Effects of Intronic SNPs in the Myostatin Gene on Growth and Carcass Traits in Colored Polish Merino Sheep
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Growth Traits
2.3. Carcass Traits
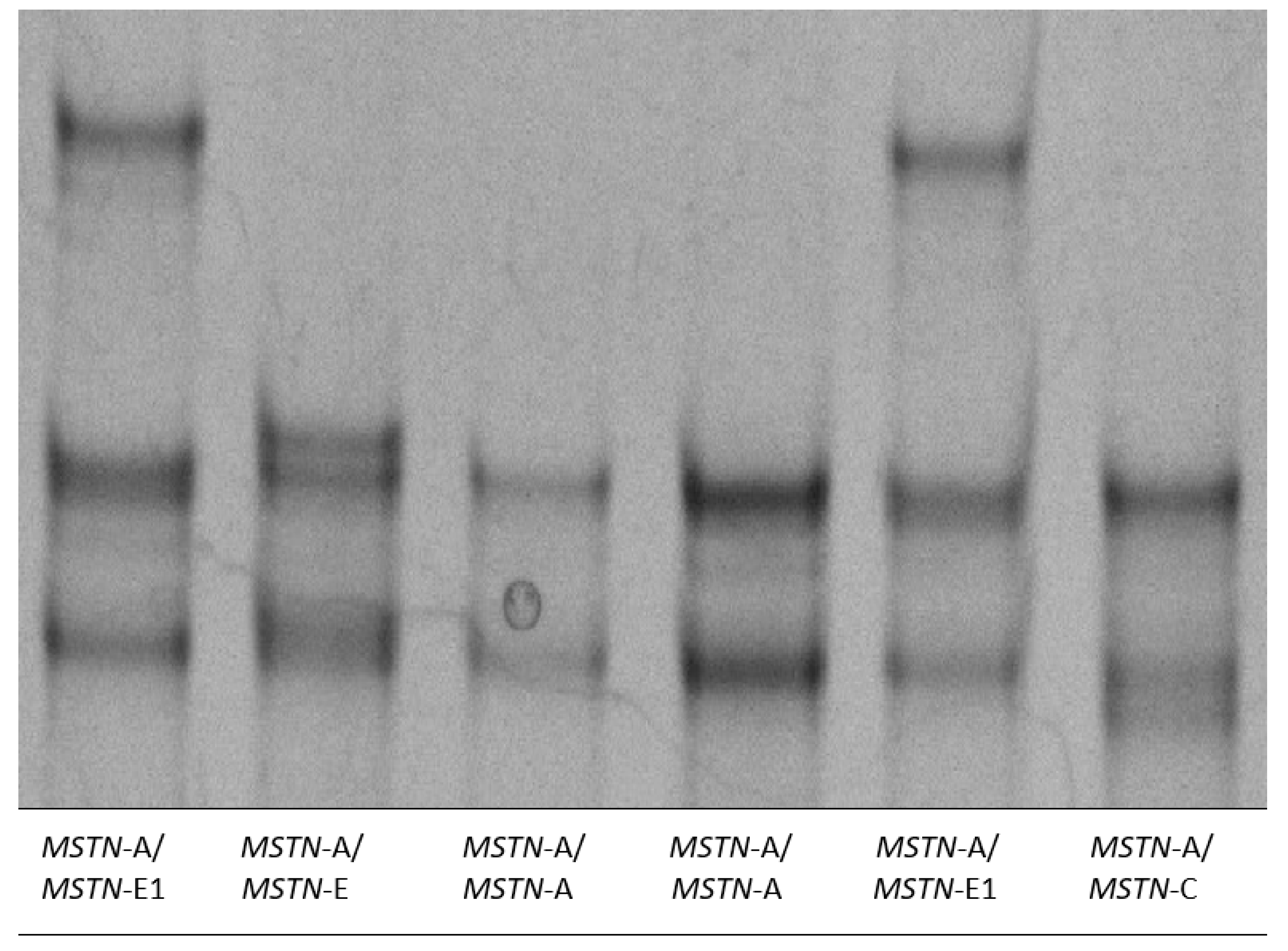
2.4. Genotyping of the Ovine MSTN Gene
2.5. Transcription Factor Binding Sites (TFBSs) Prediction
2.6. Statistical Analyses
3. Results
3.1. Detected MSTN Alleles and Genotypes and Their Frequencies
3.2. Transcription Factor Binding Sites (TFBSs)
3.3. Effects of MSTN Alleles and Genotypes on Growth Traits
3.4. Effects of MSTN Alleles and Genotypes on Slaughter Traits
4. Discussion
4.1. Detected MSTN Alleles and Genotypes and their Frequencies
4.2. Effects of MSTN Alleles and Genotypes on Growth Traits
4.3. Effects of MSTN Alleles and Genotypes on Slaughter Traits
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McPherron, A.C.; Lee, S.-J. The transforming growth factor β superfamily. In Growth Factors Cytokines Health Disease; Elsevier: Amsterdam, The Netherlands, 1996; Volume 1, pp. 357–393. [Google Scholar]
- Sharma, M.; Kambadur, R.; Matthews, K.G.; Somers, W.G.; Devlin, G.P.; Conaglen, J.V.; Fowke, P.J.; Bass, J.J. Myostatin, a transforming growth factor-β superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J. Cell. Physiol. 1999, 9, 1–9. [Google Scholar] [CrossRef]
- Zerbino, D.R.; Achuthan, P.; Akanni, W.; Amode, M.R.; Barrell, D.; Bhai, J.; Billis, K.; Cummins, C.; Gall, A.; Gil, L.; et al. Ensembl 2018. Nucleic Acids Res. 2018, 46, 754–761. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lee, S.-J. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 1997, 94, 12457–12461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boman, I.A.; Klemetsdal, G.; Blichfeldt, T.; Nafstad, O.; Våge, D.I. A frameshift mutation in the coding region of the myostatin gene (MSTN) affects carcass conformation and fatness in Norwegian White Sheep (Ovis aries). Anim. Genet. 2009, 40, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Boman, I.A.; Klemetsdal, G.; Nafstad, O.; Blichfeldt, T.; Våge, D.I. Impact of two myostatin (MSTN) mutations on weight gain and lamb carcass classification in Norwegian White Sheep (Ovis aries). Genet. Sel. Evol. 2010, 42, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boman, I.A.; Våge, D.I. An insertion in the coding region of the myostatin (MSTN) gene affects carcass conformation and fatness in the Norwegian Spælsau (Ovis aries). BMC Res. Notes 2009, 2, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjan, A.; Jeichitra, V.; Rajendran, R.; Raja, A. Polymorphism in exon 3 of myostatin (MSTN) gene and its association with growth traits in Indian sheep breeds. Small Rumin. Res. 2017, 149, 81–84. [Google Scholar]
