The Expression of Decidual Protein Induced by Progesterone (DEPP) Is Controlled by Three Distal Consensus Hypoxia Responsive Element (HRE) in Hypoxic Retinal Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Donor Eyes
2.2. Animals
2.3. Mouse Hypoxic Exposure and Tissue Collection
2.4. Cells
2.5. In Vitro Hypoxic Exposure and DMOG Treatment of Cultured Cells
2.6. Transfection of ARPE-19 Cells with Plasmids and siRNAs
2.7. RNA Isolation, cDNA Synthesis, and Real-Time PCR
2.8. Western Blotting
2.9. Luciferase/β Galactosidase Assay
2.10. In-Silico Identification of Putative Hypoxia Responsive Elements (HRE)
2.11. Cloning of the 3.8 kb Region Upstream of DEPP Transcriptional Start Site, Deletion Constructs and Sit- Directed Mutagenesis
2.12. Conservation Analysis
2.13. Statistical Analysis
3. Results
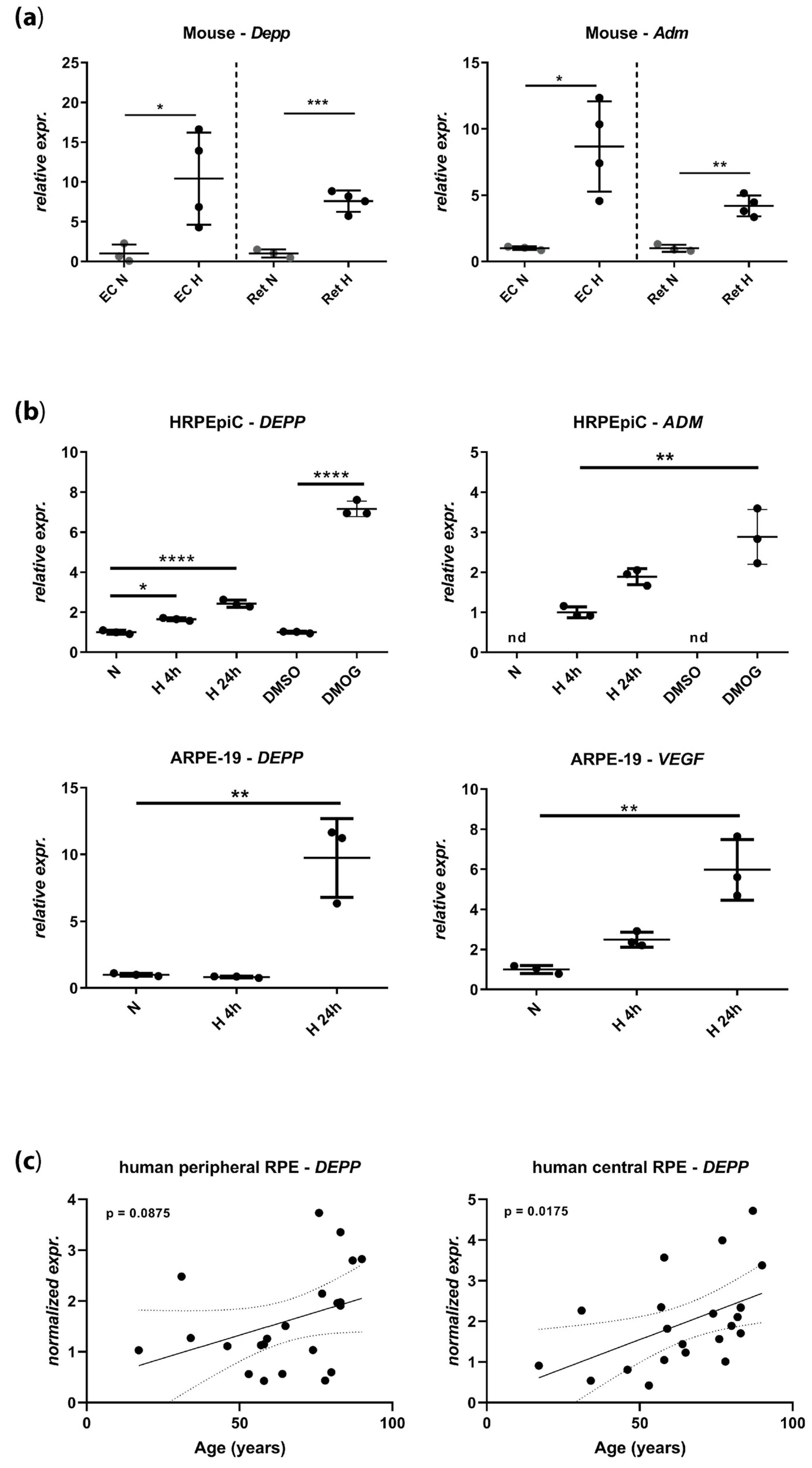
3.1. DEPP Is Upregulated in Hypoxic Ocular Tissues and Cells
3.2. DEPP Is a HIF1 and HIF2 Target Gene in the ARPE-19 Human Cell Line
3.3. A 3.8 kb Fragment Upstream of the Transcription Start Site Is Sufficient for Hypoxic Regulation of DEPP Expression
3.4. Disruption of HREs 25, 26, and 27 Individually or in Combination Abolishes the Hypoxic Response
3.5. HRE 25 Is Conserved across Species
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klein, R.; Klein, B.E.; Cruickshanks, K.J. The prevalence of age-related maculopathy by geographic region and ethnicity. Prog. Retin. Eye Res. 1999, 18, 371–389. [Google Scholar] [CrossRef]
- Hageman, G.S.; Anderson, D.H.; Johnson, L.V.; Hancox, L.S.; Taiber, A.J.; Hardisty, L.I.; Hageman, J.L.; Stockman, H.A.; Borchardt, J.D.; Gehrs, K.M.; et al. A common haplotype in the complement regulatory gene factor h (hf1/cfh) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 7227–7232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet. Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (vegf trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef] [Green Version]
- Gragoudas, E.S.; Adamis, A.P.; Cunningham, E.T., Jr.; Feinsod, M.; Guyer, D.R. Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 2004, 351, 2805–2816. [Google Scholar] [CrossRef] [Green Version]
- Morstein, J.; Trauner, D. New players in phototherapy: Photopharmacology and bio-integrated optoelectronics. Curr. Opin. Chem. Biol. 2019, 50, 145–151. [Google Scholar] [CrossRef]
- Okawa, H.; Sampath, A.P.; Laughlin, S.B.; Fain, G.L. Atp consumption by mammalian rod photoreceptors in darkness and in light. Curr. Biol. Cb 2008, 18, 1917–1921. [Google Scholar] [CrossRef] [Green Version]
- Niven, J.E.; Laughlin, S.B. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 2008, 211, 1792–1804. [Google Scholar] [CrossRef] [Green Version]
- Feeney-Burns, L.; Ellersieck, M.R. Age-related changes in the ultrastructure of bruch’s membrane. Am. J. Ophthalmol. 1985, 100, 686–697. [Google Scholar] [CrossRef]
