The Canonical Wnt Pathway as a Key Regulator in Liver Development, Differentiation and Homeostatic Renewal
Abstract
1. Introduction
1.1. Role of Wnt/β-Catenin in Gastrulation
1.2. Gut Tube Patterning
1.3. Differentiation of Hepatoblasts into Hepatocytes and Cholangiocytes
1.4. Role of Wnt in Directed Differentiation of Pluripotent Stem Cells to Hepatocyte-Like Cells
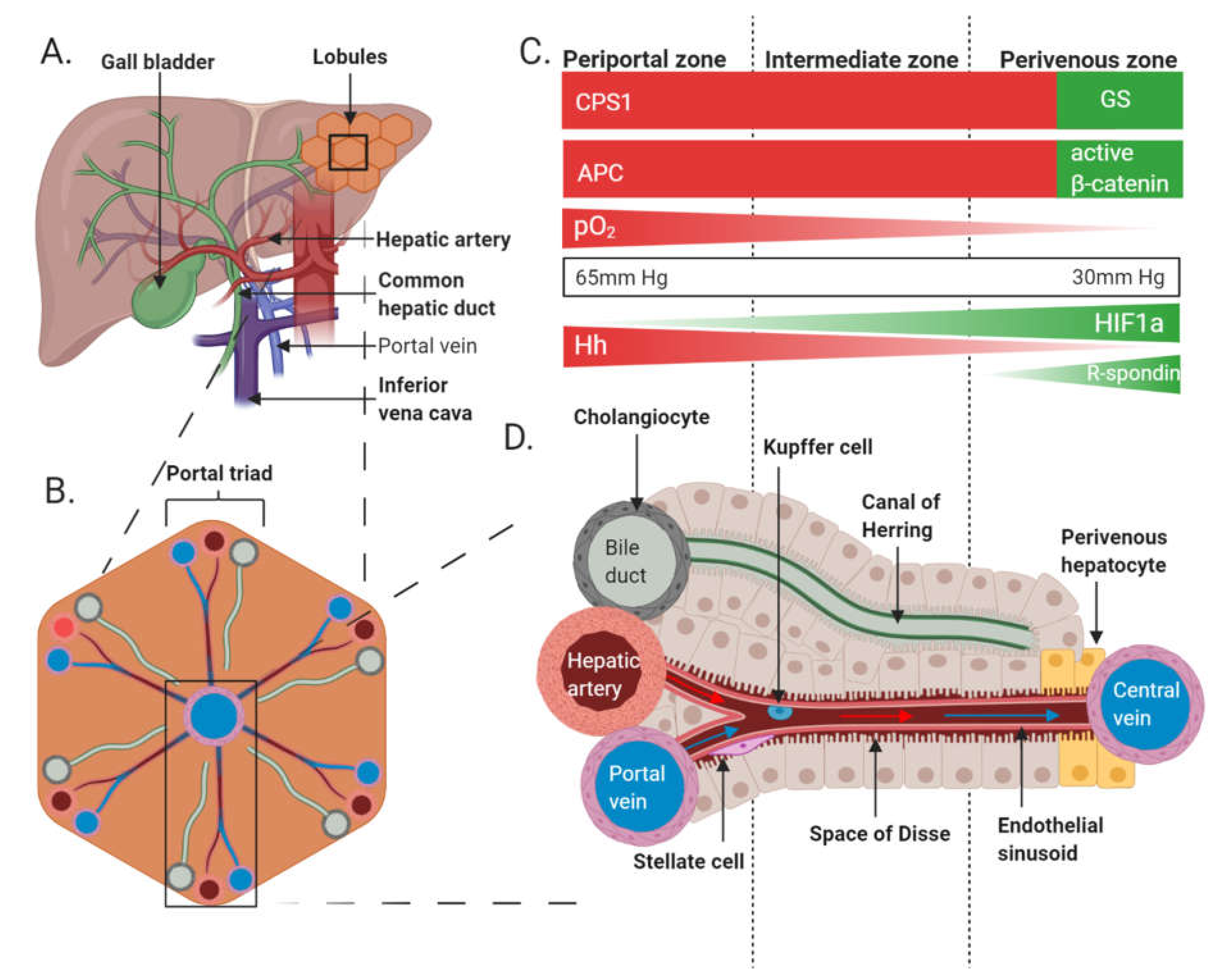
1.5. The Role of Wnt/β-Catenin Signalling in Metabolic Zonation of Adult Liver
1.6. The Role of the Wnt/β-Catenin Pathway in Metabolic Zonation of Liver
1.7. Homeostatic Renewal and Regeneration of the Liver
1.8. Wnt/β-Catenin Crosstalk with Hh and HIF Pathways
2. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Croce, J.C.; McClay, D.R. Evolution of the Wnt pathways. Methods Mol. Biol. 2008, 469, 3–18. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Clevers, H. Wnt signalling in stem cells and cancer. Nature 2005, 434, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Seidensticker, M.J.; Behrens, J. Biochemical interactions in the wnt pathway. Biochim. Biophys. Acta 2000, 1495, 168–182. [Google Scholar] [CrossRef]
- Kelly, O.G.; Pinson, K.I.; Skarnes, W.C. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development 2004, 131, 2803. [Google Scholar] [CrossRef] [PubMed]
- Ikeya, M.; Takada, S. Wnt signaling from the dorsal neural tube is required for the formation of the medial dermomyotome. Development 1998, 125, 4969. [Google Scholar] [PubMed]
- Ishikawa, T.; Tamai, Y.; Zorn, A.M.; Yoshida, H.; Seldin, M.F.; Nishikawa, S.; Taketo, M.M. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development 2001, 128, 25. [Google Scholar] [PubMed]
- Van Amerongen, R.; Berns, A. Knockout mouse models to study Wnt signal transduction. Trends Genet. 2006, 22, 678–689. [Google Scholar] [CrossRef]
- Perugorria, M.J.; Olaizola, P.; Labiano, I.; Esparza-Baquer, A.; Marzioni, M.; Marin, J.J.G.; Bujanda, L.; Banales, J.M. Wnt-β-catenin signalling in liver development, health and disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 121–136. [Google Scholar] [CrossRef]
- González, S.; Oh, D.; Baclagon, E.R.; Zheng, J.J.; Deng, S.X. Wnt Signaling Is Required for the Maintenance of Human Limbal Stem/Progenitor Cells In Vitro. Investig. Ophthalmol. Vis. Sci. 2019, 60, 107–112. [Google Scholar] [CrossRef]
- Haegel, H.; Larue, L.; Ohsugi, M.; Fedorov, L.; Herrenknecht, K.; Kemler, R. Lack of beta-catenin affects mouse development at gastrulation. Development 1995, 121, 3529–3537. [Google Scholar] [PubMed]
- Sun, T.; Pikiolek, M.; Orsini, V.; Bergling, S.; Holwerda, S.; Morelli, L.; Hoppe, P.S.; Planas-Paz, L.; Yang, Y.; Ruffner, H.; et al. AXIN2+ Pericentral Hepatocytes Have Limited Contributions to Liver Homeostasis and Regeneration. Cell Stem Cell 2020, 26, 97–107.e106. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Jimenez, R.J.; Sharma, K.; Luu, H.Y.; Hsu, B.Y.; Ravindranathan, A.; Stohr, B.A.; Willenbring, H. Broad Distribution of Hepatocyte Proliferation in Liver Homeostasis and Regeneration. Cell Stem Cell 2020, 26, 27–33.e24. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Wakefield, L.; Tarlow, B.D.; Grompe, M. In Vivo Lineage Tracing of Polyploid Hepatocytes Reveals Extensive Proliferation during Liver Regeneration. Cell Stem Cell 2020, 26, 34–47.e33. [Google Scholar] [CrossRef]
