High-Concentrate Feeding to Dairy Cows Induces Apoptosis via the NOD1/Caspase-8 Pathway in Mammary Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals, Diets, and Their Trail Plan
2.3. Collection of Samples
2.4. Detection of Apoptosis by the Tunnel Assay
2.5. Isolation of RNA, cDNA Synthesis, and Real-Time (RT-qPCR)
2.6. The Concentration of Caspase-3 and Caspase-8 in Mammary Tissue
2.7. Western Blotting Analysis
2.8. Immunofluorescence Antibody (IFA) Assay
2.9. Statistical Analysis
3. Results
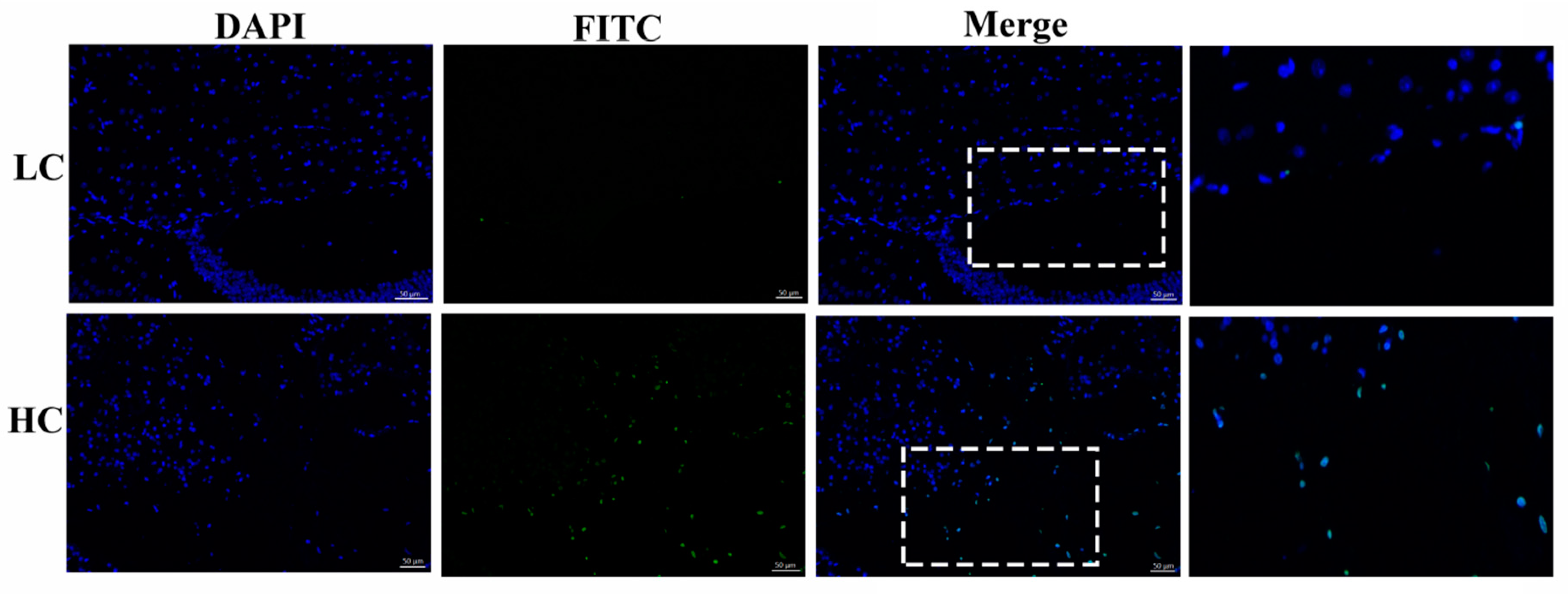
3.1. Determination of Apoptotic Cells in the Mammary Gland Tissue of Dairy Cows
3.2. mRNA Expression of NOD1, Rip-2, Bax, and Bcl2 in Mammary Gland Tissue
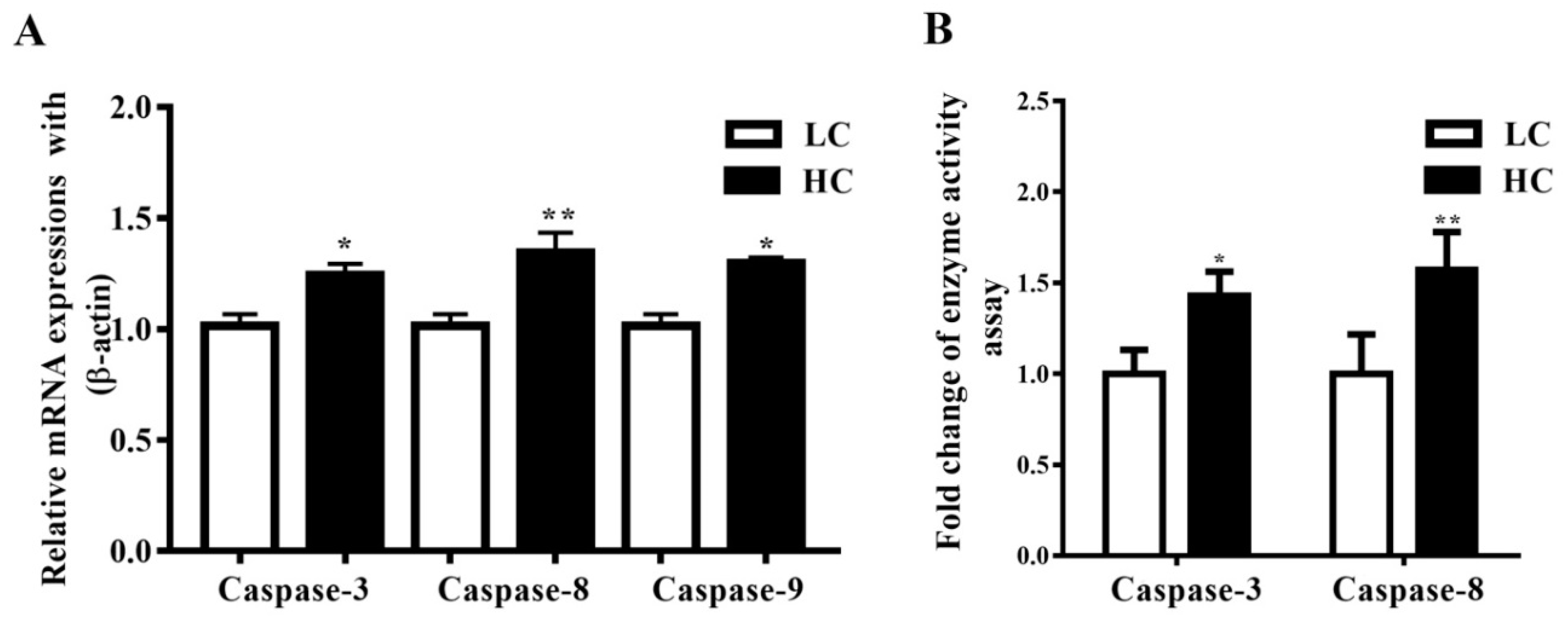
3.3. Determination of Caspase-3, Caspase-8, and Caspase-9 mRNA Expressions, and Caspase-3 and Caspase-8 Concentration in Mammary Gland Tissue
3.4. Protein Expression in Mammary Tissue
3.5. Protein Expression and Localization of NOD1 and Caspase-8
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Danscher, A.M.; Li, S.; Andersen, P.H.; Khafipour, E.; Kristensen, N.B.; Plaizier, J.C. Indicators of induced subacute ruminal acidosis (SARA) in Danish Holstein cows. Acta Vet. Scand. 2015, 57, 39. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Xu, X.; Zou, Y.; Yang, Z.; Li, S.; Cao, Z. Changes in feed intake, nutrient digestion, plasma metabolites, and oxidative stress parameters in dairy cows with subacute ruminal acidosis and its regulation with pelleted beet pulp. J. Anim. Sci. Biotechnol. 2013, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Xu, T.T.; Liu, Y.J.; Zhu, W.Y.; Mao, S.Y. A high-grain diet causes massive disruption of ruminal epithelial tight junctions in goats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R232–R241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Ma, N.; Wang, L.; Dai, H.; Bilal, M.S.; Roy, A.C.; Shen, X. Overfeeding with a high-concentrate diet activates the NOD1-NF-kappaB signalling pathway in the mammary gland of mid-lactating dairy cows. Microb. Pathog. 2019, 128, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [Green Version]
- Clarke, T.B.; Weiser, J.N. Intracellular sensors of extracellular bacteria. Immunol. Rev. 2011, 243, 9–25. [Google Scholar] [CrossRef]
- Philpott, D.J.; Girardin, S.E. Nod-like receptors: Sentinels at host membranes. Curr. Opin. Immunol. 2010, 22, 428–434. [Google Scholar] [CrossRef]