- Berenberg, T.L.; Metelitsina, T.I.; Madow, B.; Dai, Y.; Ying, G.S.; Dupont, J.C.; Grunwald, L.; Brucker, A.J.; Grunwald, J.E. The association between drusen extent and foveolar choroidal blood flow in age-related macular degeneration. Retina 2012, 32, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Bergen, A.A.; Arya, S.; Koster, C.; Pilgrim, M.G.; Wiatrek-Moumoulidis, D.; van der Spek, P.J.; Hauck, S.M.; Boon, C.J.F.; Emri, E.; Stewart, A.J.; et al. On the origin of proteins in human drusen: The meet, greet and stick hypothesis. Prog. Retin. Eye Res. 2019, 70, 55–84. [Google Scholar] [CrossRef] [Green Version]
- McHugh, K.J.; Li, D.; Wang, J.C.; Kwark, L.; Loo, J.; Macha, V.; Farsiu, S.; Kim, L.A.; Saint-Geniez, M. Computational modeling of retinal hypoxia and photoreceptor degeneration in patients with age-related macular degeneration. PLoS ONE 2019, 14, e0216215. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Wenzel, A.; Groszer, M.; Mayser, H.; Seeliger, M.; Samardzija, M.; Bauer, C.; Gassmann, M.; Reme, C.E. Hif-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat. Med. 2002, 8, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Barben, M.; Ail, D.; Storti, F.; Klee, K.; Schori, C.; Samardzija, M.; Michalakis, S.; Biel, M.; Meneau, I.; Blaser, F.; et al. Hif1a inactivation rescues photoreceptor degeneration induced by a chronic hypoxia-like stress. Cell Death Differ. 2018, 25, 2071–2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barben, M.; Schori, C.; Samardzija, M.; Grimm, C. Targeting hif1a rescues cone degeneration and prevents subretinal neovascularization in a model of chronic hypoxia. Mol. Neurodegener. 2018, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, J.; Petrovski, G.; Vereb, Z.; Facsko, A.; Kaarniranta, K. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. Biomed Res. Int. 2014, 2014, 768026. [Google Scholar] [CrossRef]
- Arjamaa, O.; Nikinmaa, M.; Salminen, A.; Kaarniranta, K. Regulatory role of hif-1alpha in the pathogenesis of age-related macular degeneration (amd). Ageing Res. Rev. 2009, 8, 349–358. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the hif hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014, 9, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Discher, D.J.; Bishopric, N.H.; Webster, K.A. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem. Biophys. Res. Commun. 1998, 245, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Resta, T.C. Estradiol attenuates hypoxia-induced pulmonary endothelin-1 gene expression. Am J. Physiol. Lung Cell Mol. Physiol. 2002, 283, L86–L93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storti, F.; Santambrogio, S.; Crowther, L.M.; Otto, T.; Abreu-Rodriguez, I.; Kaufmann, M.; Hu, C.J.; Dame, C.; Fandrey, J.; Wenger, R.H.; et al. A novel distal upstream hypoxia response element regulating oxygen-dependent erythropoietin gene expression. Haematologica 2014, 99, e45–e48. [Google Scholar] [CrossRef] [Green Version]
- Schorg, A.; Santambrogio, S.; Platt, J.L.; Schodel, J.; Lindenmeyer, M.T.; Cohen, C.D.; Schrodter, K.; Mole, D.R.; Wenger, R.H.; Hoogewijs, D. Destruction of a distal hypoxia response element abolishes trans-activation of the pag1 gene mediated by hif-independent chromatin looping. Nucleic Acids Res. 2015, 43, 5810–5823. [Google Scholar] [CrossRef]