- Lin, S.; Nascimento, E.M.; Gajera, C.R.; Chen, L.; Neuhöfer, P.; Garbuzov, A.; Wang, S.; Artandi, S.E. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature 2018, 556, 244–248. [Google Scholar] [CrossRef]
- Ozawa, M.; Baribault, H.; Kemler, R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989, 8, 1711–1717. [Google Scholar] [CrossRef]
- Dessimoz, J.; Opoka, R.; Kordich, J.J.; Grapin-Botton, A.; Wells, J.M. FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo. Mech. Dev. 2006, 123, 42–55. [Google Scholar] [CrossRef]
- Drees, F.; Pokutta, S.; Yamada, S.; Nelson, W.J.; Weis, W.I. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 2005, 123, 903–915. [Google Scholar] [CrossRef]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of β-catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef]
- Hur, J.; Jeong, S. Multitasking β-catenin: From adhesion and transcription to RNA regulation. Anim. Cells Syst. 2013, 17, 299–305. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Underhill, G.H.; Zaret, K.S.; Fox, I.J. Cell and tissue engineering for liver disease. Sci. Transl. Med. 2014, 6, 245sr2. [Google Scholar] [CrossRef] [PubMed]
- Ben-Moshe, S.; Itzkovitz, S. Spatial heterogeneity in the mammalian liver. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Deane, H.W. A cytological study of the diurnal cycle of the liver of the mouse in relation to storage and secretion. Anat. Rec. 1944, 88, 39–65. [Google Scholar] [CrossRef]
- Jungermann, K.; Sasse, D. Heterogeneity of liver parenchymal cells. Trends Biochem. Sci. 1978, 3, 198–202. [Google Scholar] [CrossRef]
- Jungermann, K.; Katz, N. Functional specialization of different hepatocyte populations. Physiol. Rev. 1989, 69, 708–764. [Google Scholar] [CrossRef] [PubMed]
- Tosh, D.; Alberti, G.M.; Agius, L. Glucagon regulation of gluconeogenesis and ketogenesis in periportal and perivenous rat hepatocytes. Heterogeneity of hormone action and of the mitochondrial redox state. Biochem. J. 1988, 256, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Kietzmann, T.; Cornesse, Y.; Brechtel, K.; Modaressi, S.; Jungermann, K. Perivenous expression of the mRNA of the three hypoxia-inducible factor alpha-subunits, HIF1alpha, HIF2alpha and HIF3alpha, in rat liver. Biochem. J. 2001, 354, 531–537. [Google Scholar] [CrossRef]
- Jungermann, K.; Kietzmann, T. Oxygen: Modulator of metabolic zonation and disease of the liver. Hepatology 2000, 31, 255–260. [Google Scholar] [CrossRef]
- Jungermann, K.; Keitzmann, T. Zonation of Parenchymal and Nonparenchymal Metabolism in Liver. Annu. Rev. Nutr. 1996, 16, 179–203. [Google Scholar] [CrossRef]
- Jungermann, K. Zonation of metabolism and gene expression in liver. Histochem. Cell Biol. 1995, 103, 81–91. [Google Scholar] [CrossRef]
- Gebhardt, R. Metabolic zonation of the liver: Regulation and implications for liver function. Pharmacol. Ther. 1992, 53, 275–354. [Google Scholar] [CrossRef]
- Gumucio, J.J.; Gebhardt, R. Cell-cell interactions: Clues to hepatocyte heterogeneity and beyond? Hepatology 1992, 16, 843–845. [Google Scholar] [CrossRef]
- Gebhardt, R.; Ebert, A.; Bauer, G. Heterogeneous expression of glutamine synthetase mRNA in rat liver parenchyma revealed by in situ hybridization and Northern blot analysis of RNA from periportal and perivenous hepatocytes. FEBS Lett. 1988, 241, 89–93. [Google Scholar] [CrossRef]