- Franchi, L.; Warner, N.; Viani, K.; Nunez, G. Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 2009, 227, 106–128. [Google Scholar] [CrossRef] [Green Version]
- Chamaillard, M.; Hashimoto, M.; Horie, Y.; Masumoto, J.; Qiu, S.; Saab, L.; Ogura, Y.; Kawasaki, A.; Fukase, K.; Kusumoto, S.; et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 2003, 4, 702–707. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujimoto, Y.; Lucas, P.C.; Nakano, H.; Fukase, K.; Nunez, G.; Inohara, N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. EMBO J. 2008, 27, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, L.O.; Zamboni, D.S. NOD1 and NOD2 Signaling in Infection and Inflammation. Front. Immunol. 2012, 3, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irving, A.T.; Mimuro, H.; Kufer, T.A.; Lo, C.; Wheeler, R.; Turner, L.J.; Thomas, B.J.; Malosse, C.; Gantier, M.P.; Casillas, L.N.; et al. The immune receptor NOD1 and kinase RIP2 interact with bacterial peptidoglycan on early endosomes to promote autophagy and inflammatory signaling. Cell Host Microbe 2014, 15, 623–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves de Souza, J.A.; Frasnelli, S.C.; Curylofo-Zotti, F.A.; Avila-Campos, M.J.; Spolidorio, L.C.; Zamboni, D.S.; Graves, D.T.; Rossa, C., Jr. NOD1 in the modulation of host-microbe interactions and inflammatory bone resorption in the periodontal disease model. Immunology 2016, 149, 374–385. [Google Scholar] [CrossRef] [Green Version]
- Girardin, S.E.; Tournebize, R.; Mavris, M.; Page, A.L.; Li, X.; Stark, G.R.; Bertin, J.; DiStefano, P.S.; Yaniv, M.; Sansonetti, P.J.; et al. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001, 2, 736–742. [Google Scholar] [CrossRef] [Green Version]
- Bertin, J.; Nir, W.J.; Fischer, C.M.; Tayber, O.V.; Errada, P.R.; Grant, J.R.; Keilty, J.J.; Gosselin, M.L.; Robison, K.E.; Wong, G.H.; et al. Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-kappaB. J. Biol. Chem. 1999, 274, 12955–12958. [Google Scholar] [CrossRef] [Green Version]
- Thompson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462. [Google Scholar] [CrossRef]
- Nagata, S.; Tanaka, M. Programmed cell death and the immune system. Nat. Rev. Immunol. 2017, 17, 333–340. [Google Scholar] [CrossRef]
- Nicholson, D.W.; Thornberry, N.A. Caspases: Killer proteases. Trends Biochem. Sci. 1997, 22, 299–306. [Google Scholar] [CrossRef]
