Genetic Architecture Underpinning Yield Components and Seed Mineral–Nutrients in Sesame
Abstract
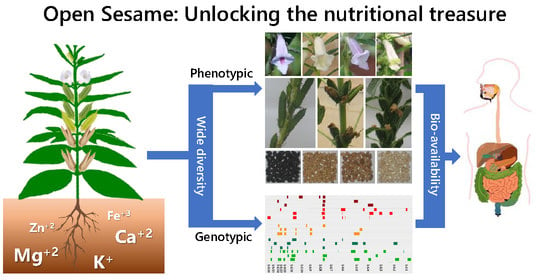
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Phenotypic Characterization
2.3. Seed Nutrient Concentration Analysis
2.4. In-Vitro Digestion Analysis
2.5. QTL Analysis
2.6. Statistical Analyses of Phenotypic Data
3. Results
3.1. Wide Phenotypic Variation in Morphological and Seed Mineral-Nutrient Concentrations
3.2. S-91 × S-297 Population Exhibited Range of Interrelationships between Plant Morphological and Seed Quality Traits
3.3. Constructing an High-Density Genetic Map and QTL Analysis
3.4. QTL for Phenology and Morphological Traits
3.5. QTL for Seed Quality Traits
3.6. Candidate Genes Associated with Seed Mineral-Nutrient Concentration
3.7. Bio-Accessibility of Seed Mineral–Nutrients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Müller, O.; Krawinkel, M. Malnutrition and health in developing countries. Can. Med. Assoc. J. 2005, 173, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Platel, K.; Srinivasan, K. Bioavailability of micronutrients from plant foods: An update. Crit. Rev. Food Sci. Nutr. 2015, 56, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets-iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Lott, J.N.; Ockenden, I.; Raboy, V.; Batten, G.D. Phytic acid and phosphorus in crop seeds and fruits: A global estimate. Seed Sci. Res. 2000, 10, 11–33. [Google Scholar] [CrossRef]
- Gadri, Y.; Williams, L.E.; Peleg, Z. Tradeoffs between yield components promote crop stability in sesame. Plant Sci. 2020, 295, 110105. [Google Scholar] [CrossRef]
- Çağırgan, M.I. Selection and morphological characterization of induced determinate mutants in sesame. Field Crop. Res. 2006, 96, 19–24. [Google Scholar] [CrossRef]
- Mushtaq, A.; Hanif, M.A.; Ayub, M.A.; Bhatti, I.A.; Jilani, M.I. Sesame. In Medicinal Plants of South Asia; Hanif, M.A., Nawaz, H., Khan, M.M., Byrne, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 601–615. [Google Scholar]
- Uzun, B.; Arslan, Ç.; Furat, Ş. Variation in fatty acid compositions, oil content and oil yield in a germplasm collection of sesame (Sesamum indicum L.). J. Am. Oil Chem. Soc. 2008, 85, 1135–1142. [Google Scholar] [CrossRef]
- Pathak, N.; Rai, A.K.; Kumari, R.; Thapa, A.; Bhat, K.V. Sesame Crop: An Underexploited Oilseed Holds Tremendous Potential for Enhanced Food Value. Agric. Sci. 2014, 5, 519–529. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.A.; Suleiman, T.M.; Lusas, E.W. Sesame protein: A review and prospectus. J. Am. Oil Chem. Soc. 1979, 56, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Dimitrios, B. Sources of natural phenolic antioxidants. Trends Food Sci. Technol. 2006, 17, 505–512. [Google Scholar] [CrossRef]
- Bedigian, D. Sesame: The genus Sesamum; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar]
- Tripathy, S.K.; Kar, J.; Sahu, D. Advances in sesame (Sesamum indicum L.) breeding. In Advances in Plant Breeding Strategies: Industrial and Food Crops; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 6, pp. 577–635. [Google Scholar]
