The Pituitary Transcriptional Response Related to Feed Conversion in Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Consent to Participate
2.2. Animals
2.3. cDNA Library Construction
2.4. Raw Read Processing
2.5. Analysis of Differentially Expressed Genes (DEGs)
2.6. Validation of RNA-Seq Results and Estimation of Transcript Abundance
3. Results
3.1. Animal Traits
3.2. Analysis of Differentially Expressed Genes
3.3. qPCR Analysis
4. Discussion
4.1. Inducing of Pituitary Gene Expression in Response to Variable Feed Conversion in Pigs
4.2. The Enriched Insulin-Like Growth Factor Pathway
4.3. The Enriched Notch Signalling Pathway
4.4. The Enriched Wnt Signalling Pathway
5. Conclusions
Supplementary Materials
Availability of Data
- and under the link—https://bit.ly/2Wtbqlb. For the others, please contact the authors.
Author Contributions
Funding
Conflicts of Interest
References
- Żak, G.; Tyra, M.; Różycki, M. Possibility of improvement of lean meat content of ham and loin in pigs by selection for growth and feed conversion rate. Anim. Sci. Pap. Rep. 2008, 26, 305–316. [Google Scholar]
- Żak, G.; Tyra, M.; Lasek, J. Ocena świń w stacjach kontroli użytkowości rzeźnej trzody chlewnej–historia, stan obecny, znaczenie i zakres. Przegląd Hod. 2018, 86, 5–6. [Google Scholar]
- Tyra, M.; Mucha, A.; Eckert, R. Zależności pomiędzy dziennym spożyciem paszy a wskaźnikami użytkowości tucznej, rzeźnej i jakości mięsa świń ocenianych w stacjach kontroli. Rocz. Nauk. Zoot. 2019, 46, 1–13. [Google Scholar]
- Melmed, S. The Pituitary; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Menche, N. PFLEGEN: Biologie Anatomie Physiologie; Elsevier Health Sciences: London, UK, 2017. [Google Scholar]
- Lupu, F.; Terwilliger, J.D.; Lee, K.; Segre, G.V.; Efstratiadis, A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev. Biol. 2001, 229, 141–162. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, Y.; Ko, W.K.; Li, W.; Wong, A.O. Paracrine regulation of growth hormone gene expression by gonadotrophin release in grass carp pituitary cells: Functional implications, molecular mechanisms and signal transduction. J. Mol. Endocrinol. 2005, 34, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Barb, C.R.; Kraeling, R.R.; Rampacek, G.B. Nutritional regulators of the hypothalamic-pituitary axis in pigs. Reprod. Suppl. 2001, 58, 1–15. [Google Scholar] [PubMed]
- Falch, C.M.; Sundaram, A.Y.; Oystese, K.A.B.; Normann, K.R.; Silamikelis, I.; Andersen, M.; Olarescu, N.C. RNA sequencing and RT-qPCR identify different gene expression profiles in fast-vs. slow-growing non-functioning pituitary adenomas. Endocr. Abstracts 2017, 49, OC83. [Google Scholar] [CrossRef]
- Ren, J.; Jian, F.; Jiang, H.; Sun, Y.; Pan, S.; Gu, C.; Sun, Q. Decreased expression of SFRP2 promotes development of the pituitary corticotroph adenoma by upregulating Wnt signaling. Int. J. Oncol. 2018, 52, 1934–1946. [Google Scholar] [CrossRef]