- Zhou, H.; Hickford, J.G.H.; Fang, Q. Variation in the coding region of the myostatin (GDF8) gene in sheep. Mol. Cell. Probes 2008, 22, 67–68. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Yu, Y.; E-er, H.; Ma, Q. SNP Genotyping of myostatin (MSTN) gene through TaqMan probe assay and association study between MSTN genotypes and growth traits of tan sheep. Anim. Husb. Feed Sci. 2016, 8, 333–335. [Google Scholar]
- Han, J.; Forrest, R.H.; Hickford, J.G.H. Genetic variations in the myostatin gene (MSTN) in New Zealand sheep breeds. Mol. Biol. Rep. 2013, 40, 6379–6384. [Google Scholar] [CrossRef]
- Trukhachev, V.; Yatsyk, O.; Telegina, E.; Krivoruchko, A.; Zhou, H.; Hickford, J.G.H. Comparison of the myostatin (MSTN) gene in Russian Stavropol Merino sheep and New Zealand Merino sheep. Small Rumin. Res. 2018, 160, 103–106. [Google Scholar] [CrossRef]
- Clop, A.; Marcq, F.; Takeda, H.; Pirottin, D.; Tordoir, X.; Bibé, B.; Bouix, J.; Caiment, F.; Elsen, J.M.; Eychenne, F.; et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat. Genet. 2006, 38, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.Q.; Du, Z.; Liu, S.R.; Yang, Y.L.; Shen, M.; Wang, X.H.; Yin, J.L.; Hu, X.X.; Fei, J.; Fan, J.J.; et al. Association of SNP haplotypes at the myostatin gene with muscular hypertrophy in sheep. Asian Australas. J. Anim. Sci. 2008, 21, 928–935. [Google Scholar] [CrossRef]
- Kijas, J.W.; McCulloch, R.; Edwards, J.E.H.; Oddy, V.H.; Lee, S.H.; Van der Werf, J. Evidence for multiple alleles effecting muscling and fatness at the Ovine GDF8 locus. BMC Genet. 2007, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhou, H.; Hu, J.; Li, S.; Luo, Y.; Hickford, J.G.H. Two single nucleotide polymorphisms in the promoter of the ovine myostatin gene (MSTN) and their effect on growth and carcass muscle traits in New Zealand Romney sheep. J. Anim. Breed. Genet. 2016, 133, 219–226. [Google Scholar] [CrossRef]
- Hickford, J.G.H.; Forrest, R.H.; Zhou, H.; Fang, Q.; Han, J.; Frampton, C.M.; Horrell, A.L. Polymorphisms in the ovine myostatin gene (MSTN) and their association with growth and carcass traits in New Zealand Romney sheep. Anim. Genet. 2010, 41, 64–72. [Google Scholar] [CrossRef]
- Sjakste, T.; Paramonova, N.; Grislis, Z.; Trapina, I.; Kairisa, D. Analysis of the single-nucleotide polymorphism in the 5′UTR and part of intron I of the sheep MSTN gene. DNA Cell Biol. 2011, 30, 433–444. [Google Scholar] [CrossRef]