- Pham, T.D.; Geleta, M.; Bui, T.M.; Bui, T.C.; Merker, A.; Carlsson, A.S. Comparative analysis of genetic diversity of sesame (Sesamum indicum L.) from Vietnam and Cambodia using agro-morphological and molecular markers. Hereditas 2011, 148, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Basak, M.; Uzun, B.; Yol, E. Genetic diversity and population structure of the Mediterranean sesame core collection with use of genome-wide SNPs developed by double digest RAD-Seq. PLoS ONE 2019, 14, e0223757. [Google Scholar] [CrossRef]
- Pandey, S.K.; Das, A.; Rai, P.; Dasgupta, T. Morphological and genetic diversity assessment of sesame (Sesamum indicum L.) accessions differing in origin. Physiol. Mol. Biol. Plants 2015, 21, 519–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whan, A.P.; Smith, A.B.; Cavanagh, C.R.; Ral, J.-P.F.; Shaw, L.M.; Howitt, C.A.; Bischof, L. GrainScan: A low cost, fast method for grain size and colour measurements. Plant Methods 2014, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Doyle, J.; Doyle, J. Genomic plant DNA preparation from fresh tissue-CTAB method. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Wang, L.; Xia, Q.; Zhang, Y.; Zhu, X.; Zhu, X.; Li, D.; Ni, X.; Gao, Y.; Xiang, H.; Wei, X.; et al. Updated sesame genome assembly and fine mapping of plant height and seed coat color QTLs using a new high-density genetic map. BMC Genom. 2016, 17, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2012, 14, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Peleg, Z.; Cakmak, I.; Ozturk, L.; Yazici, A.; Jun, Y.; Budak, H.; Korol, A.B.; Fahima, T.; Saranga, Y. Quantitative trait loci conferring grain mineral nutrient concentrations in durum wheat × wild emmer wheat RIL population. Theor. Appl. Genet. 2009, 119, 353–369. [Google Scholar] [CrossRef] [Green Version]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ nutrition in plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, G.A.; Bouraine, S.; Wege, S.; Li, Y.; De Carbonnel, M.; Berthomieu, P.; Poirier, Y.; Rouached, H. Coordination between zinc and phosphate homeostasis involves the transcription factor PHR1, the phosphate exporter PHO1, and its homologue PHO1;H3 in Arabidopsis. J. Exp. Bot. 2014, 65, 871–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connolly, E.L.; Campbell, N.H.; Grotz, N.; Prichard, C.L.; Guerinot, M.L. Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiol. 2003, 133, 1102–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, B.; Sherman, T.; Fromm, H. Cyclic nucleotide-gated channels in plants. FEBS Lett. 2007, 581, 2237–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kim, S.A.; Lee, J.; Guerinot, M.L.; An, G. Zinc deficiency-inducible OsZIP8 encodes a plasma membrane-localized zinc transporter in rice. Mol. Cells 2010, 29, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Tulchinsky, T.H. Micronutrient deficiency conditions: Global health issues. Public Heal. Rev. 2010, 32, 243–255. [Google Scholar] [CrossRef] [Green Version]
- Furat, S.; Uzun, B. The use of agro-morphological characters for the assessment of genetic diversity in sesame (Sesamum indicum L.). Plant Omics 2010, 3, 85–91. [Google Scholar]