- Cheung, L.Y.; George, A.S.; McGee, S.R.; Daly, A.Z.; Brinkmeier, M.L.; Ellsworth, B.S.; Camper, S.A. Single-Cell RNA sequencing reveals novel markers of male pituitary stem cells and hormone-producing cell types. Endocrinology 2018, 159, 3910–3924. [Google Scholar] [CrossRef]
- Hekman, J.P.; Johnson, J.L.; Edwards, W.; Vladimirova, A.V.; Gulevich, R.G.; Ford, A.L.; Trut, L.N. Anterior pituitary transcriptome suggests differences in ACTH release in tame and aggressive foxes. G3 2018, 8, 859–873. [Google Scholar] [CrossRef]
- Wysocka, D.; Sobiech, P.; Herudzińska, M.; Sachajko, M.; Pareek, C.S. Investigation of candidate genes for metabolic disorders expressed in liver and pituitary gland by comparing the RNA-seq data of Polish-HF and Polish-Red cattle. Transl. Res. Vet. Sci. 2018, 1, 69–83. [Google Scholar] [CrossRef]
- Shan, L.; Wu, Q.; Li, Y.; Shang, H.; Guo, K.; Wu, J.; Li, M.H. Transcriptome profiling identifies differentially expressed genes in postnatal developing pituitary gland of miniature pig. DNA Res. 2013, 21, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Tyra, M.; Żak, G. Analysis of the possibility of improving the indicators of pork quality through selection with particular consideration of intramuscular fat (IMF) content. Ann. Anim. Sci. 2013, 13, 33–44. [Google Scholar] [CrossRef]
- Piórkowska, K.; Żukowski, K.; Nowak, J.; Połtowicz, K.; Ropka-Molik, K.; Gurgul, A. Genome-wide RNA-Seq analysis of breast muscles of two broiler chicken groups differing in shear force. Anim. Genet. 2016, 47, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Piórkowska, K.; Ropka-Molik, K.; Eckert, R.; Różycki, M. The expression pattern of proteolytic enzymes of cathepsin family in two important porcine skeletal muscles. Livest. Sci. 2013, 157, 427–434. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2000, 29, 45. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Stulnig, T.M.; Lin, C.Y.; Yeo, A.L.; Nowotny, P.; Liu, E.T.; Steffensen, K.R. Liver X receptors regulate adrenal steroidogenesis and hypothalamic-pituitary-adrenal feedback. Mol. Endocrinol. 2007, 21, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Christoffolete, M.A.; Doleschall, M.; Egri, P.; Liposits, Z.; Zavacki, A.M.; Bianco, A.C.; Gereben, B. Regulation of thyroid hormone activation via the liver X-receptor/retinoid X-receptor pathway. J. Endocrinol. 2010, 205, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Geiger, B.; Deeb, N.; Rothschild, M.F. Liver X receptor alpha and beta genes have the potential role on loin lean and fat content in pigs. J. Anim. Breed. Gen. 2006, 123, 81–88. [Google Scholar] [CrossRef]
