Identification of Glyceraldehyde-3-Phosphate Dehydrogenase Gene as an Alternative Safe Harbor Locus in Pig Genome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids
2.2. Cell Culture and Transfection
2.3. T7EN1 Detection Assay and Sequencing
2.4. Fluorescence-Activated Cell Sorting (FACS)
2.5. Immunofluorescence Assay (IFA)
2.6. Western Blot Analysis
2.7. Off-Target Analysis of sgRNA
2.8. Cell Proliferation Assay
2.9. Quantitative RT-PCR (qPCR) and Statistical Analysis
3. Results
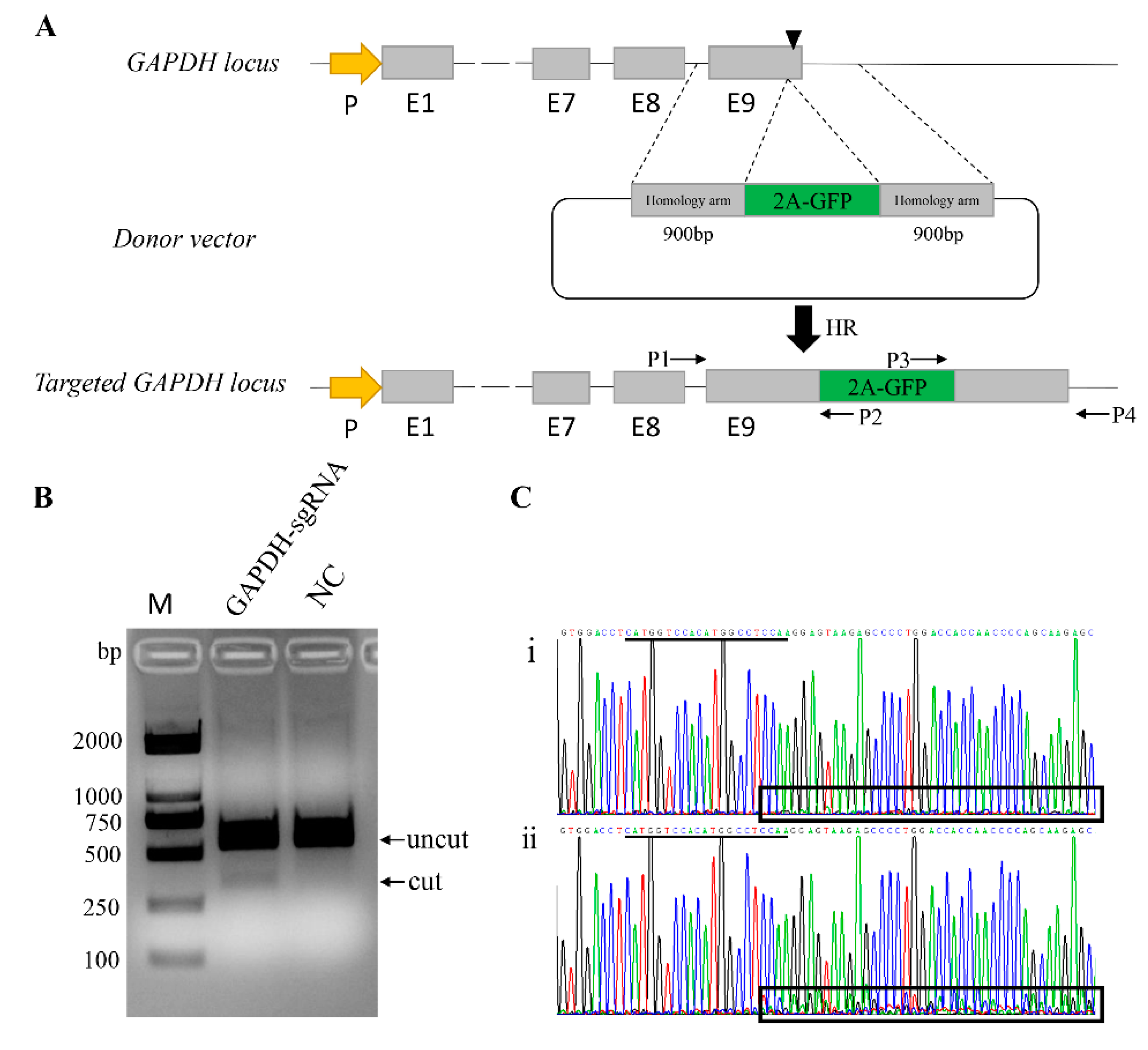
3.1. Generation of a Reporter System in Pig Genome
3.2. Quantification of HR-Mediated Knock-in in Various Pig Cells
3.3. Identification of the Protein Expression of GAPDH Gene
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Golovan, S.P.; Meidinger, R.G.; Ajakaiye, A.; Cottrill, M.; Wiederkehr, M.Z.; Barney, D.J.; Plante, C.; Pollard, J.W.; Fan, M.Z.; Hayes, M.A.; et al. Pigs expressing salivary phytase produce low-phosphorus manure. Nat. Biotechnol. 2001, 19, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.X.; Kang, J.X.; Li, R.F.; Wang, J.D.; Witt, W.T.; Yong, H.Y.; Hao, Y.H.; Wax, D.M.; Murphy, C.N.; Rieke, A.; et al. Generation of cloned transgenic pigs rich in omega-3 fatty acids. Nat. Biotechnol. 2006, 24, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhao, C.Z.; Lin, R.Y.; Li, G.L.; Li, C.C.; Wang, H.Y.; Xu, J.; Xie, S.S.; Yu, M.; Zhao, S.H. Production of homeobox A10 gene transgenic pigs by somatic cell nuclear transfer. J. Integr. Agric. 2019, 18, 1072–1079. [Google Scholar] [CrossRef]
- Yang, D.; Wang, C.E.; Zhao, B.; Li, W.; Ouyang, Z.; Liu, Z.; Yang, H.; Fan, P.; O’Neill, A.; Gu, W.; et al. Expression of Huntington’s disease protein results in apoptotic neurons in the brains of cloned transgenic pigs. Hum. Mol. Genet. 2010, 19, 3983–3994. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.J.; Steinfeld, J.B.; Greene, E.C. Single-Stranded DNA Curtains for Studying Homologous Recombination. Methods Enzymol. 2017, 582, 193–219. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Kolber-Simonds, D.; Park, K.W.; Cheong, H.T.; Greenstein, J.L.; Im, G.S.; Samuel, M.; Bonk, A.; Rieke, A.; Day, B.N.; et al. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 2002, 295, 1089–1092. [Google Scholar] [CrossRef]
- Dai, Y.; Vaught, T.D.; Boone, J.; Chen, S.H.; Phelps, C.J.; Ball, S.; Monahan, J.A.; Jobst, P.M.; McCreath, K.J.; Lamborn, A.E.; et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat. Biotechnol. 2002, 20, 251–255. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.; de Angelis, M.H.; Wurst, W.; Kuhn, R. Gene targeting by homologous recombination in mouse zygotes mediated by zinc-finger nucleases. Proc. Natl. Acad. Sci. USA 2010, 107, 15022–15026. [Google Scholar] [CrossRef] [Green Version]