- Michalopoulos, G.K. Liver regeneration. J. Cell. Physiol. 2007, 213, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Higgins, G.M. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch. Pathol. 1931, 12, 186–202. [Google Scholar]
- Monga, S.P.; Pediaditakis, P.; Mule, K.; Stolz, D.B.; Michalopoulos, G.K. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology 2001, 33, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, L.; Fish, M.; Logan, C.Y.; Nusse, R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature 2015, 524, 180–185. [Google Scholar] [CrossRef]
- Zorn, A.M.; Wells, J.M. Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 2009, 25, 221–251. [Google Scholar] [CrossRef]
- Shen, M.M. Nodal signaling: Developmental roles and regulation. Development 2007, 134, 1023–1034. [Google Scholar] [CrossRef]
- Zorn, A.M.; Wells, J.M. Molecular basis of vertebrate endoderm development. Int. Rev. Cytol. 2007, 259, 49–111. [Google Scholar] [CrossRef]
- Yoney, A.; Etoc, F.; Ruzo, A.; Carroll, T.; Metzger, J.J.; Martyn, I.; Li, S.; Kirst, C.; Siggia, E.D.; Brivanlou, A.H. WNT signaling memory is required for ACTIVIN to function as a morphogen in human gastruloids. Elife 2018, 7, e38279. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Hagos, E.G.; Xu, B.; Sias, C.; Kawakami, K.; Burdine, R.D.; Dougan, S.T. Nodal signals mediate interactions between the extra-embryonic and embryonic tissues in zebrafish. Dev. Biol. 2007, 310, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Hilton, E.; Rex, M.; Old, R. VegT activation of the early zygotic gene Xnr5 requires lifting of Tcf-mediated repression in the Xenopus blastula. Mech. Dev. 2003, 120, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.; Heisenberg, C.P. The yolk syncytial layer in early zebrafish development. Trends Cell Biol. 2010, 20, 586–592. [Google Scholar] [CrossRef]
- Norris, D.P.; Brennan, J.; Bikoff, E.K.; Robertson, E.J. The Foxh1-dependent autoregulatory enhancer controls the level of Nodal signals in the mouse embryo. Development 2002, 129, 3455–3468. [Google Scholar]
- Dufort, D.; Schwartz, L.H.; Harpal, K.; Rossant, J. The transcription factor HNF3beta is required in visceral endoderm for normal primitive streak morphogenesis. Development 1998, 125, 3015–3025. [Google Scholar]
- Kanai-Azuma, M.; Kanai, Y.; Gad, J.M.; Tajima, Y.; Taya, C.; Kurohmaru, M.; Sanai, Y.; Yonekawa, H.; Yazaki, K.; Tam, P.P.L.; et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 2002, 129, 2367. [Google Scholar]
- Zorn, A.M.; Butler, K.; Gurdon, J.B. Anterior endomesoderm specification in Xenopus by Wnt/beta-catenin and TGF-beta signalling pathways. Dev. Biol. 1999, 209, 282–297. [Google Scholar] [CrossRef]
- Ho, C.Y.; Houart, C.; Wilson, S.W.; Stainier, D.Y. A role for the extraembryonic yolk syncytial layer in patterning the zebrafish embryo suggested by properties of the hex gene. Curr. Biol. 1999, 9, 1131–1134. [Google Scholar] [CrossRef]
- Mclin, V.A.; Rankin, S.A.; Zorn, A.M. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development 2007, 134, 2207–2217. [Google Scholar] [CrossRef]
- Li, Y.; Rankin, S.A.; Sinner, D.; Kenny, A.P.; Krieg, P.A.; Zorn, A.M. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008, 22, 3050–3063. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, K.E.; Krieg, P.A. Expression of the Wnt inhibitor, sFRP5, in the gut endoderm of Xenopus. Gene Expr. Patterns 2002, 2, 369–372. [Google Scholar] [CrossRef]