- Piórkowska, K.; Żukowski, K.; Ropka-Molik, K.; Tyra, M.; Gurgul, A. A comprehensive transcriptome analysis of skeletal muscles in two Polish pig breeds differing in fat and meat quality traits. Genet. Mol. Biol. 2018, 41, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raber, J.; Akana, S.F.; Bhatnagar, S.; Dallman, M.F.; Wong, D.; Mucke, L. Hypothalamic-pituitary-adrenal dysfunction in Apoe(-/-) mice: Possible role in behavioral and metabolic alterations. J. Neurosci. 2000, 20, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Babenko, V.N.; Smagin, D.A.; Kudryavtseva, N.N. RNA-Seq mouse brain regions expression data analysis: Focus on ApoE functional network. J. Integr. Bioinform. 2017, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sui, L.; Panigrahi, S.K.; Meece, K.; Xin, Y.; Kim, J.; Leibel, R.L. PC1/3 deficiency impacts pro-opiomelanocortin processing in human embryonic stem cell-derived hypothalamic neurons. Stem Cell Rep. 2017, 8, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Saneyasu, T.; Hasegawa, S.; Kamisoyama, H.A. Comparative study of the central effects of melanocortin peptides on food intake in broiler and layer chicks. Peptides 2012, 37, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Korek, E.; Krauss, H.; Piatek, J.; Checinska, Z. Regulacja hormonalna łaknienia. Med. Ogólna Nauk. Zdr. 2013, 19, 211–217. [Google Scholar]
- Appleyard, S.M.; Hayward, M.; Young, J.I.; Butler, A.A.; Cone, R.D.; Rubinstein, M.; Low, M.J. A role for the endogenous opioid β-endorphin in energy homeostasis. Endocrinology 2003, 144, 1753–1760. [Google Scholar] [CrossRef]
- Dallman, M.F.; Warne, J.P.; Foster, M.T.; Pecoraro, N.C. Glucocorticoids and insulin both modulate caloric intake through actions on the brain. J. Physiol. 2007, 583, 431–436. [Google Scholar] [CrossRef]
- Fonseca, T.L.; Correa-Medina, M.; Campos, M.P.; Wittmann, G.; Werneck-de-Castro, J.P.; Drigo, R.A.; Gereben, B. Coordination of hypothalamic and pituitary T3 production regulates TSH expression. J. Clin. Investig. 2013, 123, 1492–1500. [Google Scholar] [CrossRef] [Green Version]
- Martin, N.M.; Small, C.J.; Sajedi, A.; Liao, X.H.; Weiss, R.E.; Gardiner, J.V.; Bloom, S.R. Abnormalities of the hypothalamo-pituitary-thyroid axis in the pro-opiomelanocortin deficient mouse. Regul. Pept. 2004, 122, 169–172. [Google Scholar] [CrossRef]
- Bachelot, A.; Binart, N. Reproductive role of prolactin. Reproduction 2007, 133, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, K.M. A review of the hormone prolactin during lactation. Prog. Food Nutr. Sci. 1990, 14, 1–43. [Google Scholar] [PubMed]
- Saiardi, A.; Bozzi, Y.; Baik, J.H.; Borrelli, E. Antiproliferative role of dopamine: Loss of D2 receptors causes hormonal dysfunction and pituitary hyperplasia. Neuron 1997, 19, 115–126. [Google Scholar] [CrossRef]