- Sartori, R.; Milan, G.; Patron, M.; Mammucari, C.; Blaauw, B.; Abraham, R.; Sandri, M. Smad2 and 3 transcription factors control muscle mass in adulthood. AJP Cell Physiol. 2009, 296, C1248–C1257. [Google Scholar] [CrossRef] [Green Version]
- Mann, R.S.; Lelli, K.M.; Joshi, R. Hox specificity: Unique roles for cofactors and collaborators. Curr. Top. Dev. Biol. 2009, 88, 63–101. [Google Scholar]
- Han, J.; Zhou, H.; Forrest, R.H.; Sedcole, J.R.; Frampton, C.M.; Hickford, J.G.H. Effect of myostatin (MSTN) g + 6223G > A on production and carcass traits in New Zealand Romney sheep. Asian Australas. J. Anim. Sci. 2010, 23, 863–866. [Google Scholar] [CrossRef]
- Kawęcka, A.; Sikora, J. Owce. In Polskie Rasy Zachowawcze. Atlas Zwierząt Gospodarskich Objętych Programem Ochrony w Polsce; Krupiński, J., Ed.; National Research Institute of Animal Production: Cracow, Poland, 2012; pp. 33–52. [Google Scholar]
- Grochowska, E.; Borys, B.; Janiszewski, P.; Knapik, J.; Mroczkowski, S. Effect of the IGF-I gene polymorphism on growth, body size, carcass and meat quality traits in Coloured Polish Merino sheep. Arch. Anim. Breed. 2017, 60, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Grochowska, E.; Borys, B.; Lisiak, D.; Mroczkowski, S. Genotypic and allelic effects of the myostatin gene (MSTN) on carcass, meat quality, and biometric traits in Colored Polish Merino sheep. Meat Sci. 2019, 151, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Nawara, W.; Osikowski, M.; Kultz, I.; Modelska, M. Wycena Tryków na Podstawie Badania Wartości Potomstwa w Stacjach Oceny Tryków Instytutu Zootechniki za Rok 1962, 166th ed.; National Research Institute of Animal Production: Cracow, Poland, 1963. [Google Scholar]
- Krupiński, J.; Borys, B.; Kmieć, W.; Knapik, J.; Korman, K.; Osikowski, M.; Pompa-Roborzyński, M.; Rzepecki, R. Ocena Użytkowości Mięsnej Jagniąt na Tle Wymogów oraz Metod Stosowanych w Krajach Unii Europejskiej; National Research Institute of Animal Production: Cracow, Poland, 2009. [Google Scholar]
- Hu, H.; Miao, Y.R.; Jia, L.H.; Yu, Q.Y.; Zhang, Q.; Guo, A.Y. AnimalTFDB 3.0: A comprehensive resource for annotation and prediction of animal transcription factors. Nucleic Acids Res. 2019, 47, D33–D38. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT 9.4 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2014. [Google Scholar]
- Kolenda, M.; Grochowska, E.; Milewski, S.; Mroczkowski, S. The association between the polymorphism in the myostatin gene and growth traits in Kamieniec and Pomeranian sheep breeds. Small Rumin. Res. 2019, 177, 29–35. [Google Scholar] [CrossRef]
