Mandibulofacial Dysostosis Attributed to a Recessive Mutation of CYP26C1 in Hereford Cattle
Abstract
:1. Introduction
2. Materials and Methods
3. Results
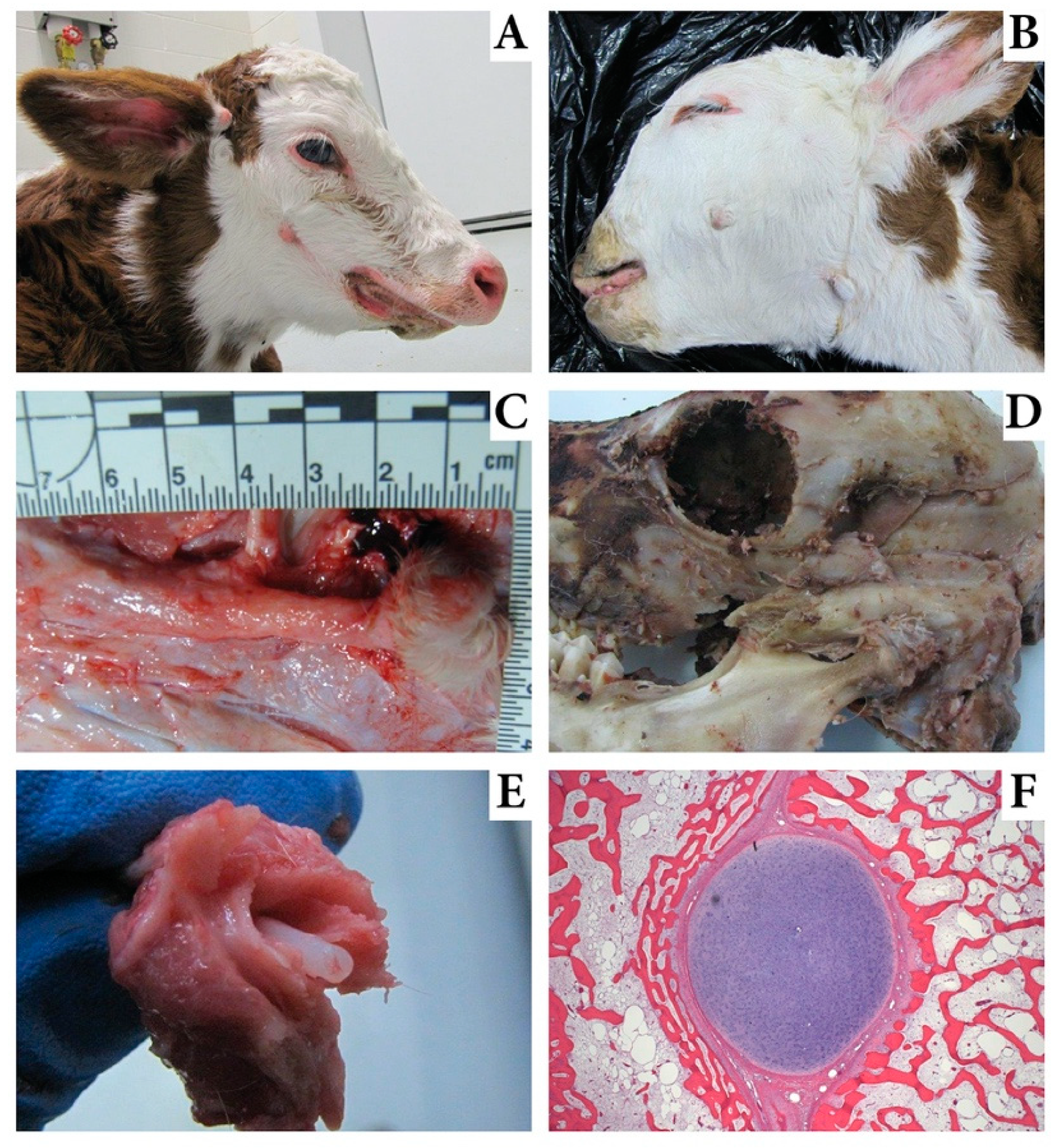
3.1. Pathologic Characteristics of Affected Calves
3.2. Whole-Genome Sequencing
3.3. Candidate Variant Filtering
3.4. CYP26C1 Variant Genotyping
3.5. Predicted Impact on Protein Function
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ciepłoch, A.; Rutkowska, K.; Oprządek, J.; Poławska, E. Genetic disorders in beef cattle: A review. Genes Genom. 2017, 39, 461–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, H.A.; Sonstegard, T.S.; VanRaden, P.M.; Null, D.J.; Van Tassell, C.P.; Larkin, D.M.; Lewin, H.A. Identification of a nonsense mutation in APAF1 that is likely causal for a decrease in reproductive efficiency in Holstein dairy cattle. J. Dairy Sci. 2016, 99, 6693–6701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Zhao, Y.; Shen, G.; Dai, J. Etiology and Pathogenesis of Hemifacial Microsomia. J. Dent. Res. 2018, 97, 1297–1305. [Google Scholar] [CrossRef]
- Gitton, Y.; Heude, É.; Vieux-Rochas, M.; Benouaiche, L.; Fontaine, A.; Sato, T.; Kurihara, Y.; Kurihara, H.; Couly, G.; Levi, G. Evolving maps in craniofacial development. Semin. Cell Dev. Biol. 2010, 21, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Vieux-Rochas, M.; Coen, L.; Sato, T.; Kurihara, Y.; Gitton, Y.; Barbieri, O.; Le Blay, K.; Merlo, G.; Ekker, M.; Kurihara, H.; et al. Molecular Dynamics of Retinoic Acid-Induced Craniofacial Malformations: Implications for the Origin of Gnathostome Jaws. PLoS ONE 2007, 2. [Google Scholar] [CrossRef] [PubMed]
- Parada, C.; Chai, Y. Mandible and Tongue Development. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 115, pp. 31–58. ISBN 0070-2153. [Google Scholar]
- Petersen, J.L.; Tietze, S.M.; Burrack, R.M.; Steffen, D.J. Evidence for a de novo, dominant germ-line mutation causative of osteogenesis imperfecta in two Red Angus calves. Mamm. Genome 2019, 30, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadept removes adapter sequences from high-throughput sequencing reads. Embnet.J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. Subgroup, 1000 Genome Project Data Processing The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [Green Version]
- Petersen, J.L.; Kalbfleisch, T.S.; Parris, M.; Tietze, S.M.; Cruickshank, J. MC1R and KIT Haplotypes Associate With Pigmentation Phenotypes of North American Yak (Bos grunniens). J. Hered. 2020, 111, 182–193. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühnel, K.; Ke, N.; Cryle, M.J.; Sligar, S.G.; Schuler, M.A.; Schlichting, I. Crystal Structures of Substrate-Free and Retinoic Acid-Bound Cyanobacterial Cytochrome P450 CYP120A1. Biochemistry 2008, 47, 6552–6559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinforma. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daetwyler, H.D.; Capitan, A.; Pausch, H.; Stothard, P.; Van Binsbergen, R.; Brøndum, R.F.; Liao, X.; Djari, A.; Rodriguez, S.C.; Grohs, C.; et al. Whole-genome sequencing of 234 bulls facilitates mapping of monogenic and complex traits in cattle. Nat. Genet. 2014, 46, 858–865. [Google Scholar] [CrossRef] [PubMed]