- Russell, J.O.; Monga, S.P. Wnt/β-Catenin Signaling in Liver Development, Homeostasis, and Pathobiology. Annu. Rev. Pathol. 2018, 13, 351–378. [Google Scholar] [CrossRef]
- Rossi, J.M.; Dunn, N.R.; Hogan, B.L.; Zaret, K.S. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001, 15, 1998–2009. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Zheng, M.; Goldfarb, M.; Zaret, K.S. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science 1999, 284, 1998–2003. [Google Scholar] [CrossRef]
- Poulain, M.; Ober, E.A. Interplay between Wnt2 and Wnt2bb controls multiple steps of early foregut-derived organ development. Development 2011, 138, 3557–3568. [Google Scholar] [CrossRef]
- Rodríguez-Seguel, E.; Mah, N.; Naumann, H.; Pongrac, I.M.; Cerdá-Esteban, N.; Fontaine, J.F.; Wang, Y.; Chen, W.; Andrade-Navarro, M.A.; Spagnoli, F.M. Mutually exclusive signaling signatures define the hepatic and pancreatic progenitor cell lineage divergence. Genes Dev. 2013, 27, 1932–1946. [Google Scholar] [CrossRef]
- Ober, E.A.; Verkade, H.; Field, H.A.; Stainier, D.Y. Mesodermal Wnt2b signalling positively regulates liver specification. Nature 2006, 442, 688–691. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.C.; Wang, X.; Wang, W.H.; Wang, Y.C.; Xu, C.R. The contributions of mesoderm-derived cells in liver development. Semin. Cell Dev. Biol. 2019, 92, 63–76. [Google Scholar] [CrossRef]
- Tan, X.; Yuan, Y.; Zeng, G.; Apte, U.; Thompson, M.D.; Cieply, B.; Stolz, D.B.; Michalopoulos, G.K.; Kaestner, K.H.; Monga, S.P. Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology 2008, 47, 1667–1679. [Google Scholar] [CrossRef]
- Berg, T.; Rountree, C.B.; Lee, L.; Estrada, J.; Sala, F.G.; Choe, A.; Veltmaat, J.M.; De Langhe, S.; Lee, R.; Tsukamoto, H.; et al. Fibroblast growth factor 10 is critical for liver growth during embryogenesis and controls hepatoblast survival via beta-catenin activation. Hepatology 2007, 46, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Monga, S.P.; Mars, W.M.; Pediaditakis, P.; Bell, A.; Mulé, K.; Bowen, W.C.; Wang, X.; Zarnegar, R.; Michalopoulos, G.K. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002, 62, 2064–2071. [Google Scholar] [PubMed]
- Hussain, S.Z.; Sneddon, T.; Tan, X.; Micsenyi, A.; Michalopoulos, G.K.; Monga, S.P. Wnt impacts growth and differentiation in ex vivo liver development. Exp. Cell Res. 2004, 292, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Cordi, S.; Godard, C.; Saandi, T.; Jacquemin, P.; Monga, S.P.; Colnot, S.; Lemaigre, F.P. Role of β-catenin in development of bile ducts. Differentiation 2016, 91, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sison-Young, R.L.; Kia, R.; Heslop, J.; Kelly, L.; Rowe, C.; Cross, M.J.; Kitteringham, N.R.; Hanley, N.; Park, B.K.; Goldring, C.E. Human pluripotent stem cells for modeling toxicity. Adv. Pharmacol. 2012, 63, 207–256. [Google Scholar] [CrossRef]
- Sauer, V.; Roy-Chowdhury, N.; Guha, C.; Roy-Chowdhury, J. Induced pluripotent stem cells as a source of hepatocytes. Curr. Pathobiol. Rep. 2014, 2, 11–20. [Google Scholar] [CrossRef]
- Nejak-Bowen, K.; Monga, S.P. Wnt/beta-catenin signaling in hepatic organogenesis. Organogenesis 2008, 4, 92–99. [Google Scholar] [CrossRef]