- Michel, R.N.; Dunn, S.E.; Chin, E.R. Calcineurin and skeletal muscle growth. Proc. Nutr. Soc. 2004, 63, 341–349. [Google Scholar] [CrossRef]
- Trukhachev, V.; Belyaev, V.; Kvochko, A.; Kulichenko, A.; Kovalev, D.; Pisarenko, S.; Volynkina, A.; Selionova, M.; Aybazov, M.; Shumaenko, S.; et al. Myostatin gene (MSTN) polymorphism with a negative effect on meat productivity in Dzhalginsky Merino sheep breed. J. Biosci. Biotechnol. 2015, 4, 191–199. [Google Scholar]
- Farhadian, M.; Hashemi, A.; Mardani, K.; Darvishzadeh, R.; Jafari, S. Polymorphisms in the ovine myostatin gene are associated with birth weight but not with weight gain in Iranian Makoei sheep. Genet. Mol. Res. 2012, 11, 20121004. [Google Scholar] [CrossRef]
- Haynes, F.E.M.; Greenwood, P.L.; McDonagh, M.B.; McMahon, C.D.; Nicholas, G.D.; Berry, C.J.; Oddy, V.H. Lack of association between allelic status and myostatin content in lambs with the myostatin g + 6723G > A allele. J. Anim. Sci. 2013, 91, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Masri, A.Y.; Lambe, N.R.; Macfarlane, J.M.; Brotherstone, S.; Haresign, W.; Bünger, L. Evaluating the effects of a single copy of a mutation in the myostatin gene (c.*1232G > A) on carcass traits in crossbred lambs. Meat Sci. 2011, 87, 412–418. [Google Scholar] [CrossRef]
- Masri, A.Y.; Lambe, N.R.; Macfarlane, J.M.; Brotherstone, S.; Haresign, W.; Bünger, L. Evaluating the effects of the c.*1232G > A mutation and TM-QTL in Texel × Welsh Mountain lambs using ultrasound and video image analyses. Small Rumin. Res. 2011, 99, 99–109. [Google Scholar] [CrossRef]
- Han, J.; Forrest, R.H.; Sedcole, J.R.; Hickford, J.G.H. Myostatin (MSTN) gene haplotypes and their association with growth and carcass traits in New Zealand Romney lambs. Small Rumin. Res. 2015, 127, 8–19. [Google Scholar] [CrossRef]
- Johnson, P.L.; Dodds, K.G.; Bain, W.E.; Greer, G.J.; McLean, N.J.; McLaren, R.J.; Galloway, S.M.; van Stijn, T.C.; McEwan, J.C. Investigations into the GDF8 g + 6723G-A polymorphism in New Zealand Texel sheep. J. Anim. Sci. 2009, 87, 1856–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, E.; Keele, J.W.; Fahrenkrug, S.C.; Smith, T.P.L.; Cundiff, L.V.; Stone, R.T. Quantitative analysis of birth, weaning, and yearling weights and calving difficulty in piedmontese crossbreds segregating an inactive myostatin allele. J. Anim. Sci. 1999, 77, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Short, R.E.; MacNeil, M.D.; Grosz, M.D.; Gerrard, D.E.; Grings, E.E. Pleiotropic effects in Hereford, Limousin, and Piedmontese F2 crossbred calves of genes controlling muscularity including the Piedmontese myostatin allele. J. Anim. Sci. 2002, 80, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Crispo, M.; Mulet, A.P.; Tesson, L.; Barrera, N.; Cuadro, F.; Dos Santos-Neto, P.C.; Nguyen, T.H.; Crénéguy, A.; Brusselle, L.; Anegón, I.; et al. Efficient generation of myostatin knock-out sheep using CRISPR/Cas9 technology and microinjection into zygotes. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, G.; Hao, Z.; Zhang, G.; Qing, Y.; Liu, S.; Qing, L.; Pan, W.; Chen, L.; Liu, G.; et al. Generation of biallelic knock-out sheep via gene-editing and somatic cell nuclear transfer. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hadjipavlou, G.; Matika, O.; Clop, A.; Bishop, S.C. Two single nucleotide polymorphisms in the myostatin (GDF8) gene have significant association with muscle depth of commercial Charollais sheep. Anim. Genet. 2008, 39, 346–353. [Google Scholar] [CrossRef]

| Nucleotide Position | SNP | Allele | |||
|---|---|---|---|---|---|
| MSTN-A | MSTN-C | MSTN-E | MSTN-E1 | ||
| c.373+18 | G/T | G | T | T | T |
| c.373+101 | C/T | C | C | C | T |
| c.373+240 | T | T | T | T | T |
| c.373+241 | T | T | T | T | T |
| c.373+243 | A/G | G | A | G | G |
| c.373+246 | T | T | T | T | T |
| c.373+249 | C/T | T | C | T | T |
| c.373+259 | G/T | G | T | T | T |
| c.373+323 | C/T | C | T | C | C |
| Allele/Genotype (n = 264) | Frequency (%) | |
|---|---|---|
| Allele | MSTN-A | 87.7 |
| MSTN-C | 2.3 | |
| MSTN-E | 5.5 | |
| MSTN-E1 | 4.5 | |
| Genotype | MSTN-A/MSTN-A | 75.4 |
| MSTN-A/MSTN-C | 4.5 | |
| MSTN-A/MSTN-E | 11.0 | |
| MSTN-A/MSTN-E1 | 9.1 | |
| Trait | Unit | LSM 1 ± Standard Error | p-Value | |||
|---|---|---|---|---|---|---|
| MSTN-A/MSTN-A | MSTN-A/MSTN-C | MSTN-A/MSTN-E | MSTN-A/MSTN-E1 | |||
| n = 264 | 199 | 12 | 29 | 24 | ||
| Body weight at 2nd day of life | kg | 5.0 ± 0.08 a | 5.3 ± 0.22 a,b | 5.1 ± 0.14 a,b | 5.5 ± 0.18 b | 0.042 |
| Body weight at 30th day of life | kg | 12.7 ± 0.17 | 12.9 ± 0.54 | 12.5 ± 0.35 | 13.1 ± 0.42 | 0.676 |
| Body weight at 56th day of life | kg | 19.2 ± 0.32 | 19.3 ± 0.81 | 19.2 ± 0.55 | 19.3 ± 0.69 | 0.999 |
| Body weight at 78th day of life | kg | 26.4 ± 0.48 | 24.9 ± 1.71 | 26.9 ± 0.76 | 26.7 ± 0.98 | 0.721 |
| Average daily gain between 2nd and 30th day of life | g | 256 ± 4.06 | 253 ± 14.48 | 246 ± 9.27 | 252 ± 10.41 | 0.808 |
| Average daily gain between 30th and 56th day of life | g | 249 ± 6.75 | 243 ± 15.40 | 258 ± 10.74 | 243 ± 13.33 | 0.686 |
| Average daily gain between 56th and 78th day of life | g | 329.8 ± 8.33 | 326.7 ± 23.30 | 319.1 ± 15.54 | 330.0 ± 19.21 | 0.914 |
| Trait | Unit | Allele | LSM 1 ± Standard Error | p-Value | |||