- McGeady, T.A.; Quinn, P.J.; FitzPatrick, E.S.; Ryan, M.T.; Kilroy, D.; Lonergan, P. Veterinary Embryology; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 111894061X. [Google Scholar]
- Granstrom, G.; Zellin, G.; Magnusson, B.C.; Mangs, H. Enzyme histochemical analysis of Meckel’s cartilage. J. Anat. 1988, 160, 101–108. [Google Scholar] [PubMed]
- Schilling, T.F.; Sosnik, J.; Nie, Q. Visualizing retinoic acid morphogen gradients. Methods Cell Biol. 2016, 133, 139–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruest, L.B.; Xiang, X.; Lim, K.C.; Levi, G.; Clouthier, D.E. Endothelin-A receptor-dependent and -independent signaling pathways in establishing mandibular identity. Development 2004, 131, 4413–4423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serbedzija, G.N.; Bronner-Fraser, M.; Fraser, S.E. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development 1992, 116, 297–307. [Google Scholar]
- Noden, P.A.N.N. Cardiogenesis in the Bovine to 35 Somites. Master’s Thesis, Kansas State University, Manhatten, KS, USA, 1966. [Google Scholar]
- Thomsen, P.T.-N.P.D. Morphological assessmentof preimplantation embryo quality in cattle. Reproduction 2003, 61, 103–116. [Google Scholar]
- Tahayato, A.; Dollé, P.; Petkovich, M. Cyp26C1 encodes a novel retinoic acid-metabolizing enzyme expressed in the hindbrain, inner ear, first branchial arch and tooth buds during murine development. Gene Expr. Patterns 2003, 3, 449–454. [Google Scholar] [CrossRef]
- Williams, A.L.; Bohnsack, B.L. What’s retinoic acid got to do with it? Retinoic acid regulation of the neural crest in craniofacial and ocular development. Genesis 2019, 57, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, A.; Juergensen, L.; Roeth, R.; Weiss, B.; Fukami, M.; Fricke-Otto, S.; Binder, G.; Ogata, T.; Decker, E.; Nuernberg, G.; et al. Retinoic acid catabolizing enzyme CYP 26C1 is a genetic modifier in SHOX deficiency. Embo Mol. Med. 2016, 8, 1455–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uehara, M.; Yashiro, K.; Mamiya, S.; Nishino, J.; Chambon, P.; Dolle, P.; Sakai, Y. CYP26A1 and CYP26C1 cooperatively regulate anterior-posterior patterning of the developing brain and the production of migratory cranial neural crest cells in the mouse. Dev. Biol. 2007, 302, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Slavotinek, A.M.; Mehrotra, P.; Nazarenko, I.; Tang, P.L.F.; Lao, R.; Cameron, D.; Li, B.; Chu, C.; Chou, C.; Marqueling, A.L.; et al. Focal facial dermal dysplasia, type IV, is caused by mutations in CYP26C1. Hum. Mol. Genet. 2013, 22, 696–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.K.; Kang, Y.K. Positional preference of proline in α-helices. Protein Sci. 1999, 8, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Agerholm, J.S.; McEvoy, F.J.; Heegaard, S.; Charlier, C.; Jagannathan, V.; Drögemüller, C. A de novo missense mutation of FGFR2 causes facial dysplasia syndrome in Holstein cattle. BMC Genet. 