- Takayama, K.; Mizuguchi, H. Generation of human pluripotent stem cell-derived hepatocyte-like cells for drug toxicity screening. Drug Metab. Pharmacokinet. 2017, 32, 12–20. [Google Scholar] [CrossRef]
- Hay, D.C.; Fletcher, J.; Payne, C.; Terrace, J.D.; Gallagher, R.C.J.; Snoeys, J.; Black, J.R.; Wojtacha, D.; Samuel, K.; Hannoun, Z.; et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 12301–12306. [Google Scholar] [CrossRef]
- Toivonen, S.; Lundin, K.; Balboa, D.; Ustinov, J.; Tamminen, K.; Palgi, J.; Trokovic, R.; Tuuri, T.; Otonkoski, T. Activin A and Wnt-dependent specification of human definitive endoderm cells. Exp. Cell Res. 2013, 319, 2535–2544. [Google Scholar] [CrossRef]
- Bone, H.K.; Nelson, A.S.; Goldring, C.E.; Tosh, D.; Welham, M.J. A novel chemically directed route for the generation of definitive endoderm from human embryonic stem cells based on inhibition of GSK-3. J. Cell Sci. 2011, 124, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Hewitt, N.J.; Albrecht, U.; Andersen, M.E.; Ansari, N.; Bhattacharya, S.; Bode, J.G.; Bolleyn, J.; Borner, C.; Böttger, J.; et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 2013, 87, 1315–1530. [Google Scholar] [CrossRef] [PubMed]
- Halpern, K.B.; Shenhav, R.; Matcovitch-Natan, O.; Tóth, B.; Lemze, D.; Golan, M.; Massasa, E.E.; Baydatch, S.; Landen, S.; Moor, A.E.; et al. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature 2017, 542, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, J.M.; Gieling, R.G.; Markman, M.M.; Hagoort, J.; Lamers, W.H. Stereological measurement of porto-central gradients in gene expression in mouse liver. Hepatology 2004, 39, 343–352. [Google Scholar] [CrossRef]
- Kietzmann, T. Metabolic zonation of the liver: The oxygen gradient revisited. Redox Biol. 2017, 11, 622–630. [Google Scholar] [CrossRef]
- Kietzmann, T.; Dimova, E.Y.; Flügel, D.; Scharf, J.G. Oxygen: Modulator of physiological and pathophysiological processes in the liver. Zeitschrift Gastroenterol. 2006, 44, 67–76. [Google Scholar] [CrossRef]
- Burke, Z.D.; Reed, K.R.; Yeh, S.-W.; Meniel, V.; Sansom, O.J.; Clarke, A.R.; Tosh, D. Spatiotemporal regulation of liver development by the Wnt/β-catenin pathway. Sci. Rep. 2018, 8, 2735. [Google Scholar] [CrossRef]
- Planas-Paz, L.; Orsini, V.; Boulter, L.; Calabrese, D.; Pikiolek, M.; Nigsch, F.; Xie, Y.; Roma, G.; Donovan, A.; Marti, P.; et al. The RSPO-LGR4/5-ZNRF3/RNF43 module controls liver zonation and size. Nat. Cell Biol. 2016, 18, 467–479. [Google Scholar] [CrossRef]
- Burke, Z.D.; Reed, K.R.; Phesse, T.J.; Sansom, O.J.; Clarke, A.R.; Tosh, D. Liver zonation occurs through a beta-catenin-dependent, c-Myc-independent mechanism. Gastroenterology 2009, 136, 2316–2324.e2311–2313. [Google Scholar] [CrossRef]
- Kuo, F.C.; Darnell, J.E., Jr. Evidence that interaction of hepatocytes with the collecting (hepatic) veins triggers position-specific transcription of the glutamine synthetase and ornithine aminotransferase genes in the mouse liver. Mol. Cell. Biol. 1991, 11, 6050–6058. [Google Scholar] [CrossRef]