- Boatright, K.M.; Salvesen, G.S. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 2003, 15, 725–731. [Google Scholar] [CrossRef]
- Shi, Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Porter, A.G.; Janicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Chandra Roy, A.; Wang, Y.; Zhang, H.; Roy, S.; Dai, H.; Chang, G.; Shen, X. Sodium Butyrate Mitigates iE-DAP Induced Inflammation Caused by High-Concentrate Feeding in Liver of Dairy Goats. J. Agric. Food Chem. 2018, 66, 8999–9009. [Google Scholar] [CrossRef] [PubMed]
- Travassos, L.H.; Carneiro, L.A.; Girardin, S.E.; Boneca, I.G.; Lemos, R.; Bozza, M.T.; Domingues, R.C.; Coyle, A.J.; Bertin, J.; Philpott, D.J.; et al. Nod1 participates in the innate immune response to Pseudomonas aeruginosa. J. Biol. Chem. 2005, 280, 36714–36718. [Google Scholar] [CrossRef] [Green Version]
- Chang, G.; Liu, X.; Ma, N.; Yan, J.; Dai, H.; Roy, A.C.; Shen, X. Dietary Addition of Sodium Butyrate Contributes to Attenuated Feeding-Induced Hepatocyte Apoptosis in Dairy Goats. J. Agric. Food Chem. 2018, 66, 9995–10002. [Google Scholar] [CrossRef]
- Chang, G.; Yan, J.; Ma, N.; Liu, X.; Dai, H.; Bilal, M.S.; Shen, X. Dietary Sodium Butyrate Supplementation Reduces High-Concentrate Diet Feeding-Induced Apoptosis in Mammary Cells in Dairy Goats. J. Agric. Food Chem. 2018, 66, 2101–2107. [Google Scholar] [CrossRef]
- Khafipour, E.; Li, S.; Plaizier, J.C.; Krause, D.O. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl. Environ. Microbiol. 2009, 75, 7115–7124. [Google Scholar] [CrossRef] [Green Version]
- Khafipour, E.; Krause, D.O.; Plaizier, J.C. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J. Dairy Sci. 2009, 92, 1060–1070. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Khafipour, E.; Krause, D.O.; Kroeker, A.; Rodriguez-Lecompte, J.C.; Gozho, G.N.; Plaizier, J.C. Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows. J. Dairy Sci. 2012, 95, 294–303. [Google Scholar] [CrossRef]
- Keunen, J.E.; Plaizier, J.C.; Kyriazakis, L.; Duffield, T.F.; Widowski, T.M.; Lindinger, M.I.; McBride, B.W. Effects of a subacute ruminal acidosis model on the diet selection of dairy cows. J. Dairy Sci. 2002, 85, 3304–3313. [Google Scholar] [CrossRef]
- Gozho, G.N.; Plaizier, J.C.; Krause, D.O.; Kennedy, A.D.; Wittenberg, K.M. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J. Dairy Sci. 2005, 88, 1399–1403. [Google Scholar] [CrossRef] [Green Version]