|---|---|---|---|---|---|---|---|
| Number of Copies of the Allele | |||||||
| MSTN-A-1; MSTN-C, MSTN-E and MSTN-E1-0 | n | MSTN-A-2; MSTN-C, MSTN-E and MSTN-E1-1 | n | ||||
| Body weight at 2nd day of life | kg | MSTN-A | 5.2 ± 0.12 | 65 | 5.1 ± 0.09 | 199 | 0.093 |
| MSTN-C | 5.1 ± 0.09 | 252 | 5.3 ± 0.23 | 12 | 0.347 | ||
| MSTN-E | 5.1 ± 0.09 | 235 | 5.06 ± 0.16 | 29 | 0.754 | ||
| MSTN-E1 | 5.1 ± 0.07 a | 240 | 5.5 ± 0.18 b | 24 | 0.006 | ||
| Body weight at 30th day of life | kg | MSTN-A | 12.4 ± 0.27 | 65 | 12.7 ± 0.18 | 199 | 0.996 |
| MSTN-C | 12.7 ± 0.18 | 252 | 12.9 ± 0.54 | 12 | 0.709 | ||
| MSTN-E | 12.8 ±0.18 | 235 | 12.5 ± 0.36 | 29 | 0.386 | ||
| MSTN-E1 | 12.7 ± 0.15 | 240 | 13.1 ± 0.41 | 24 | 0.298 | ||
| Body weight at 56th day of life | kg | MSTN-A | 19.2 ± 0.44 | 65 | 19.2 ± 0.32 | 199 | 0.961 |
| MSTN-C | 19.2 ± 0.31 | 252 | 19.3 ± 0.80 | 12 | 0.937 | ||
| MSTN-E | 19.1 ± 0.32 | 235 | 19.6 ± 0.58 | 29 | 0.415 | ||
| MSTN-E1 | 19.2 ± 0.31 | 240 | 19.3 ± 0.68 | 24 | 0.896 | ||
| Body weight at 78th day of life | kg | MSTN-A | 26.4 ± 0.59 | 65 | 26.5 ± 0.47 | 199 | 0.829 |
| MSTN-C | 26.5 ± 0.46 | 252 | 26.6 ± 1.03 | 12 | 0.877 | ||
| MSTN-E | 26.2 ± 0.44 | 235 | 27.2 ±0.77 | 29 | 0.263 | ||
| MSTN-E1 | 26.5 ± 0.46 | 240 | 26.5 ± 0.90 | 24 | 0.966 | ||
| Average daily gain between 2nd and 30th day of life | g | MSTN-A | 250 ± 6.43 | 65 | 256 ± 4.04 | 199 | 0.388 |
| MSTN-C | 254 ± 3.68 | 252 | 253 ± 14.45 | 12 | 0.937 | ||
| MSTN-E | 255 ± 3.79 | 235 | 246 ± 9.24 | 29 | 0.360 | ||
| MSTN-E1 | 254 ± 3.78 | 240 | 252 ± 10.38 | 24 | 0.822 | ||
| Average daily gain between 30th and 56th day of life | g | MSTN-A | 251 ± 8.71 | 65 | 249 ± 6.71 | 199 | 0.784 |
| MSTN-C | 249 ± 6.43 | 252 | 242 ± 15.32 | 12 | 0.629 | ||
| MSTN-E | 247 ± 6.61 | 235 | 265 ± 11.17 | 29 | 0.077 | ||
| MSTN-E1 | 250 ± 6.65 | 240 | 243 ± 13.33 | 24 | 0.593 | ||
| Average daily gain between 56th and 78th day of life | g | MSTN-A | 324 ± 11.88 | 65 | 330 ± 8.30 | 199 | 0.603 |
| MSTN-C | 329 ± 7.95 | 252 | 327 ± 23.24 | 12 | 0.935 | ||
| MSTN-E | 328 ± 7.68 | 235 | 332 ± 16.30 | 29 | 0.793 | ||
| MSTN-E1 | 328 ± 8.10 | 240 | 330 ± 19.21 | 24 | 0.918 | ||
| Trait | Unit | LSM 1 ± Standard Error | p-Value | |
|---|---|---|---|---|
| MSTN-A/MSTN-A | MSTN-A/MSTN-E | |||
| n | 79 | 12 | ||
| Carcass parts | ||||
| Fore part of the carcass weight | g | 2628 ± 13.23 | 2645 ± 29.98 | 0.599 |
| Full loin part weight | g | 1711 ± 10.96 | 1698 ± 24.18 | 0.539 |
| Leg part weight | g | 2217 ± 9.13 | 2239 ± 22.79 | 0.392 |
| Carcass cuts | ||||
| Scrag weight | g | 370 ± 5.25 | 328 ± 10.30 | 0.2671 |