- Hailfinger, S.; Jaworski, M.; Braeuning, A.; Buchmann, A.; Schwarz, M. Zonal gene expression in murine liver: Lessons from tumors. Hepatology 2006, 43, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, R.; Baldysiak-Figiel, A.; Krügel, V.; Ueberham, E.; Gaunitz, F. Hepatocellular expression of glutamine synthetase: An indicator of morphogen actions as master regulators of zonation in adult liver. Prog. Histochem. Cytochem. 2007, 41, 201–266. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Martínez-Ramírez, A.S.; Borders, T.L.; Gao, F.; Sosa-Pineda, B. Metabolic and non-metabolic liver zonation is established non-synchronously and requires sinusoidal Wnts. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Benhamouche, S.; Decaens, T.; Godard, C.; Chambrey, R.; Rickman, D.S.; Moinard, C.; Vasseur-Cognet, M.; Kuo, C.J.; Kahn, A.; Perret, C.; et al. Apc Tumor Suppressor Gene Is the “Zonation-Keeper“ of Mouse Liver. Dev. Cell 2006, 10, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Burke, Z.D.; Tosh, D. The Wnt/beta-catenin pathway: Master regulator of liver zonation? Bioessays 2006, 28, 1072–1077. [Google Scholar] [CrossRef]
- Lamers, W.H.; Gaasbeek Janzen, J.W.; Kortschot, A.T.; Charles, R.; Moorman, A.F. Development of enzymic zonation in liver parenchyma is related to development of acinar architecture. Differentiation 1987, 35, 228–235. [Google Scholar] [CrossRef]
- Gebhardt, R.; Mecke, D. Heterogeneous distribution of glutamine synthetase among rat liver parenchymal cells in situ and in primary culture. EMBO J. 1983, 2, 567–570. [Google Scholar] [CrossRef]
- Loeppen, S.; Schneider, D.; Gaunitz, F.; Gebhardt, R.; Kurek, R.; Buchmann, A.; Schwarz, M. Overexpression of glutamine synthetase is associated with beta-catenin-mutations in mouse liver tumors during promotion of hepatocarcinogenesis by phenobarbital. Cancer Res. 2002, 62, 5685–5688. [Google Scholar]
- Cadoret, A.; Ovejero, C.; Terris, B.; Souil, E.; Lévy, L.; Lamers, W.H.; Kitajewski, J.; Kahn, A.; Perret, C. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene 2002, 21, 8293–8301. [Google Scholar] [CrossRef]
- Nicholes, K.; Guillet, S.; Tomlinson, E.; Hillan, K.; Wright, B.; Frantz, G.D.; Pham, T.A.; Dillard-Telm, L.; Tsai, S.P.; Stephan, J.P.; et al. A mouse model of hepatocellular carcinoma: Ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am. J. Pathol. 2002, 160, 2295–2307. [Google Scholar] [CrossRef]
- Colnot, S.; Decaens, T.; Niwa-Kawakita, M.; Godard, C.; Hamard, G.; Kahn, A.; Giovannini, M.; Perret, C. Liver-targeted disruption of Apc in mice activates β-catenin signaling and leads to hepatocellular carcinomas. Proc. Natl. Acad. Sci. USA 2004, 101, 17216–17221. [Google Scholar] [CrossRef] [PubMed]
- He, T.-C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYCas a Target of the APC Pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Halpern, K.B.; Shenhav, R.; Massalha, H.; Toth, B.; Egozi, A.; Massasa, E.E.; Medgalia, C.; David, E.; Giladi, A.; Moor, A.E.; et al. Paired-cell sequencing enables spatial gene expression mapping of liver endothelial cells. Nat. Biotechnol. 2018, 36, 962–970. [Google Scholar] [CrossRef]
- Aird, W.C. Phenotypic Heterogeneity of the Endothelium. Circ. Res. 2007, 100, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.S.; Vidal, V.; Mertz, M.; Kendall, T.J.; Charlet, A.; Okamoto, H.; Schedl, A. The Angiocrine Factor Rspondin3 Is a Key Determinant of Liver Zonation. Cell Rep. 2015, 13, 1757–1764. [Google Scholar] [CrossRef]
- Notenboom, R.G.; de Boer, P.A.; Moorman, A.F.; Lamers, W.H. The establishment of the hepatic architecture is a prerequisite for the development of a lobular pattern of gene expression. Development 1996, 122, 321–332. [Google Scholar]