- Arriel, N.H.C.; Di Mauro, A.O.; Arriel, E.F.; Unêda-Trevisoli, S.H.; Costa, M.M.; Bárbaro, I.M.; Muniz, F.R.S. Genetic divergence in sesame based on morphological and agronomic traits. Crop Breed. Appl. Biotechnol. 2007, 7, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Liu, K.; Zhang, Y.; Feng, Q.; Wang, L.; Zhao, Y.; Li, D.; Zhao, Q.; Zhu, X.; Zhu, X.; et al. Genetic discovery for oil production and quality in sesame. Nat. Commun. 2015, 6, 8609. [Google Scholar] [CrossRef] [Green Version]
- Cai, G.; Yang, Q.-Y.; Chen, H.; Yang, Q.; Zhang, C.; Fan, C.; Zhou, Y. Genetic dissection of plant architecture and yield-related traits in Brassica napus. Sci. Rep. 2016, 6, 21625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langham, D.R. Phenology of sesame. In Issues in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2007; pp. 144–182. [Google Scholar]
- Zhao, F.; Su, Y.; Dunham, S.; Rakszegi, M.; Bedo, Z.; McGrath, S.; Shewry, P. Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. J. Cereal Sci. 2009, 49, 290–295. [Google Scholar] [CrossRef]
- Menkir, A. Genetic variation for grain mineral content in tropical-adapted maize inbred lines. Food Chem. 2008, 110, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Pinson, S.R.M.; Tarpley, L.; Yan, W.; Yeater, K.; Lahner, B.; Yakubova, E.; Huang, X.-Y.; Zhang, M.; Guerinot, M.L.; Salt, D.E. Worldwide genetic diversity for mineral element concentrations in rice grain. Crop Sci. 2015, 55, 294–311. [Google Scholar] [CrossRef]
- Mamo, B.E.; Barber, B.L.; Steffenson, B.J. Genome-wide association mapping of zinc and iron concentration in barley landraces from Ethiopia and Eritrea. J. Cereal Sci. 2014, 60, 497–506. [Google Scholar] [CrossRef]
- Chatzav, M.; Peleg, Z.; Ozturk, L.; Yazici, A.; Fahima, T.; Cakmak, I.; Saranga, Y. Genetic diversity for grain nutrients in wild emmer wheat: Potential for wheat improvement. Ann. Bot. 2010, 105, 1211–1220. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Oliveira, A.L.; Tan, L.; Fu, Y.; Sun, C. Genetic identification of quantitative trait loci for contents of mineral nutrients in rice grain. J. Integr. Plant Biol. 2009, 51, 84–92. [Google Scholar] [CrossRef]
- Blair, M.W.; Astudillo, C.; Grusak, M.A.; Graham, R.; Beebe, S.E. Inheritance of seed iron and zinc concentrations in common bean (Phaseolus vulgaris L.). Mol. Breed. 2008, 23, 197–207. [Google Scholar] [CrossRef]
- Grotz, N.; Guerinot, M.L. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2006, 1763, 595–608. [Google Scholar] [CrossRef] [Green Version]
- Ajeesh Krishna, T.P.; Maharajan, T.; Victor Roch, G.; Ignacimuthu, S.; Ceasar, S.A. Structure, function, regulation and phylogenetic relationship of ZIP family tansporters of plants. Front. Plant Sci. 2020, 11, 662. [Google Scholar] [CrossRef]
- Šimić, D.; Sudar, R.; Ledenčan, T.; Jambrović, A.; Zdunić, Z.; Brkić, I.; Kovačević, V. Genetic variation of bioavailable iron and zinc in grain of a maize population. J. Cereal Sci. 2009, 50, 392–397. [Google Scholar] [CrossRef]
- Shunmugam, A.; Bock, C.; Arganosa, G.C.; Georges, F.; Gray, G.R.; Warkentin, T.D. Accumulation of Phosphorus-containing compounds in developing seeds of low-phytate pea (Pisum sativum L.) mutants. Plants 2015, 4, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abebe, Y.; Bogale, A.; Hambidge, K.M.; Stoecker, B.J.; Bailey, K.; Gibson, R.S. Phytate, zinc, iron and calcium content of selected raw and prepared foods consumed in rural Sidama, Southern Ethiopia, and implications for bioavailability. J. Food Compos. Anal. 2007, 20, 161–168. [Google Scholar] [CrossRef]