- Mebel, D.M.; Wong, J.C.; Dong, Y.J.; Borgland, S.L. Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. Eur. J. Neurosci. 2012, 36, 2336–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cianfarani, S.; Rossi, P. Neuroblastoma and insulin-like growth factor system. Eur. J. Pediatr. 1997, 156, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Frasca, F.; Pandini, G.; Scalia, P.; Sciacca, L.; Mineo, R.; Costantino, A.; Vigneri, R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol. Cell Biol. 1999, 19, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.M.; Rotwein, P. Control of MyoD function during initiation of muscle differentiation by an autocrine signaling pathway activated by insulin-like growth factor-II. J. Biol. Chem. 2006, 281, 29962–29971. [Google Scholar] [CrossRef]
- Uchimura, T.; Hollander, J.M.; Nakamura, D.S.; Liu, Z.; Rosen, C.J.; Georgakoudi, I.; Zeng, L. An essential role for IGF2 in cartilage development and glucose metabolism during postnatal long bone growth. Development 2017, 144, 3533–3546. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Xu, J.; He, X.; Xu, H.; Li, G.; Du, H.; Zhang, X. A genome-wide mRNA screen and functional analysis reveal FOXO3 as a candidate gene for chicken growth. PLoS ONE 2015, 10, e0137087. [Google Scholar] [CrossRef]
- Clark, D.L.; Clark, D.I.; Beever, J.E.; Dilger, A.C. Increased prenatal IGF2 expression due to the porcine IGF2 intron3-G3072A mutation may be responsible for increased muscle mass. J. Anim. Sci. 2015, 93, 2546–2558. [Google Scholar] [CrossRef]
- Oczkowicz, M.; Tyra, M.; Walinowicz, K.; Różycki, M.; Rejduch, B. Known mutation (A3072G) in intron 3 of theIGF2 gene is associated with growth and carcass composition in Polish pig breeds. J. Appl. Genet. 2009, 50, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Nantie, L.B.; Himes, A.D.; Getz, D.R.; Raetzman, L.T. Notch signaling in postnatal pituitary expansion: Proliferation, progenitors, and cell specification. Mol. Endocrinol. 2014, 28, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Pajvani, U.B.; Qiang, L.; Kangsamaksin, T.; Kitajewski, J.; Ginsberg, H.N.; Accili, D. Inhibition of notch uncouples akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat. Med. 2013, 19, 1054. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Levy, J.D.; Zhang, Y.; Frontini, A.; Kolodin, D.P.; Svensson, K.J.; Wu, J. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014, 156, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, Y.; Cai, D. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat. Cell Biol. 2012, 14, 999. [Google Scholar] [CrossRef] [PubMed]
- Bartholdi, D.; Roelfsema, J.H.; Papadia, F.; Breuning, M.H.; Niedrist, D.; Hennekam, R.C.; Peters, D.J. Genetic heterogeneity in Rubinstein–Taybi syndrome: Delineation of the phenotype of the first patients carrying mutations in EP 300. J. Med. Genet. 2007, 44, 327–333. [Google Scholar] [CrossRef]
- Hegedus, B.; Yeh, T.H.; Lee, D.Y.; Emnett, R.J.; Li, J.; Gutmann, D.H. Neurofibromin regulates somatic growth through the hypothalamic–pituitary axis. Hum. Mol. Genet. 2008, 17, 2956–2966. [Google Scholar] [CrossRef] [PubMed]