| Middle neck weight | g | 526 ± 8.90 | 535 ± 15.18 | 0.535 |
| Shoulder weight | g | 1049 ± 5.24 | 1038 ± 11.93 | 0.387 |
| Fore shank weight | g | 308 ± 2.03 a | 327 ± 5.27 b | 0.0018 |
| Breast and flank weight | g | 1029 ± 8.08 | 1050 ± 19.77 | 0.329 |
| Rib weight | g | 473 ± 4.54 | 456 ± 10.04 | 0.104 |
| Loin weight | g | 518 ± 4.73 a | 486 ± 12.44 b | 0.025 |
| Tenderloin weight | g | 58 ± 0.95 | 60 ± 1.87 | 0.218 |
| Leg weight | g | 1815 ±9.10 | 1834 ± 20.47 | 0.365 |
| Hind shank weight | g | 401 ± 2.66 | 402 ± 6.31 | 0.844 |
| Leg | ||||
| Muscle tissue yield | % | 71.8 ± 0.30 | 71.7 ± 0.67 | 0.914 |
| Fat tissue yield | % | 12.4 ± 0.27 | 12.1 ± 0.62 | 0.655 |
| Bone tissue yield | % | 15.2 ± 0.17 | 15.7 ± 0.37 | 0.173 |
| Trait | Unit | Allele | LSM 1 ± Standard Error | p-Value | |||
|---|---|---|---|---|---|---|---|
| Number of Copies of the Allele | |||||||
| MSTN-A-1; MSTN-E and MSTN-E1-0 | n | MSTN-A-2; MSTN-E and MSTN-E1-1 | n | ||||
| Carcass parts | |||||||
| Fore part of the carcass weight | g | MSTN-A | 2645 ± 21.56 | 25 | 2628 ± 12.60 | 79 | 0.491 |
| MSTN-E | 2627 ± 12.25 | 92 | 2633 ± 26.52 | 14 | 0.871 | ||
| MSTN-E1 | 2624 ± 12.45 | 96 | 2624 ± 35.15 | 10 | 0.903 | ||
| Full loin part weight | g | MSTN-A | 1707 ± 17.48 | 25 | 1711 ± 10.33 | 79 | 0.824 |
| MSTN-E | 1711 ± 9.89 | 92 | 1709 ± 21.43 | 14 | 0.947 | ||
| MSTN-E1 | 1708 ± 9.81 | 96 | 1740 ± 27.95 | 10 | 0.266 | ||
| Leg part weight | g | MSTN-A | 2255 ± 17.92 | 25 | 2214 ± 8.91 | 79 | 0.055 |
| MSTN-E | 2219 ± 7.91 | 92 | 2228 ± 22.06 | 14 | 0.695 | ||
| MSTN-E1 | 2221 ± 8.01 | 96 | 2192 ± 24.95 | 10 | 0.266 | ||
| Carcass cuts | |||||||
| Scrag weight | kg | MSTN-A | 389 ± 7.53 a | 25 | 370 ± 4.65 b | 79 | 0.016 |
| MSTN-E | 371 ± 4.92 | 92 | 384 ± 9.43 | 14 | 0.207 | ||
| MSTN-E1 | 373 ± 5.58 | 96 | 363 ± 13.53 | 10 | 0.450 | ||
| Middle neck weight | g | MSTN-A | 535 ± 11.52 | 25 | 526 ± 7.72 | 79 | 0.429 |
| MSTN-E | 526 ± 7.62 | 92 | 530 ± 13.39 | 14 | 0.759 | ||
| MSTN-E1 | 527 ± 8.39 | 96 | 510 ± 19.05 | 10 | 0.367 | ||
| Shoulder weight | g | MSTN-A | 1054 ± 11.90 | 25 | 1051 ± 7.09 | 79 | 0.811 |
| MSTN-E | 1053 ± 6.05 | 92 | 1040 ± 13.90 | 14 | 0.404 | ||
| MSTN-E1 | 1051 ± 6.06 | 96 | 1044 ± 17.94 | 10 | 0.674 | ||
| Fore shank weight | g | MSTN-A | 320 ± 3.82 a | 25 | 308 ± 2.10 b | 79 | 0.005 |
| MSTN-E | 308 ± 2.07 a | 92 | 322 ± 4.91 b | 14 | 0.009 | ||
| MSTN-E1 | 311 ± 2.31 | 96 | 299 ± 7.87 | 10 | 0.147 | ||
| Breast and flank weight | g | MSTN-A | 1049 ± 14.25 | 25 | 1031 ± 8.02 | 79 | 0.268 |
| MSTN-E | 1032 ± 7.65 | 92 | 1050 ± 17.70 | 14 | 0.340 | ||