- Cichy, K.A.; Caldas, G.V.; Snapp, S.S.; Blair, M.W. QTL Analysis of Seed Iron, Zinc, and Phosphorus Levels in an Andean Bean Population. Crop Sci. 2009, 49, 1742–1750. [Google Scholar] [CrossRef] [Green Version]
- Stangoulis, J.C.; Huynh, B.-L.; Welch, R.M.; Choi, E.-Y.; Graham, R.D. Quantitative trait loci for phytate in rice grain and their relationship with grain micronutrient content. Euphytica 2007, 154, 289–294. [Google Scholar] [CrossRef]
- Zhang, H.; Miao, H.; Wei, L.; Li, C.; Duan, Y.; Xu, F.; Qu, W.; Zhao, R.; Ju, M.; Chang, S. Identification of a SiCL1 gene controlling leaf curling and capsule indehiscence in sesame via cross-population association mapping and genomic variants screening. BMC Plant Biol. 2018, 18, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaymard, F.; Pilot, G.; Lacombe, B.; Bouchez, D.; Bruneau, D.; Boucherez, J.; Michaux-Ferrière, N.; Thibaud, J.-B.; Sentenac, H. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 1998, 94, 647–655. [Google Scholar] [CrossRef] [Green Version]
- Zelman, A.K.; Dawe, A.; Gehring, C.; Berkowitz, G.A. Evolutionary and structural perspectives of plant cyclic nucleotide-gated cation channels. Front. Plant Sci. 2012, 3, 95. [Google Scholar] [CrossRef] [Green Version]
- Guerinot, M.L. The ZIP family of metal transporters. Biochim. Biophys. Acta BBA-Biomembr. 2000, 1465, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Rossander-Hultén, L.; Brune, M.; Sandström, B.; Lönnerdal, B.; Hallberg, L. Competitive inhibition of iron absorption by manganese and zinc in humans. Am. J. Clin. Nutr. 1991, 54, 152–156. [Google Scholar] [CrossRef]
- Hallberg, L.; Hulthén, L. Prediction of dietary iron absorption: An algorithm for calculating absorption and bioavailability of dietary iron. Am. J. Clin. Nutr. 2000, 71, 1147–1160. [Google Scholar] [CrossRef]
- Seshadri, S. Prevalence of micronutrient deficiency particularly of iron, zinc and folic acid in pregnant women in South East Asia. Br. J. Nutr. 2001, 85, S87. [Google Scholar] [CrossRef]
- Scheers, N. Regulatory Effects of Cu, Zn, and Ca on Fe absorption: The intricate play between nutrient transporters. Nutrients 2013, 5, 957–970. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.-I.; Egger, L.; Portmann, R.; Ménard, O.; Marze, S.; Minekus, M.; Le Feunteun, S.; Sarkar, A.; Grundy, M.M.L.; Carrière, F.; et al. A standardised semi-dynamic in vitro digestion method suitable for food-an international consensus. Food Funct. 2020, 11, 1702–1720. [Google Scholar] [CrossRef] [Green Version]




| Trait | Code | S-91 | S-297 | F2 Population | |
|---|---|---|---|---|---|
| Mean | Range | ||||
| Flowering date (days) | FD | 25 ± 0.14 | 18 ± 0.11 | 17.8 | 14–29 |
| Flowering Node | FN | 5 ± 0.7 | 3 ± 0.2 | 3.4 | 2–5 |
| Node Number | NN | 29 ± 1.9 | 36 ± 1.3 | 34.1 | 20–43 |
| Internode Length (cm) | IL | 4.9 ± 0.1 | 4.1 ± 0.2 | 4.1 | 2.7–6.5 |
| Number of Secondary Branches | SB | 1 ± 0.5 | 0 ± 0.2 | 0.7 | 0–5 |