2017, 18, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Oryan, A.; Shirian, S.; Samadian, M.R. Congenital craniofacial and skeletal defects with arthrogryposis in two newborn male Holstein Friesian calves. Comp. Clin. Path. 2011, 20, 43–46. [Google Scholar] [CrossRef]
- Sartelet, A.; Stauber, T.; Coppieters, W.; Ludwig, C.F.; Fasquelle, C.; Druet, T.; Zhang, Z.; Ahariz, N.; Cambisano, N.; Jentsch, T.J.; et al. A missense mutation accelerating the gating of the lysosomal Cl -/H+-exchanger ClC-7/Ostm1 causes osteopetrosis with gingival hamartomas in cattle. Dmm Dis. Model. Mech. 2014, 7, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Kumar, C.R.; Shaikh, H.A.; Kandarpalle, A.V.; Allure, K. Surgical Correction of Congenital Macrostomia in Cattle Calf. Int. J. Sci. Eng. Res. 2019, 10, 529–531. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Ye, X.; Taub, P.J. Review of the Genetic Basis of Jaw Malformations. J. Pediatr. Genet. 2016, 5, 209–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieczorek, D. Human facial dysostoses. Clin. Genet. 2013, 83, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Lines, M.A.; Huang, L.; Schwartzentruber, J.; Douglas, S.L.; Lynch, D.C.; Beaulieu, C.; Guion-Almeida, M.L.; Zechi-Ceide, R.M.; Gener, B.; Gillessen-Kaesbach, G. Haploinsufficiency of a spliceosomal GTPase encoded by EFTUD2 causes mandibulofacial dysostosis with microcephaly. Am. J. Hum. Genet. 2012, 90, 369–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammer, E.J.; Chen, D.T.; Hoar, R.M.; Agnish, N.D.; Benke, P.J.; Braun, J.T.; Curry, C.J.; Fernhoff, P.M.; Grix, A.W., Jr.; Lott, I.T. Retinoic acid embryopathy. N. Engl. J. Med. 1985, 313, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Wiszniak, S.; Mackenzie, F.E.; Anderson, P.; Kabbara, S.; Ruhrberg, C.; Schwarz, Q. Neural crest cell-derived VEGF promotes embryonic jaw extension. Proc. Natl. Acad. Sci. USA 2015, 112, 6086–6091. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Pathologic Description | Calf 1 | Calf 2 | Calf 3 | Calf 4 | Calf 5 |
|---|---|---|---|---|---|
| Bilateral bone-wrapped Meckel’s cartilage | yes | yes | yes | yes | yes |
| Bilateral skin tags 2–10 cm caudal to the commissure of the lips | yes | yes | yes | yes | yes |
| Skin tags near or below the external acoustic meatus | - | yes | yes | yes | yes |
| Low set and/or drooped pinnae | yes | yes | yes | yes | yes |
| Hypoplasia of the masseter and temporalis muscles | yes | yes | yes | yes | yes |
| Megastomia | yes | yes | no | no | yes |
| Brachgnathia inferior | yes | no | no | yes | yes |
| Campylognathia involving mandible and maxilla | no | no | yes | no | yes |
| Asymmetry of the orbits | no | no | yes | no | yes |
| Cleft palate | yes | no | no | no | no |
| Sex of calf | female | male | male | male | female |