- Thiersch, M.; Raffelsberger, W.; Frigg, R.; Samardzija, M.; Wenzel, A.; Poch, O.; Grimm, C. Analysis of the retinal gene expression profile after hypoxic preconditioning identifies candidate genes for neuroprotection. BMC Genom. 2008, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Nonoguchi, K.; Sakurai, T.; Masuda, T.; Itoh, K.; Fujita, J. A novel protein depp, which is induced by progesterone in human endometrial stromal cells activates elk-1 transcription factor. Mol. Hum. Reprod. 2005, 11, 471–476. [Google Scholar] [CrossRef]
- Ragel, B.T.; Couldwell, W.T.; Gillespie, D.L.; Jensen, R.L. Identification of hypoxia-induced genes in a malignant glioma cell line (u-251) by cdna microarray analysis. Neurosurg. Rev. 2007, 30, 181–187. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kuriyama, H.; Kihara, S.; Kishida, K.; Maeda, N.; Hibuse, T.; Nishizawa, H.; Matsuda, M.; Funahashi, T.; Shimomura, I. Insulin-mediated regulation of decidual protein induced by progesterone (depp) in adipose tissue and liver. Horm. Metab. Res. Horm. Stoffwechs. Horm. Metab. 2010, 42, 173–177. [Google Scholar] [CrossRef]
- Salcher, S.; Hagenbuchner, J.; Geiger, K.; Seiter, M.A.; Rainer, J.; Kofler, R.; Hermann, M.; Kiechl-Kohlendorfer, U.; Ausserlechner, M.J.; Obexer, P. C10orf10/depp, a transcriptional target of foxo3, regulates ros-sensitivity in human neuroblastoma. Mol. Cancer 2014, 13, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yabuta, N.; Onda, H.; Watanabe, M.; Yoshioka, N.; Nagamori, I.; Funatsu, T.; Toji, S.; Tamai, K.; Nojima, H. Isolation and characterization of the tiga genes, whose transcripts are induced by growth arrest. Nucleic Acids Res. 2006, 34, 4878–4892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salcher, S.; Hermann, M.; Kiechl-Kohlendorfer, U.; Ausserlechner, M.J.; Obexer, P. C10orf10/depp-mediated ros accumulation is a critical modulator of foxo3-induced autophagy. Mol. Cancer 2017, 16, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Gai, J.; Wang, Y.; Li, H. Foxo regulates expression of decidual protein induced by progesterone (depp) in human endothelial cells. FEBS Lett. 2011, 585, 1796–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kast, B.; Schori, C.; Grimm, C. Hypoxic preconditioning protects photoreceptors against light damage independently of hypoxia inducible transcription factors in rods. Exp. Eye Res. 2016, 146, 60–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storti, F.; Raphael, G.; Griesser, V.; Klee, K.; Drawnel, F.; Willburger, C.; Scholz, R.; Langmann, T.; von Eckardstein, A.; Fingerle, J.; et al. Regulated efflux of photoreceptor outer segment-derived cholesterol by human rpe cells. Exp. Eye Res. 2017, 165, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Siepel, A.; Bejerano, G.; Pedersen, J.S.; Hinrichs, A.S.; Hou, M.; Rosenbloom, K.; Clawson, H.; Spieth, J.; Hillier, L.W.; Richards, S.; et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005, 15, 1034–1050. [Google Scholar] [CrossRef] [Green Version]
- Karolchik, D.; Hinrichs, A.S.; Furey, T.S.; Roskin, K.M.; Sugnet, C.W.; Haussler, D.; Kent, W.J. The ucsc table browser data retrieval tool. Nucleic Acids Res. 2004, 32, D493–D496. [Google Scholar] [CrossRef]