- Mahfouz, M.M.; Li, L.X.; Shamimuzzaman, M.; Wibowo, A.; Fang, X.Y.; Zhu, J.K. De novo-engineered transcription activator-like effector (TALE) hybrid nuclease with novel DNA binding specificity creates double-strand breaks. Proc. Natl. Acad. Sci. USA 2011, 108, 2623–2628. [Google Scholar] [CrossRef] [Green Version]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.L.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.B.; Jiang, W.Y.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Yang, H.; Shivalila, C.S.; Dawlaty, M.M.; Cheng, A.W.; Zhang, F.; Jaenisch, R. One-Step Generation of Mice Carrying Mutations in Multiple Genes by CRISPR/Cas-Mediated Genome Engineering. Cell 2013, 153, 910–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, N.N.; Sun, C.H.; Gao, L.; Zhu, D.; Xu, X.F.; Zhu, X.J.; Xiong, J.W.; Xi, J.J. Genome editing with RNA-guided Cas9 nuclease in Zebrafish embryos. Cell Res. 2013, 23, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mashhadi, R.H.; Sorensen, C.B.; Kragh, P.M.; Christoffersen, C.; Mortensen, M.B.; Tolbod, L.P.; Thim, T.; Du, Y.T.; Li, J.; Liu, Y.; et al. Familial Hypercholesterolemia and Atherosclerosis in Cloned Minipigs Created by DNA Transposition of a Human PCSK9 Gain-of-Function Mutant. Sci. Transl. Med. 2013, 5, 166ra1. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Yang, Y.; Bu, L.; Guo, X.G.; Tang, C.C.; Song, J.; Fan, N.N.; Zhao, B.T.; Ouyang, Z.; Liu, Z.M.; et al. Rosa26-targeted swine models for stable gene over-expression and Cre-mediated lineage tracing. Cell Res. 2014, 24, 501–504. [Google Scholar] [CrossRef] [Green Version]
- Ruan, J.; Li, H.; Xu, K.; Wu, T.; Wei, J.; Zhou, R.; Liu, Z.; Mu, Y.; Yang, S.; Ouyang, H.; et al. Highly efficient CRISPR/Cas9-mediated transgene knockin at the H11 locus in pigs. Sci. Rep. 2015, 5, 14253. [Google Scholar] [CrossRef]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Zheng, X.G.; Qu, W.B.; Li, G.L.; Li, X.Y.; Miao, Y.L.; Han, X.S.; Liu, X.D.; Li, Z.H.; Ma, Y.L.; et al. CRISPR-offinder: A CRISPR guide RNA design and off-target searching tool for user-defined protospacer adjacent motif. Int. J. Biol. Sci. 2017, 13, 1470–1478. [Google Scholar] [CrossRef]
- Yao, J.; Huang, J.; Hai, T.; Wang, X.; Qin, G.; Zhang, H.; Wu, R.; Cao, C.; Xi, J.J.; Yuan, Z.; et al. Efficient bi-allelic gene knockout and site-specific knock-in mediated by TALENs in pigs. Sci. Rep. 2014, 4, 6926. [Google Scholar] [CrossRef]