| MSTN-E1 | 1034 ± 7.22 | 96 | 1045 ± 22.07 | 10 | 0.637 | ||
| Rib weight | g | MSTN-A | 462 ± 6.88 | 25 | 471 ± 4.01 | 79 | 0.223 |
| MSTN-E | 470 ± 3.86 | 92 | 455 ± 8.72 | 14 | 0.100 | ||
| MSTN-E1 | 469 ± 3.79 | 96 | 460 ± 11.16 | 10 | 0.483 | ||
| Loin weight | g | MSTN-A | 495 ± 9.61 | 25 | 516 ± 5.26 | 79 | 0.053 |
| MSTN-E | 515 ± 5.05 | 92 | 500 ± 12.39 | 14 | 0.279 | ||
| MSTN-E1 | 510 ± 4.40 | 96 | 537 ± 14.42 | 10 | 0.083 | ||
| Tenderloin weight | g | MSTN-A | 60 ± 1.38 | 25 | 58 ± 0.85 | 79 | 0.114 |
| MSTN-E | 58 ± 0.77 | 92 | 58 ± 1.64 | 14 | 0.837 | ||
| MSTN-E1 | 58 ± 0.77 | 96 | 56 ± 2.51 | 10 | 0.359 | ||
| Leg weight | g | MSTN-A | 1856 ± 16.45 a | 25 | 1813 ± 8.75 b | 79 | 0.020 |
| MSTN-E | 1817 ± 8.93 | 92 | 1820 ± 18.95 | 14 | 0.899 | ||
| MSTN-E1 | 1820 ± 8.35 | 96 | 1798 ± 24.17 | 10 | 0.372 | ||
| Hind shank weight | g | MSTN-A | 397 ± 4.99 | 25 | 401 ± 3.04 | 79 | 0.499 |
| MSTN-E | 399 ± 2.90 a | 92 | 414 ± 6.30 b | 14 | 0.027 | ||
| MSTN-E1 | 401 ± 2.99 | 96 | 415 ± 9.83 | 10 | 0.155 | ||
| Leg | |||||||
| Muscle tissue yield | % | MSTN-A | 71.6 ± 0.49 | 25 | 71.8 ± 0.29 | 79 | 0.752 |
| MSTN-E | 71.8 ± 0.29 | 92 | 71.6 ± 0.60 | 14 | 0.753 | ||
| MSTN-E1 | 71.8 ± 0.27 | 96 | 71.2 ± 0.78 | 10 | 0.457 | ||
| Fat tissue yield | % | MSTN-A | 12.8 ± 0.56 | 25 | 12.4 ± 0.25 | 79 | 0.510 |
| MSTN-E | 12.6 ± 0.28 | 92 | 12.3 ± 0.59 | 14 | 0.664 | ||
| MSTN-E1 | 12.5 ± 0.25 | 96 | 13.7 ± 0.73 | 10 | 0.094 | ||
| Bone tissue yield | % | MSTN-A | 15.1 ± 0.28 | 25 | 15.2 ± 0.16 | 79 | 0.690 |
| MSTN-E | 15.1 ± 0.15 | 92 | 15.7 ± 0.34 | 14 | 0.123 | ||
| MSTN-E1 | 15.2 ± 0.15 | 96 | 14.6 ± 0.44 | 10 | 0.151 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grochowska, E.; Borys, B.; Mroczkowski, S. Effects of Intronic SNPs in the Myostatin Gene on Growth and Carcass Traits in Colored Polish Merino Sheep. Genes 2020, 11, 2. https://doi.org/10.3390/genes11010002
Grochowska E, Borys B, Mroczkowski S. Effects of Intronic SNPs in the Myostatin Gene on Growth and Carcass Traits in Colored Polish Merino Sheep. Genes. 2020; 11(1):2. https://doi.org/10.3390/genes11010002
Chicago/Turabian StyleGrochowska, Ewa, Bronisław Borys, and Sławomir Mroczkowski. 2020. "Effects of Intronic SNPs in the Myostatin Gene on Growth and Carcass Traits in Colored Polish Merino Sheep" Genes 11, no. 1: 2. https://doi.org/10.3390/genes11010002
APA StyleGrochowska, E., Borys, B., & Mroczkowski, S. (2020). Effects of Intronic SNPs in the Myostatin Gene on Growth and Carcass Traits in Colored Polish Merino Sheep. Genes, 11(1), 2. https://doi.org/10.3390/genes11010002