- Corpet, F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988, 16, 10881–10890. [Google Scholar] [CrossRef]
- Stepp, M.W.; Folz, R.J.; Yu, J.; Zelko, I.N. The c10orf10 gene product is a new link between oxidative stress and autophagy. Biochim. Biophys. Acta 2014, 1843, 1076–1088. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.T.; Kong, G.; Denardo, D.; Li, Y.; Uray, I.; Pal, S.; Mohsin, S.; Hilsenbeck, S.G.; Bissonnette, R.; Lamph, W.W.; et al. Identification of biomarkers modulated by the rexinoid lgd1069 (bexarotene) in human breast cells using oligonucleotide arrays. Cancer Res. 2006, 66, 12009–12018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Ji, M.; Lin, Y.; Miao, Y.; Chen, S.; Li, H. Depp/depp1/c10orf10 regulates hepatic glucose and fat metabolism partly via ros-induced fgf21. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 5459–5469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schodel, J.; Oikonomopoulos, S.; Ragoussis, J.; Pugh, C.W.; Ratcliffe, P.J.; Mole, D.R. High-resolution genome-wide mapping of hif-binding sites by chip-seq. Blood 2011, 117, e207–e217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenger, R.H.; Stiehl, D.P.; Camenisch, G. Integration of oxygen signaling at the consensus hre. Sci. STKE Signal Transduct. Knowl. Environ. 2005, 2005, re12. [Google Scholar] [CrossRef] [Green Version]
- Fukasawa, M.; Tsuchiya, T.; Takayama, E.; Shinomiya, N.; Uyeda, K.; Sakakibara, R.; Seki, S. Identification and characterization of the hypoxia-responsive element of the human placental 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene. J. Biochem. 2004, 136, 273–277. [Google Scholar] [CrossRef]
- Chan, K.; Kan, Y.W. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 12731–12736. [Google Scholar] [CrossRef] [Green Version]
- Toth, R.K.; Warfel, N.A. Strange bedfellows: Nuclear factor, erythroid 2-like 2 (nrf2) and hypoxia-inducible factor 1 (hif-1) in tumor hypoxia. Antioxidants 2017, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.; Anderson, D.J. Isolation of arterial-specific genes by subtractive hybridization reveals molecular heterogeneity among arterial endothelial cells. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 233, 1589–1604. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on rpe degeneration in non-neovascular amd. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]




| Age | Gender | Known Disease | Cause of Death |
|---|---|---|---|
| 17 | M | - | Brain trauma and multiple contusions |
| 31 | M | - | Trauma |
| 34 | F | Alcohol addiction | Brain trauma and multiple contusions |
| 46 | M | Leukaemia | Chickenpox (varicella) virus (VZV) infection with encephalitis |
| 53 | M | Hypertension | Brain hemorrhage |
| 57 | M | - | Hemorrhage |
| 58 | M | Colitis ulcerosa | Brain trauma |
| 58 | F | Depression | Acute liver failure |
| 59 | F | Diabetes Type 1 | Heart failure |
| 64 | M | - | Heart failure |
| 65 | M | Diabetes Type 2 | Hemorrhage |
| 74 | M | Adenocarcinoma | Prostate adenocarcinoma |
| 76 | M | Adenocarcinoma | Adenocarcinoma at the intestine + lung metastasis |