- Brinkman, E.K.; Chen, T.; Amendola, M.; van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014, 42, e168. [Google Scholar] [CrossRef] [PubMed]
- Phadke, M.; Krynetskaia, N.; Mishra, A.; Krynetskiy, E. Accelerated cellular senescence phenotype of GAPDH-depleted human lung carcinoma cells. Biochem. Biophys. Res. Commun. 2011, 411, 409–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phadke, M.S.; Krynetskaia, N.F.; Mishra, A.K.; Krynetskiy, E. Glyceraldehyde 3-phosphate dehydrogenase depletion induces cell cycle arrest and resistance to antimetabolites in human carcinoma cell lines. J. Pharmacol. Exp. Ther. 2009, 331, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Qiao, J.; Hu, S.W.; Zhao, X.X.; Regouski, M.; Yang, M.; Polejaeva, I.A.; Chen, C.F. Efficient Gene Knockout in Goats Using CRISPR/Cas9 System. PLoS ONE 2014, 9, e106718. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, X.M.; Shi, H.; Yan, Q.M.; Zheng, M.; Li, J.; Zhang, Q.J.; Qin, Y.M.; Zhong, Y.G.; Mi, J.D.; et al. Generation of ApoE deficient dogs via combination of embryo injection of CRISPR/Cas9 with somatic cell nuclear transfer. J. Genet. Genom. 2018, 45, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cai, Y.J.; Liao, Z.D.; Xu, Y.T.; Wang, Y.; Wang, Z.Y.; Jiang, X.Y.; Li, Y.Z.; Lu, Y.; Nie, Y.H.; et al. Cloning of a gene-edited macaque monkey by somatic cell nuclear transfer. Natl. Sci. Rev. 2019, 6, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Wang, M.; Ye, C.; Fang, J.; Duan, Y.; Zhang, Z.; Hua, Q.; Shi, C.; Zhang, L.; Zhang, R.; et al. One-step generation of mice carrying a conditional allele together with an HA-tag insertion for the delta opioid receptor. Sci. Rep. 2017, 7, 44476. [Google Scholar] [CrossRef]
- Wang, K.K.; Ouyang, H.S.; Xie, Z.C.; Yao, C.G.; Guo, N.N.; Li, M.J.; Jiao, H.P.; Pang, D.X. Efficient Generation of Myostatin Mutations in Pigs Using the CRISPR/Cas9 System. Sci. Rep. 2015, 5, 16623. [Google Scholar] [CrossRef]
- Whitworth, K.M.; Rowland, R.R.R.; Ewen, C.L.; Trible, B.R.; Kerrigan, M.A.; Cino-Ozuna, A.G.; Samuel, M.S.; Lightner, J.E.; McLaren, D.G.; Mileham, A.J.; et al. Gene-edited pigs are protected from porcine reproductive and respiratory syndrome virus. Nat. Biotechnol. 2016, 34, 20–22. [Google Scholar] [CrossRef]
- Yan, S.; Tu, Z.; Liu, Z.; Fan, N.; Yang, H.; Yang, S.; Yang, W.; Zhao, Y.; Ouyang, Z.; Lai, C.; et al. A Huntingtin Knockin Pig Model Recapitulates Features of Selective Neurodegeneration in Huntington’s Disease. Cell 2018, 173, 989–1002. [Google Scholar] [CrossRef]