- Zhao, L.; Jin, Y.; Donahue, K.; Tsui, M.; Fish, M.; Logan, C.Y.; Wang, B.; Nusse, R. Tissue Repair in the Mouse Liver Following Acute Carbon Tetrachloride Depends on Injury-Induced Wnt/β-Catenin Signaling. Hepatology 2019, 69, 2623–2635. [Google Scholar] [CrossRef]
- Yanger, K.; Knigin, D.; Zong, Y.; Maggs, L.; Gu, G.; Akiyama, H.; Pikarsky, E.; Stanger, B.Z. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell 2014, 15, 340–349. [Google Scholar] [CrossRef]
- Lowes, K.N.; Croager, E.J.; Olynyk, J.K.; Abraham, L.J.; Yeoh, G.C. Oval cell-mediated liver regeneration: Role of cytokines and growth factors. J. Gastroenterol. Hepatol. 2003, 18, 4–12. [Google Scholar] [CrossRef]
- Farber, E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3′-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956, 16, 142–148. [Google Scholar]
- Fausto, N.; Thompson, N.L.; Braun, L. Chapter 3—Purification and Culture of Oval Cells from Rat Liver. In Cell Separation; Pretlow, T.G., Pretlow, T.P., Eds.; Academic Press: Cambridge, MA, USA, 1987; pp. 45–77. [Google Scholar]
- Popper, H.; Kent, G.; Stein, R. Ductular cell reaction in the liver in hepatic injury. J. Mt. Sinai Hosp. N. Y. 1957, 24, 551–556. [Google Scholar] [PubMed]
- Michalopoulos, G.K.; Barua, L.; Bowen, W.C. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology 2005, 41, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, Y.; Ebato, K.; Kato, H.; Arakawa, S.; Shimizu, S.; Miyajima, A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr. Biol. 2012, 22, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Font-Burgada, J.; Shalapour, S.; Ramaswamy, S.; Hsueh, B.; Rossell, D.; Umemura, A.; Taniguchi, K.; Nakagawa, H.; Valasek, M.A.; Ye, L.; et al. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell 2015, 162, 766–779. [Google Scholar] [CrossRef] [PubMed]
- Schaub, J.R.; Huppert, K.A.; Kurial, S.N.T.; Hsu, B.Y.; Cast, A.E.; Donnelly, B.; Karns, R.A.; Chen, F.; Rezvani, M.; Luu, H.Y.; et al. De novo formation of the biliary system by TGFβ-mediated hepatocyte transdifferentiation. Nature 2018, 557, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Goessling, W.; North, T.E.; Lord, A.M.; Ceol, C.; Lee, S.; Weidinger, G.; Bourque, C.; Strijbosch, R.; Haramis, A.P.; Puder, M.; et al. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev. Biol. 2008, 320, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, R.; Hovhannisyan, A. Organ patterning in the adult stage: The role of Wnt/β-catenin signaling in liver zonation and beyond. Dev. Dyn. 2010, 239, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R. Wnt signaling and stem cell control. Cell Res. 2008, 18, 523–527. [Google Scholar] [CrossRef]
- Nhieu, J.T.; Renard, C.A.; Wei, Y.; Cherqui, D.; Zafrani, E.S.; Buendia, M.A. Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am. J. Pathol. 1999, 155, 703–710. [Google Scholar] [CrossRef]
- Taniguchi, K.; Roberts, L.R.; Aderca, I.N.; Dong, X.; Qian, C.; Murphy, L.M.; Nagorney, D.M.; Burgart, L.J.; Roche, P.C.; Smith, D.I.; et al. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene 2002, 21, 4863–4871. [Google Scholar] [CrossRef] [PubMed]
- Waddington, C.H. The Strategy of the Genes: A Discussion of Some Aspects of Theoretical Biology; Allen & Unwin: London, UK, 1957. [Google Scholar]