| 77 | M | - | Brain hemorrhage |
| 78 | M | Coronary disease | Heart failure |
| 80 | F | Coronary disease | Cerebral ischemia |
| 82 | M | Adenocarcinoma | Gastroesophageal adenocarcinoma |
| 83 | F | - | Septic shock |
| 83 | F | Hypertension | Cerebral ischemia |
| 83 | F | Epilepsy | Brain trauma |
| 87 | M | Pancreas carcinoma | Collapse |
| 90 | M | - | Heart failure |
| siRNA | Sequence (5′-3′) | Antisense (5′-3′) |
|---|---|---|
| siHIF1A | CUGAUGACCAGCAACUUGA-dTdT | UCAAGUUGCUGGUCAUCAG -dTdT |
| siHIF2A | CAGGUGGAGCUAACAGGACAUAGUA-dTdT | UACUAUGUCCUGUUAGCUCCACCUG -dTdT |
| Gene | Forward (5′-3′) | Reverse (5′-3′) | Product Length |
|---|---|---|---|
| Adm (mouse) | TCCTGGTTTCTCGGCTTCTC | ATTCTGTGGCGATGCTCTGA | 133 bp |
| ADM (human) | TTGGACTTCGGAGTTTTGCC | CCCACTTATTCCACTTCTTTCG | 149 bp |
| Actb (mouse) | CAACGGCTCCGGCATGTGC | CTCTTGCTCTGGGCCTCG | 153 bp |
| ACTB (human) | CCTGGCACCCAGCACAAT | GGGCCGGACTCGTCATAC | 144 bp |
| Depp (mouse) | CCCTGACTGCTGACTTACA | TTCCCGAATCGTTGGCA | 76 bp |
| DEPP (human) | TGTCCCTGCTCATCCATTCTC | CTCACGTAGTCATCCAGGC | 199 bp |
| HIF1A (human) | TTCACCTGAGCCTAATAGTCCC | TCATCCATTGATTGCCCCAGCAGTC | 276 bp |
| HIF2A (human) | CGGAGAGGAGGAAGGAGAAG | AGAGCAAACTGAGGAGAGGAG | 191 bp |
| VEGF (human) | GTGGACATCTTCCAGGAGTACC | TGTTGTGCTGTAGGAAGCTCAT | 205 bp |
| Antibody | Dilution | Catalogue Number | Company |
|---|---|---|---|
| Anti-HIF1A | 1:2000 | NB100-479 | Novus Biologicals |
| Anti-HIF2A | 1:2000 | PAB12124 | Abnova |
| Anti-ACTB | 1:10,000 | A5441 | Sigma-Aldrich |
| HRP conj. Sec. Antibody anti-Mouse | 1:10,000 | sc-2031 | Santa-Cruz |
| HRP conj. Sec. Antibody anti-Rabbit | 1:10,000 | #7074 | Cell Signalling |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klee, K.; Storti, F.; Maggi, J.; Todorova, V.; Karademir, D.; Berger, W.; Samardzija, M.; Grimm, C. The Expression of Decidual Protein Induced by Progesterone (DEPP) Is Controlled by Three Distal Consensus Hypoxia Responsive Element (HRE) in Hypoxic Retinal Epithelial Cells. Genes 2020, 11, 111. https://doi.org/10.3390/genes11010111
Klee K, Storti F, Maggi J, Todorova V, Karademir D, Berger W, Samardzija M, Grimm C. The Expression of Decidual Protein Induced by Progesterone (DEPP) Is Controlled by Three Distal Consensus Hypoxia Responsive Element (HRE) in Hypoxic Retinal Epithelial Cells. Genes. 2020; 11(1):111. https://doi.org/10.3390/genes11010111
Chicago/Turabian StyleKlee, Katrin, Federica Storti, Jordi Maggi, Vyara Todorova, Duygu Karademir, Wolfgang Berger, Marijana Samardzija, and Christian Grimm. 2020. "The Expression of Decidual Protein Induced by Progesterone (DEPP) Is Controlled by Three Distal Consensus Hypoxia Responsive Element (HRE) in Hypoxic Retinal Epithelial Cells" Genes 11, no. 1: 111. https://doi.org/10.3390/genes11010111
APA StyleKlee, K., Storti, F., Maggi, J., Todorova, V., Karademir, D., Berger, W., Samardzija, M., & Grimm, C. (2020). The Expression of Decidual Protein Induced by Progesterone (DEPP) Is Controlled by Three Distal Consensus Hypoxia Responsive Element (HRE) in Hypoxic Retinal Epithelial Cells. Genes, 11(1), 111. https://doi.org/10.3390/genes11010111