- Struthers, R.S.; Vale, W.W.; Arias, C.; Sawchenko, P.E.; Montminy, M.R. Somatotroph hypoplasia and dwarfism in transgenic mice expressing a non-phosphorylatable CREB mutant. Nature 1991, 350, 622. [Google Scholar] [CrossRef]
- Burton, F.H.; Hasel, K.W.; Bloom, F.E.; Sutcliffe, J.G. Pituitary hyperplasia and gigantism in mice caused by a cholera toxin transgene. Nature 1991, 350, 74. [Google Scholar] [CrossRef]
- Roof, A.K.; Gutierrez-Hartmann, A. Consider the context: Ras/ERK and PI3K/AKT/mTOR signaling outcomes are pituitary cell type-specific. Mol. Cell Endocrinol. 2018, 463, 87–96. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Kibardin, A.; Ossipova, O.; Sokol, S.Y. Metastasis-associated kinase modulates Wnt signaling to regulate brain patterning and morphogenesis. Development 2006, 133, 2845–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florez, J.C.; Jablonski, K.A.; Bayley, N.; Pollin, T.I.; de Bakker, P.I.; Shuldiner, A.R.; Altshuler, D. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N. Engl. J. Med. 2006, 355, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, S.H.; Shepherd, P.R. Glucose induces an autocrine activation of the Wnt/b-catenin pathway in macrophage cell lines. Biochem. J. 2008, 416, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Helfer, G.; Tups, A. Hypothalamic Wnt signalling and its role in energy balance regulation. J. Neuroendocr. 2016, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.G.; Giles, A.; Brabant, G.; Davis, J.R.E. Wnt signalling in pituitary development and tumorigenesis. Endocr. Relat. Cancer 2013, 20, R101–R111. [Google Scholar] [CrossRef] [Green Version]
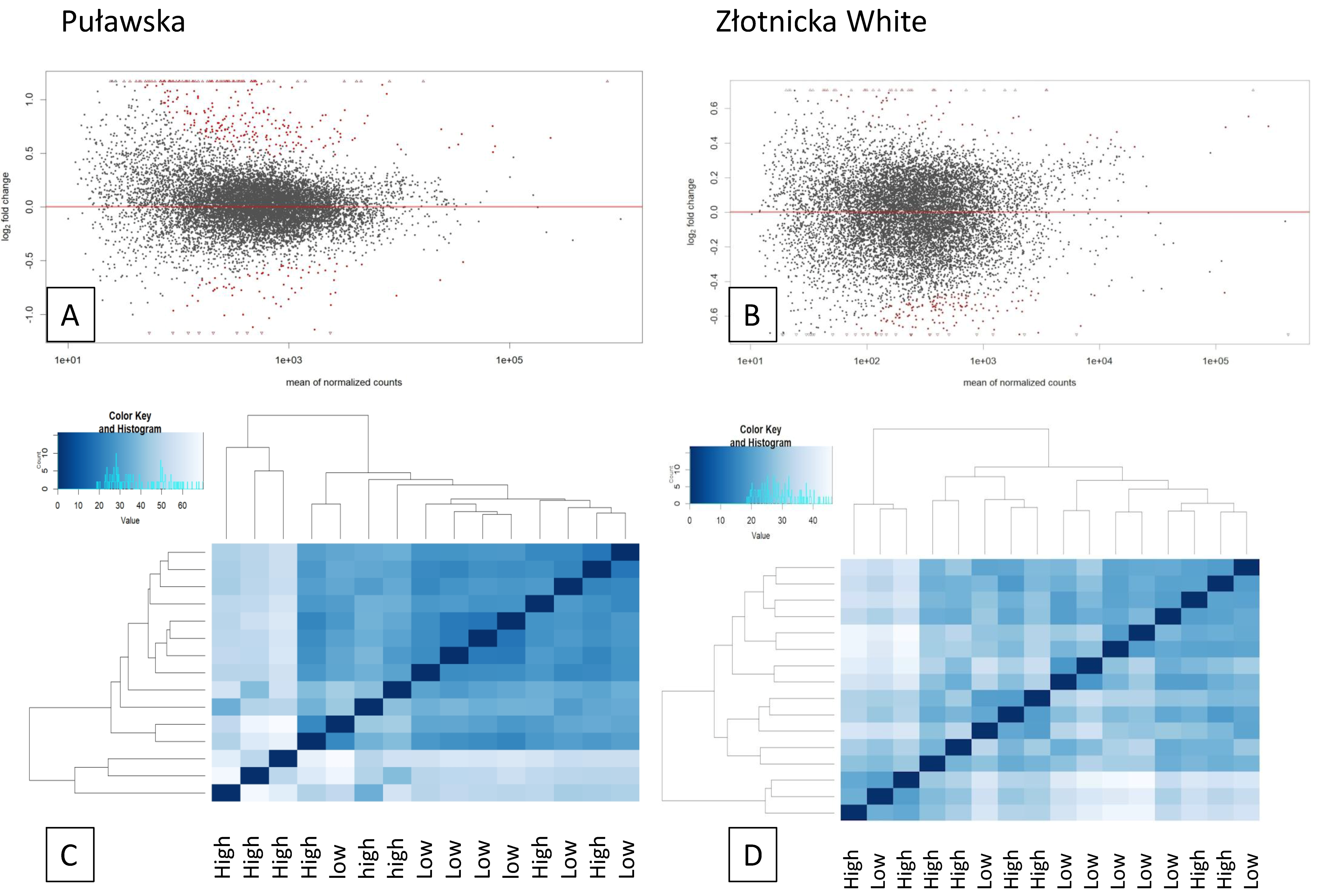

 ,
,  , high feed conversion Złotnicka White pigs:
, high feed conversion Złotnicka White pigs:  ECM receptor interaction (ssc04512),
ECM receptor interaction (ssc04512),  the thyroid hormone signaling pathway (ssc04919),
the thyroid hormone signaling pathway (ssc04919),  thermogenesis (ssc04714),
thermogenesis (ssc04714),  endocrine resistance (ssc01522),
endocrine resistance (ssc01522),  the AMPK signaling pathway (ssc04152),
the AMPK signaling pathway (ssc04152),  the Wnt signaling pathway (ssc04310),
the Wnt signaling pathway (ssc04310),  the Insulin signaling pathway (ssc04910),
the Insulin signaling pathway (ssc04910),  the Notch signaling pathway (ssc04330); low feed conversion Złotnicka White pigs:
the Notch signaling pathway (ssc04330); low feed conversion Złotnicka White pigs:  non-alcoholic fatty liver disease (NAFLD) (ssc04932),
non-alcoholic fatty liver disease (NAFLD) (ssc04932),  ribosome (ssc03010),
ribosome (ssc03010),  GO:0006412 translation, and
GO:0006412 translation, and  GO:0010817 regulation of hormone levels.
GO:0010817 regulation of hormone levels.