| Chr | Position (bp) | Reference | Alternative | Variant Annotation | Gene |
|---|---|---|---|---|---|
| 7 | 15413 | C | T | Intergenic | |
| 26 | 10588403 | T | A | Intronic | STAMBPL1 |
| 26 | 10616433 | C | T | Downstream gene variant | STAMBPL1 |
| 26 | 10713132 | G | A | Downstream gene variant | FAS |
| 26 | 10794674 | G | A | Intergenic | |
| 26 | 10982292 | TGAGAGAGGAT | TGAGAGGAT | Intronic | LIPA |
| 26 | 14404993 | T | C | Missense (p.L188P; SIFT = 0, deleterious) | CYP26C1 |
| 26 | 15898152 | C | T | Upstream gene variant | TBC1D12 |
| Reporting Herd 1 | TT | TC | CC | Total Animals |
|---|---|---|---|---|
| Founder in either maternal or paternal pedigree | 95 | 50 | 0 | 145 |
| Founder in both maternal and paternal pedigree | 2 | 5 | 2 | 9 |
| No ties to founder | 91 | 0 | 0 | 91 |
| Total Herd 1 | 188 | 55 | 2 | 245 |
| Reporting Herd 2 | ||||
| Founder in either maternal or paternal pedigree | 114 | 35 | 0 | 149 |
| Founder in both maternal and paternal pedigree | 4 | 5 | 1 | 10 |
| No ties to founder | 239 | 0 | 0 | 239 |
| Total Herd 2 | 357 | 40 | 1 | 398 |
| Other | ||||
| Founder in either maternal or paternal pedigree | 13 | 20 | 0 | 33 |
| Founder in both maternal and paternal pedigree | 48 | 37 | 2 | 87 |
| No ties to founder | 18 | 0 | 0 | 18 |
| Animal is founder | 0 | 1 | 0 | 1 |
| Total Other Genotypes | 79 | 58 | 2 | 139 |
| Total Animals Genotyped | 624 | 153 | 5 | 782 |
| CYP26C1 Genotype Source | Number of Animals |
|---|---|
| WGS for this project * | 20 |
| Hereford cattle genotyped for this project * | 762 |
| Other WGS variant data generated in our lab | 101 |
| 1000 bulls [16] and American Hereford Association (WGS) | 1705 |
| Sequence Read Archive | 783 |
| Total | 3371 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sieck, R.L.; Fuller, A.M.; Bedwell, P.S.; Ward, J.A.; Sanders, S.K.; Xiang, S.-H.; Peng, S.; Petersen, J.L.; Steffen, D.J. Mandibulofacial Dysostosis Attributed to a Recessive Mutation of CYP26C1 in Hereford Cattle. Genes 2020, 11, 1246. https://doi.org/10.3390/genes11111246
Sieck RL, Fuller AM, Bedwell PS, Ward JA, Sanders SK, Xiang S-H, Peng S, Petersen JL, Steffen DJ. Mandibulofacial Dysostosis Attributed to a Recessive Mutation of CYP26C1 in Hereford Cattle. Genes. 2020; 11(11):1246. https://doi.org/10.3390/genes11111246
Chicago/Turabian StyleSieck, Renae L., Anna M. Fuller, Patrick S. Bedwell, Jack A. Ward, Stacy K. Sanders, Shi-Hua Xiang, Sichong Peng, Jessica L. Petersen, and David J. Steffen. 2020. "Mandibulofacial Dysostosis Attributed to a Recessive Mutation of CYP26C1 in Hereford Cattle" Genes 11, no. 11: 1246. https://doi.org/10.3390/genes11111246
APA StyleSieck, R. L., Fuller, A. M., Bedwell, P. S., Ward, J. A., Sanders, S. K., Xiang, S.-H., Peng, S., Petersen, J. L., & Steffen, D. J. (2020). Mandibulofacial Dysostosis Attributed to a Recessive Mutation of CYP26C1 in Hereford Cattle. Genes, 11(11), 1246. https://doi.org/10.3390/genes11111246