| Plant Height (cm) | PH | 142 ± 11.7 | 147 ± 8.3 | 134.2 | 100–170 |
| Capsule Length (mm) | CL | 42.9 ± 1.3 | 28.8 ± 0.7 | 34.5 | 20.6–48.0 |
| Capsule Width (mm) | CW | 6.46 ± 0.22 | 5.71 ± 0.1 | 6.34 | 5.22–8.43 |
| Capsule Dehiscence Index | CDI | 1 | 5 | 4.2 | 1–5 |
| Seed Length (mm) | SL | 3.31 ± 0.02 | 3.41 ± 0.03 | 3.50 | 3.08–3.86 |
| Seed Width (mm) | SW | 2.1 ± 0.0 | 2.1 ± 0.0 | 2.1 | 1.9–2.3 |
| Seed Perimeter (mm) | SP | 10.6±0.1 | 10.9 ± 0.1 | 11.21 | 10.1–12.3 |
| Seed Area (mm2) | SA | 5.32 ± 0.11 | 5.56 ± 0.10 | 5.80 | 4.75–6.90 |
| Thousand Seed Weight (g) | TSW | 3.35 ± 0.14 | 2.97 ± 0.07 | 3.11 | 0.97–3.99 |
| Seed Color (RGB index) | SC | 153.1 ± 2.3 | 159.1 ± 2.5 | 155.3 | 128.1–198.3 |
| Zinc (mg/kg) | Zn | 87.5 ± 5.5 | 58.4 ± 1.7 | 71.1 | 45.5–108.6 |
| Iron (mg/kg) | Fe | 97.3 ± 11.22 | 76.4 ± 6.6 | 82.9 | 53.8–116.0 |
| Copper (mg/kg) | Cu | 23.2 ± 1.1 | 14.5 ± 1.0 | 17.2 | 11.5–23.5 |
| Manganese (mg/kg) | Mn | 19.3 ± 0.5 | 17.8 ± 1.6 | 16.0 | 11.8–22.2 |
| Calcium (mg/kg) | Ca | 12,591.2 ± 397.9 | 11,334.6 ± 947.9 | 10,693.6 | 4171.2–19,857.3 |
| Magnesium (mg/kg) | Mg | 4109.7 ± 122.2 | 4357.9 ± 90.8 | 4232.9 | 3433.0–4749.7 |
| Potassium (mg/kg) | K | 5577.2 ± 149.9 | 5519.8 ± 214.9 | 5434.4 | 4061.4–7201.0 |
| Phosphorus (mg/kg) | P | 8574.9 ± 239.4 | 9165.3 ± 81.1 | 8945.3 | 7777.9–9895.5 |
| Sulfur (mg/kg) | S | 3635.7 ± 64.1 | 3607.9 ± 183.2 | 3703.5 | 3082.6–4248.0 |
| Trait | #QTL | LOD a | PVE (%) b | Favorable Allele c | |
|---|---|---|---|---|---|
| S-91 | S-297 | ||||
| Plant Phenology and Morphology | |||||
| Flowering date | 6 | 2.1–4.92 | 6.3–14 | 0 | 6 |
| Flowering node | 8 | 2.56–7.57 | 7.6–20.7 | 2 | 6 |
| Node number | 6 | 2.59–19.46 | 7.7–45 | 1 | 5 |
| Internode length | 10 | 2–14.93 | 5.0–31.2 | 1 | 9 |
| Number of secondary branches | 5 | 3.03–4 | 8.9–11.6 | 0 | 5 |
| Plant height | 13 | 2–4.66 | 5.3–11.9 | 10 | 3 |
| Capsule Morphology | |||||
| Capsule length | 4 | 2.39–19.73 | 7.1–45.4 | 3 | 1 |
| Capsule width | 7 | 2.1–3.99 | 6.2–11.5 | 4 | 3 |
| Capsule dehiscence index | 4 | 2.66–95.1 | 6.3–76.8 | 4 | 0 |
| Seed Morphology | |||||
| Seed length | 7 | 2–6.4 | 5.9–17.8 | 2 | 5 |
| Seed width | 3 | 2.81–5.11 | 8.3–14.5 | 1 | 2 |
| Seed perimeter | 6 | 2.45–6.35 | 7.2–17.7 | 3 | 3 |
| Seed area | 5 | 2.3–5.77 | 6.8–16.2 | 2 | 3 |
| Seed Quality | |||||
| Thousand seed weight | 3 | 2.63–4.77 | 7.7–13.6 | 2 | 1 |
| Seed color | 4 | 2.14–12.09 | 6.3–31 | 0 | 4 |
| Seed Zn concentration | 6 | 2.43–19.92 | 7.2–45.7 | 5 | 1 |
| Seed Fe concentration | 6 | 2.05–7.94 | 6.1–21.6 | 5 | 1 |
| Seed Cu concentration | 6 | 2.13–11.8 | 6.3–30.4 | 6 | 0 |
| Seed Mn concentration | 6 | 2.4–8.17 | 7.1–22.2 | 4 | 1 |
| Seed Ca concentration | 3 | 2.05–7.84 | 6.1–21.4 | 2 | 1 |
| Seed Mg concentration | 3 | 2.28–4.16 | 6.8–12 | 1 | 2 |
| Seed K concentration | 4 | 2.55–3.7 | 7.5–10.7 | 2 | 2 |
| Seed P concentration | 6 | 2–2.65 | 5.9–7.8 | 5 | 1 |
| Seed S concentration | 3 | 2.45–4.34 | 7.2–12.5 | 2 | 1 |
| Total | 134 | 67 | 66 | ||
| LG | Interval | Trait | CG ID | Annotated Function |
|---|---|---|---|---|
| LG8 | 4550182–7125811 | FD, FN, NN, IL, SB, CL, CDI, SL, TSW, SC, Zn, Fe, Cu, Mn, Ca, Mg, K | LOC105167760 | Potassium channel SKOR-like |
