adult T-Cell Leukemia/Lymphoma vs. Normal | 10.084 | 3.46E-05 | 7.407 | Choi [24] | |
| HOXA2 | NA | NA | NA | NA | NA |
| HOXA3 | Acute Myeloid Leukemia vs. Normal | 2.230 | 2.48E-68 | 19.863 | Haferlach [25] |
| Pro-B Acute Lymphoblastic Leukemia vs. Normal | 3.975 | 8.57E-19 | 11.883 | Haferlach [25] | |
| HOXA4 | Acute Myeloid Leukemia vs. Normal | 8.083 | 4.21E-06 | 6.951 | Andersson [26] |
| Pro-B Acute Lymphoblastic Leukemia vs. Normal | 2.065 | 8.86E-16 | 10.006 | Haferlach [25] | |
| HOXA5 | Acute Myeloid Leukemia vs. Normal | 6.584 | 7.75E-05 | 5.292 | Stegmaier [27] |
| Acute Myeloid Leukemia vs. Normal | 2.522 | 2.78E-38 | 14.237 | Haferlach [25] | |
| Pro-B Acute Lymphoblastic Leukemia vs. Normal | 4.359 | 1.35E-15 | 9.826 | Haferlach [25] | |
| HOXA6 | Pro-B Acute Lymphoblastic Leukemia vs. Normal | 2.012 | 2.30E-14 | 9.174 | Haferlach [25] |
| HOXA7 | Acute Myeloid Leukemia vs. Normal | 2.027 | 1.40E-67 | 19.782 | Haferlach [25] |
| HOXA9 | Acute Myeloid Leukemia vs. Normal | 53.046 | 3.68E-07 | 9.445 | Stegmaier [27] |
| Acute Myeloid Leukemia vs. Normal | 2.483 | 2.88E-33 | 13.493 | Haferlach [25] | |
| Acute Myeloid Leukemia vs. Normal | 2.986 | 9.55E-58 | 17.841 | Haferlach [25] | |
| Pro-B Acute Lymphoblastic Leukemia vs. Normal | 7.209 | 1.32E-20 | 12.798 | Haferlach [25] | |
| HOXA10 | Acute Myeloid Leukemia vs. Normal | 8.483 | 8.82E-06 | 7.123 | Stegmaier [27] |
| Acute Adult T-Cell Leukemia/Lymphoma vs. Normal | 3.946 | 4.70E-05 | 4.888 | Choi [24] | |
| Acute Myeloid Leukemia vs. Normal | 2.174 | 1.30E-40 | 14.641 | Haferlach [25] | |
| Pro-B Acute Lymphoblastic Leukemia vs. Normal | 4.802 | 7.72E-19 | 11.645 | Haferlach [25] | |
| HOXA11 | NA | NA | NA | NA | NA |
| HOXA13 | NA | NA | NA | NA | NA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-L.; Qin, Z.-Y.; Hu, F.; Wang, Y.; Dai, Y.-J.; Liang, Y. The Role of the HOXA Gene Family in Acute Myeloid Leukemia. Genes 2019, 10, 621. https://doi.org/10.3390/genes10080621
Chen S-L, Qin Z-Y, Hu F, Wang Y, Dai Y-J, Liang Y. The Role of the HOXA Gene Family in Acute Myeloid Leukemia. Genes. 2019; 10(8):621. https://doi.org/10.3390/genes10080621
Chicago/Turabian StyleChen, Si-Liang, Zhe-Yuan Qin, Fang Hu, Yun Wang, Yu-Jun Dai, and Yang Liang. 2019. "The Role of the HOXA Gene Family in Acute Myeloid Leukemia" Genes 10, no. 8: 621. https://doi.org/10.3390/genes10080621
APA StyleChen, S.-L., Qin, Z.-Y., Hu, F., Wang, Y., Dai, Y.-J., & Liang, Y. (2019). The Role of the HOXA Gene Family in Acute Myeloid Leukemia. Genes, 10(8), 621. https://doi.org/10.3390/genes10080621