 ,
,  , high feed conversion Złotnicka White pigs:
, high feed conversion Złotnicka White pigs:  ECM receptor interaction (ssc04512),
ECM receptor interaction (ssc04512),  the thyroid hormone signaling pathway (ssc04919),
the thyroid hormone signaling pathway (ssc04919),  thermogenesis (ssc04714),
thermogenesis (ssc04714),  endocrine resistance (ssc01522),
endocrine resistance (ssc01522),  the AMPK signaling pathway (ssc04152),
the AMPK signaling pathway (ssc04152),  the Wnt signaling pathway (ssc04310),
the Wnt signaling pathway (ssc04310),  the Insulin signaling pathway (ssc04910),
the Insulin signaling pathway (ssc04910),  the Notch signaling pathway (ssc04330); low feed conversion Złotnicka White pigs:
the Notch signaling pathway (ssc04330); low feed conversion Złotnicka White pigs:  non-alcoholic fatty liver disease (NAFLD) (ssc04932),
non-alcoholic fatty liver disease (NAFLD) (ssc04932),  ribosome (ssc03010),
ribosome (ssc03010),  GO:0006412 translation, and
GO:0006412 translation, and  GO:0010817 regulation of hormone levels.
GO:0010817 regulation of hormone levels.
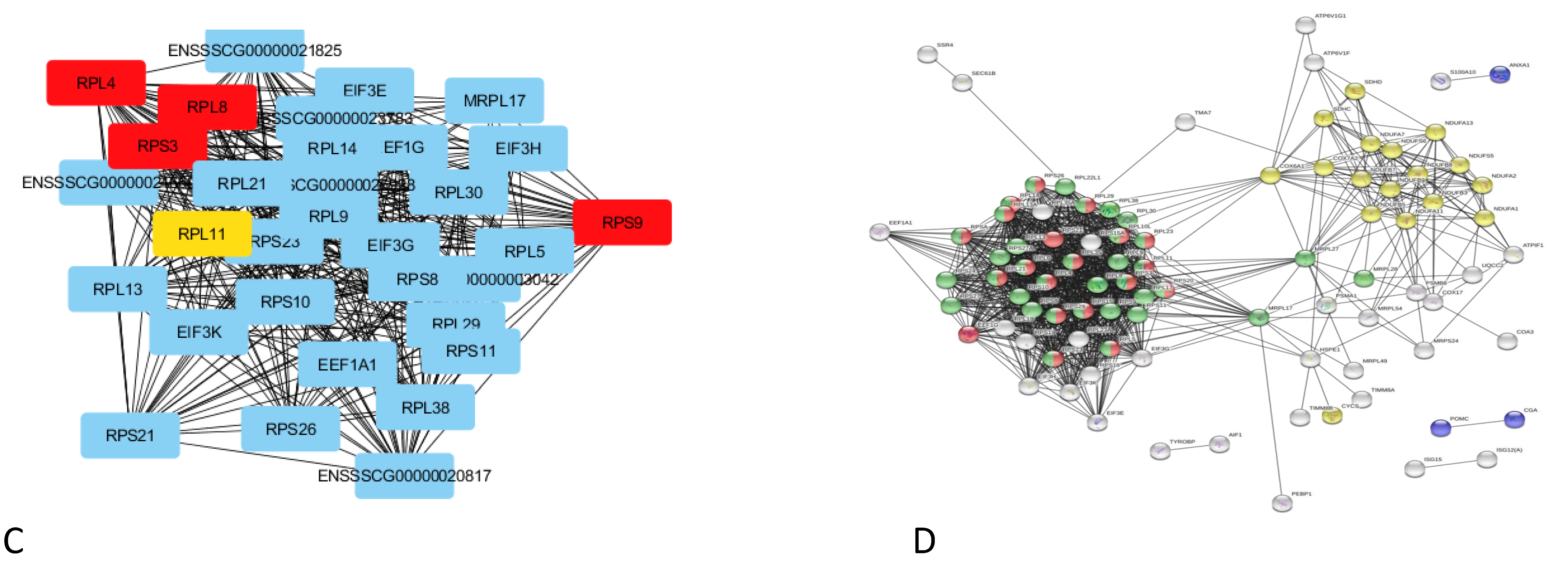
 ,
,  ,
,  respiratory electron transport, ATP synthesis by chemiosmotic coupling, and heat production by the uncoupling proteins (Reactome pathway SSC-163200),
respiratory electron transport, ATP synthesis by chemiosmotic coupling, and heat production by the uncoupling proteins (Reactome pathway SSC-163200),  thermogenesis (ssc04714),
thermogenesis (ssc04714),  and the ATP metabolic process (GO:0046034).
and the ATP metabolic process (GO:0046034).