| LOC105167785 | Potassium channel SKOR | |||
| LOC105167762 | Inositol hexakisphosphate and diphosphoinositol-pentakisphosphate kinase 2-like | |||
| LOC105167788 | Ethylene-responsive transcription factor 1B-like | |||
| LOC105167789 | Ethylene-responsive transcription factor 1B-like | |||
| LOC105167791 | Ethylene-responsive transcription factor 1B-like | |||
| LOC105167765 | Transcription repressor KAN1 | |||
| LOC105167815 | Isocitrate dehydrogenase [NADP] | |||
| LG11 | 310665–1216709 | NN, CDI, SL, SW, SP, SA, Zn, Fe, S | LOC110012885 | Ethylene-responsive transcription factor ERF023-like |
| LOC105173138 | Cyclic nucleotide-gated ion channel 1-like | |||
| LOC105173087 | Cyclic nucleotide-gated ion channel 1-like | |||
| LOC105173088 | Cyclic nucleotide-gated ion channel 1-like | |||
| LOC105173140 | MYB-like transcription factor ETC3 | |||
| LOC105173141 | Probable WRKY transcription factor 30 | |||
| LOC105173122 | Heavy metal-associated isoprenylated plant protein 39-like | |||
| LOC105173253 | Probable polygalacturonase | |||
| LOC105173155 | Ferric reduction oxidase 2 | |||
| LOC105173156 | Ferric reduction oxidase 2-like | |||
| LOC105173161 | Ascorbate transporter | |||
| LG11 | 5566003–5767772 | NN, IL, SB, PH, CDI, Fe, Zn | LOC105173373 | Protein PHOSPHATE STARVATION RESPONSE 1-like |
| LOC105173380 | Citrate synthase | |||
| LG11 | 14205927–14426041 | NN, SB, PH, CL, CW, SL, P | LOC105174482 | Transcription factor LHW |
| LOC105174515 | Transcription factor MYB1 | |||
| LG16 | 14816-3048510 | CDI, Zn, Mn | LOC105178592 | Transcription factor bHLH30-like |
| LOC105178450 | Transcription factor TCP10-like | |||
| LOC105178476 | Nicotianamine aminotransferase A | |||
| LOC105178598 | WRKY transcription factor 6 | |||
| LOC105178495 | Transcription factor MYB101 | |||
| LOC105178506 | MYB-like transcription factor ETC1 | |||
| LOC105178507 | Isocitrate lyase | |||
| LOC105178516 | Aconitate hydratase | |||
| LOC105178613 | Calcium-binding protein PBP1-like | |||
| LOC105178537 | Transcription factor MYB39-like | |||
| LOC105178559 | Ethylene-responsive transcription factor 4-like | |||
| LOC105178589 | Zinc transporter 8-like | |||
| LOC105178590 | Zinc transporter 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teboul, N.; Gadri, Y.; Berkovich, Z.; Reifen, R.; Peleg, Z. Genetic Architecture Underpinning Yield Components and Seed Mineral–Nutrients in Sesame. Genes 2020, 11, 1221. https://doi.org/10.3390/genes11101221
Teboul N, Gadri Y, Berkovich Z, Reifen R, Peleg Z. Genetic Architecture Underpinning Yield Components and Seed Mineral–Nutrients in Sesame. Genes. 2020; 11(10):1221. https://doi.org/10.3390/genes11101221
Chicago/Turabian StyleTeboul, Naama, Yaron Gadri, Zipi Berkovich, Ram Reifen, and Zvi Peleg. 2020. "Genetic Architecture Underpinning Yield Components and Seed Mineral–Nutrients in Sesame" Genes 11, no. 10: 1221. https://doi.org/10.3390/genes11101221
APA StyleTeboul, N., Gadri, Y., Berkovich, Z., Reifen, R., & Peleg, Z. (2020). Genetic Architecture Underpinning Yield Components and Seed Mineral–Nutrients in Sesame. Genes, 11(10), 1221. https://doi.org/10.3390/genes11101221