- Maskaľová, I.; Vajda, V.; Bujnak, L. 2,6-Diaminopimelic acid as a biological marker of rumen synthesis and fermentation capacities in the transition period and early lactation of dairy cows. Acta Vet. Brno 2014, 83, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Uehara, A.; Fujimoto, Y.; Kawasaki, A.; Kusumoto, S.; Fukase, K.; Takada, H. Meso-diaminopimelic acid and meso-lanthionine, amino acids specific to bacterial peptidoglycans, activate human epithelial cells through NOD1. J. Immunol. 2006, 177, 1796–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabata, T.; Tani, T.; Endo, Y.; Hanasawa, K. Bacterial translocation and peptidoglycan translocation by acute ethanol administration. J. Gastroenterol. 2002, 37, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Tani, T.; Endo, Y.; Hanasawa, K.; Tsuchiya, M.; Kodama, M. Elevation of plasma peptidoglycan and peripheral blood neutrophil activation during hemorrhagic shock: Plasma peptidoglycan reflects bacterial translocation and may affect neutrophil activation. Crit. Care Med. 2002, 30, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Ferri, K.F.; Kroemer, G. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 2001, 3, E255–E263. [Google Scholar] [CrossRef]
- Da Silva Correia, J.; Miranda, Y.; Leonard, N.; Hsu, J.; Ulevitch, R.J. Regulation of Nod1-mediated signaling pathways. Cell Death Differ. 2007, 14, 830–839. [Google Scholar] [CrossRef] [Green Version]
- Miramar, M.D.; Costantini, P.; Ravagnan, L.; Saraiva, L.M.; Haouzi, D.; Brothers, G.; Penninger, J.M.; Peleato, M.L.; Kroemer, G.; Susin, S.A. NADH oxidase activity of mitochondrial apoptosis-inducing factor. J. Biol. Chem. 2001, 276, 16391–16398. [Google Scholar] [CrossRef] [Green Version]
- Donepudi, M.; Mac Sweeney, A.; Briand, C.; Grutter, M.G. Insights into the regulatory mechanism for caspase-8 activation. Mol. Cell 2003, 11, 543–549. [Google Scholar] [CrossRef]
- Woo, M.; Hakem, R.; Soengas, M.S.; Duncan, G.S.; Shahinian, A.; Kagi, D.; Hakem, A.; McCurrach, M.; Khoo, W.; Kaufman, S.A.; et al. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 1998, 12, 806–819. [Google Scholar] [CrossRef] [Green Version]
- Hardwick, J.M.; Soane, L. Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korsmeyer, S.J. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999, 59, 1693s–1700s. [Google Scholar] [CrossRef]