 ,
,  ,
,  respiratory electron transport, ATP synthesis by chemiosmotic coupling, and heat production by the uncoupling proteins (Reactome pathway SSC-163200),
respiratory electron transport, ATP synthesis by chemiosmotic coupling, and heat production by the uncoupling proteins (Reactome pathway SSC-163200),  thermogenesis (ssc04714),
thermogenesis (ssc04714),  and the ATP metabolic process (GO:0046034).
and the ATP metabolic process (GO:0046034).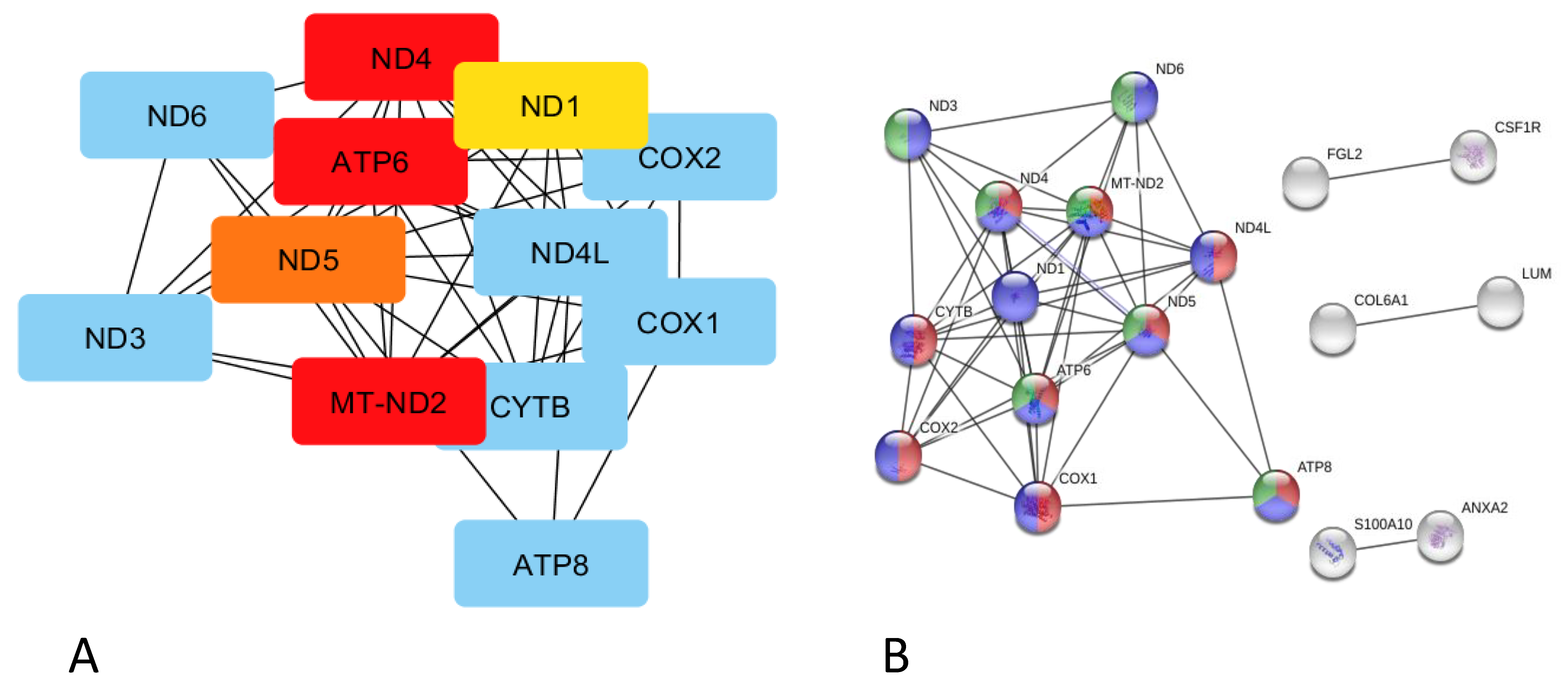


| Trait | Group of Native Pigs | |||
|---|---|---|---|---|
| Puławska | Złotnicka White | |||
| LFC (High Benefit) n = 8 | HFC (Low Benefit) n = 8 | LFC (High Benefit) n = 8 | HFC (Low Benefit) n = 8 | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Feed conversion ratio | 2.57 ± 0.06 A | 3.64 ± 0.37 B | 2.84 ± 0.71 A | 4.12 ± 0.89 B |
| Daily gain (g) | 919 ± 103 A | 715 ± 82 B | 811 ± 98 A | 561 ± 165 B |
| Daily feed intake (kg/day) | 2.35 ± 0.27 a | 2.60 ± 0.23 b | 2.30 ± 0.12 | 2.32 ± 0.16 |
| Day in test | 78 ± 9 A | 95 ± 10 B | 84 ± 15 A | 122 ± 28 B |
| Back ham (kg) | 11.1 ± 0.71 a | 10.3 ± 0.61 b | 10.2 ± 0.51 | 9.8 ± 0.58 |
| Ham (kg) | 9.3 ± 0.64 | 8.74 ± 0.60 | 8.21 ± 0.59 A | 7.34 ± 0.65 B |
| Ham fat (cm) | 1.57 ± 0.13 a | 1.38 ± 0.21 b | 1.76 ± 0.21 a | 2.22 ± 0.38 b |
| Backfat (cm) * | 1.28 ± 0.44 a | 1.64 ± 0.19 b | 1.7 ± 0.47 | 1.64 ± 0.53 |
| Knuckle fat | 0.20 ± 0.01 | 0.20 ± 0.01 | 0.20 ± 0.01 a | 0.23 ± 0.01 b |