- Ercolani, L.; Florence, B.; Denaro, M.; Alexander, M. Isolation and complete sequence of a functional human glyceraldehyde-3-phosphate dehydrogenase gene. J. Biol. Chem. 1988, 263, 15335–15341. [Google Scholar] [PubMed]
- Suzuki, K.; Tsunekawa, Y.; Hernandez-Benitez, R.; Wu, J.; Zhu, J.; Kim, E.J.; Hatanaka, F.; Yamamoto, M.; Araoka, T.; Li, Z.; et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 2016, 540, 144. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, T.; Nakade, S.; Sakane, Y.; Suzuki, K.T.; Yamamoto, T. MMEJ-assisted gene knock-in using TALENs and CRISPR-Cas9 with the PITCh systems. Nat. Protoc. 2016, 11, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Wang, X.; Hu, X.D.; Liu, Z.; Liu, J.L.; Zhou, H.B.; Shen, X.W.; Wei, Y.; Huang, Z.J.; Ying, W.Q.; et al. Homology-mediated end joining-based targeted integration using CRISPR/Cas9. Cell Res. 2017, 27, 801–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| # | Predicted OTS | Sequence | Indel |
|---|---|---|---|
| GAPDH-sgR | CATGGTCCACATGGCCTCCA AGG | ||
| 1 | Prediated-OFF-Target1 | CATGGTCCCCATGGCCTGCC TGG | NO |
| 2 | Prediated-OFF-Target2 | CATGATCCGCATGGCCTCCA TGG | NO |
| 3 | Prediated-OFF-Target3 | CACGGTCCACATGGCCTCCC TGG | NO |
| 4 | Prediated-OFF-Target4 | CATGGTCTCCATGGCCTCCA GGG | NO |
| 5 | Prediated-OFF-Target5 | CATGGTGAACATGTCCTCCA TGG | NO |
| 6 | Prediated-OFF-Target6 | GATGCTCCACCTGGCCTCCA GGG | NO |
| 7 | Prediated-OFF-Target7 | CAGGGTCCAGATGGTCTCCA GGG | NO |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Xiong, Y.; Zhao, C.; Xie, S.; Li, C.; Li, X.; Liu, X.; Li, K.; Zhao, S.; Ruan, J. Identification of Glyceraldehyde-3-Phosphate Dehydrogenase Gene as an Alternative Safe Harbor Locus in Pig Genome. Genes 2019, 10, 660. https://doi.org/10.3390/genes10090660
Han X, Xiong Y, Zhao C, Xie S, Li C, Li X, Liu X, Li K, Zhao S, Ruan J. Identification of Glyceraldehyde-3-Phosphate Dehydrogenase Gene as an Alternative Safe Harbor Locus in Pig Genome. Genes. 2019; 10(9):660. https://doi.org/10.3390/genes10090660
Chicago/Turabian StyleHan, Xiaosong, Youcai Xiong, Changzhi Zhao, Shengsong Xie, Changchun Li, Xinyun Li, Xiangdong Liu, Kui Li, Shuhong Zhao, and Jinxue Ruan. 2019. "Identification of Glyceraldehyde-3-Phosphate Dehydrogenase Gene as an Alternative Safe Harbor Locus in Pig Genome" Genes 10, no. 9: 660. https://doi.org/10.3390/genes10090660
APA StyleHan, X., Xiong, Y., Zhao, C., Xie, S., Li, C., Li, X., Liu, X., Li, K., Zhao, S., & Ruan, J. (2019). Identification of Glyceraldehyde-3-Phosphate Dehydrogenase Gene as an Alternative Safe Harbor Locus in Pig Genome. Genes, 10(9), 660. https://doi.org/10.3390/genes10090660