- Soomro, J.; Lu, Z.; Gui, H.; Zhang, B.; Shen, Z. Synchronous and Time-Dependent Expression of Cyclins, Cyclin-Dependant Kinases, and Apoptotic Genes in the Rumen Epithelia of Butyrate-Infused Goats. Front. Physiol. 2018, 9, 496. [Google Scholar] [CrossRef] [PubMed]



| Gene | Accession Number | Primer Sequence (5′–3′) | Product Length (bp) | |
|---|---|---|---|---|
| NOD1 | NM_001256563.1 | Forward | TCAACACTGACCCAGTGAGC | 147 |
| Reverse | TGAAGTTGACCAGCTCCACC | |||
| β-actin | AY141970 | Forward | CTCTTCCAGCCTTCCTTCCT | 178 |
| Reverse | GGGCAGTGATCTCTTTCTGC | |||
| Rip-2 | NM_001034610.2 | Forward | ATTCTGGTCCACGGGAGGAGTC | 94 |
| Reverse | TCTTGAGGAGCTGACAGGGACC | |||
| Caspase-3 | NM_001077840.1 | Forward | CAGCGTCGTAGCTGAACGTAA | 227 |
| Reverse | ATCGACAGGCCATGCCAGTAT | |||
| Caspase-8 | NM_001045970.2 | Forward | AGCAAATGGTCCAGGCTTTG | 250 |
| Reverse | GCTCTTGTTGACCTGCTCAC | |||
| Caspase-9 | NM_001205504.1 | Forward | AGCAAATGGTCCAGGCTTTG | 160 |
| Reverse | ATTCTCTCGACGGACACAGG | |||
| Bcl-2 | NM_001075417-2 | Forward | AGGTTGGTAACCGGACCCTA | 174 |
| Reverse | TTCCTGCCTGTCCTCGAATG | |||
| Bax | NM_173894.1 | Forward | GCTGTGGACACAGACTCTC | 158 |
| Reverse | CTGATCAACTGGGCACCTT | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
ul Aabdin, Z.; Cheng, X.; Dai, H.; Wang, Y.; Sahito, B.; Roy, A.C.; Memon, M.A.; Shen, X. High-Concentrate Feeding to Dairy Cows Induces Apoptosis via the NOD1/Caspase-8 Pathway in Mammary Epithelial Cells. Genes 2020, 11, 107. https://doi.org/10.3390/genes11010107
ul Aabdin Z, Cheng X, Dai H, Wang Y, Sahito B, Roy AC, Memon MA, Shen X. High-Concentrate Feeding to Dairy Cows Induces Apoptosis via the NOD1/Caspase-8 Pathway in Mammary Epithelial Cells. Genes. 2020; 11(1):107. https://doi.org/10.3390/genes11010107
Chicago/Turabian Styleul Aabdin, Zain, Xiaoye Cheng, Hongyu Dai, Yan Wang, Benazir Sahito, Animesh Chandra Roy, Meena Arif Memon, and Xiangzhen Shen. 2020. "High-Concentrate Feeding to Dairy Cows Induces Apoptosis via the NOD1/Caspase-8 Pathway in Mammary Epithelial Cells" Genes 11, no. 1: 107. https://doi.org/10.3390/genes11010107
APA Styleul Aabdin, Z., Cheng, X., Dai, H., Wang, Y., Sahito, B., Roy, A. C., Memon, M. A., & Shen, X. (2020). High-Concentrate Feeding to Dairy Cows Induces Apoptosis via the NOD1/Caspase-8 Pathway in Mammary Epithelial Cells. Genes, 11(1), 107. https://doi.org/10.3390/genes11010107