| Backfat in C1 point * | 1.30 ± 0.38 | 1.03 ± 0.28 | 1.53 ± 0.40 a | 2.03 ± 0.41 b |
| Backfat in the K1 point * | 1.30 ± 0.38 | 1.06 ± 0.26 | 1.53 ± 0.39 a | 2.01 ± 0.40 b |
| %meat in primary cuts | 70 ± 2.2 | 70 ± 2.4 | 66 ± 3.2 A | 61 ± 3.7 B |
| %meat in carcass | 61.4 ± 1.82 | 60.7 ± 2.85 | 57.5 ± 3.0 A | 52.9 ± 3.7 B |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piórkowska, K.; Żukowski, K.; Tyra, M.; Szyndler-Nędza, M.; Szulc, K.; Skrzypczak, E.; Ropka-Molik, K. The Pituitary Transcriptional Response Related to Feed Conversion in Pigs. Genes 2019, 10, 712. https://doi.org/10.3390/genes10090712
Piórkowska K, Żukowski K, Tyra M, Szyndler-Nędza M, Szulc K, Skrzypczak E, Ropka-Molik K. The Pituitary Transcriptional Response Related to Feed Conversion in Pigs. Genes. 2019; 10(9):712. https://doi.org/10.3390/genes10090712
Chicago/Turabian StylePiórkowska, Katarzyna, Kacper Żukowski, Mirosław Tyra, Magdalena Szyndler-Nędza, Karolina Szulc, Ewa Skrzypczak, and Katarzyna Ropka-Molik. 2019. "The Pituitary Transcriptional Response Related to Feed Conversion in Pigs" Genes 10, no. 9: 712. https://doi.org/10.3390/genes10090712
APA StylePiórkowska, K., Żukowski, K., Tyra, M., Szyndler-Nędza, M., Szulc, K., Skrzypczak, E., & Ropka-Molik, K. (2019). The Pituitary Transcriptional Response Related to Feed Conversion in Pigs. Genes, 10(9), 712. https://doi.org/10.3390/genes10090712