- Zajicek, G.; Oren, R.; Weinreb, M., Jr. The streaming liver. Liver 1985, 5, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Jho, E.H.; Zhang, T.; Domon, C.; Joo, C.K.; Freund, J.N.; Costantini, F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 2002, 22, 1172–1183. [Google Scholar] [CrossRef]
- Sarin, K.Y.; Cheung, P.; Gilison, D.; Lee, E.; Tennen, R.I.; Wang, E.; Artandi, M.K.; Oro, A.E.; Artandi, S.E. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature 2005, 436, 1048–1052. [Google Scholar] [CrossRef]
- Gebhardt, R.; Matz-Soja, M. Liver zonation: Novel aspects of its regulation and its impact on homeostasis. World J. Gastroenterol. 2014, 20, 8491–8504. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, E.; Aleithe, S.; Rennert, C.; Spormann, L.; Ott, F.; Meierhofer, D.; Gajowski, R.; Stöpel, C.; Hoehme, S.; Kücken, M.; et al. Mutual Zonated Interactions of Wnt and Hh Signaling Are Orchestrating the Metabolism of the Adult Liver in Mice and Human. Cell Rep. 2019, 29, 4553–4567.e4557. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, W.; Cheng, M.; Wang, J.; Liu, Z.; He, S.; Luo, X.; Huang, W.; Chen, T.; Yan, W.; et al. Hypoxia activates Wnt/β-catenin signaling by regulating the expression of BCL9 in human hepatocellular carcinoma. Sci. Rep. 2017, 7, 40446. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr.; Ratcliffe, P.J.; Semenza, G.L. Pathways for Oxygen Regulation and Homeostasis: The 2016 Albert Lasker Basic Medical Research Award. JAMA 2016, 316, 1252–1253. [Google Scholar] [CrossRef]
- Varela-Nallar, L.; Rojas-Abalos, M.; Abbott, A.C.; Moya, E.A.; Iturriaga, R.; Inestrosa, N.C. Chronic hypoxia induces the activation of the Wnt/β-catenin signaling pathway and stimulates hippocampal neurogenesis in wild-type and APPswe-PS1ΔE9 transgenic mice in vivo. Front. Cell Neurosci. 2014, 8, 17. [Google Scholar] [CrossRef]
- Mazumdar, J.; O‘Brien, W.T.; Johnson, R.S.; LaManna, J.C.; Chavez, J.C.; Klein, P.S.; Simon, M.C. O2 regulates stem cells through Wnt/β-catenin signalling. Nat. Cell Biol. 2010, 12, 1007–1013. [Google Scholar] [CrossRef]
- Kaidi, A.; Williams, A.C.; Paraskeva, C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat. Cell Biol. 2007, 9, 210–217. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wild, S.L.; Elghajiji, A.; Grimaldos Rodriguez, C.; Weston, S.D.; Burke, Z.D.; Tosh, D. The Canonical Wnt Pathway as a Key Regulator in Liver Development, Differentiation and Homeostatic Renewal. Genes 2020, 11, 1163. https://doi.org/10.3390/genes11101163
Wild SL, Elghajiji A, Grimaldos Rodriguez C, Weston SD, Burke ZD, Tosh D. The Canonical Wnt Pathway as a Key Regulator in Liver Development, Differentiation and Homeostatic Renewal. Genes. 2020; 11(10):1163. https://doi.org/10.3390/genes11101163
Chicago/Turabian StyleWild, Sebastian L., Aya Elghajiji, Carmen Grimaldos Rodriguez, Stephen D. Weston, Zoë D. Burke, and David Tosh. 2020. "The Canonical Wnt Pathway as a Key Regulator in Liver Development, Differentiation and Homeostatic Renewal" Genes 11, no. 10: 1163. https://doi.org/10.3390/genes11101163
APA StyleWild, S. L., Elghajiji, A., Grimaldos Rodriguez, C., Weston, S. D., Burke, Z. D., & Tosh, D. (2020). The Canonical Wnt Pathway as a Key Regulator in Liver Development, Differentiation and Homeostatic Renewal. Genes, 11(10), 1163. https://doi.org/10.3390/genes11101163



