Genetic Mutations and Variants in the Susceptibility of Familial Non-Medullary Thyroid Cancer
Abstract
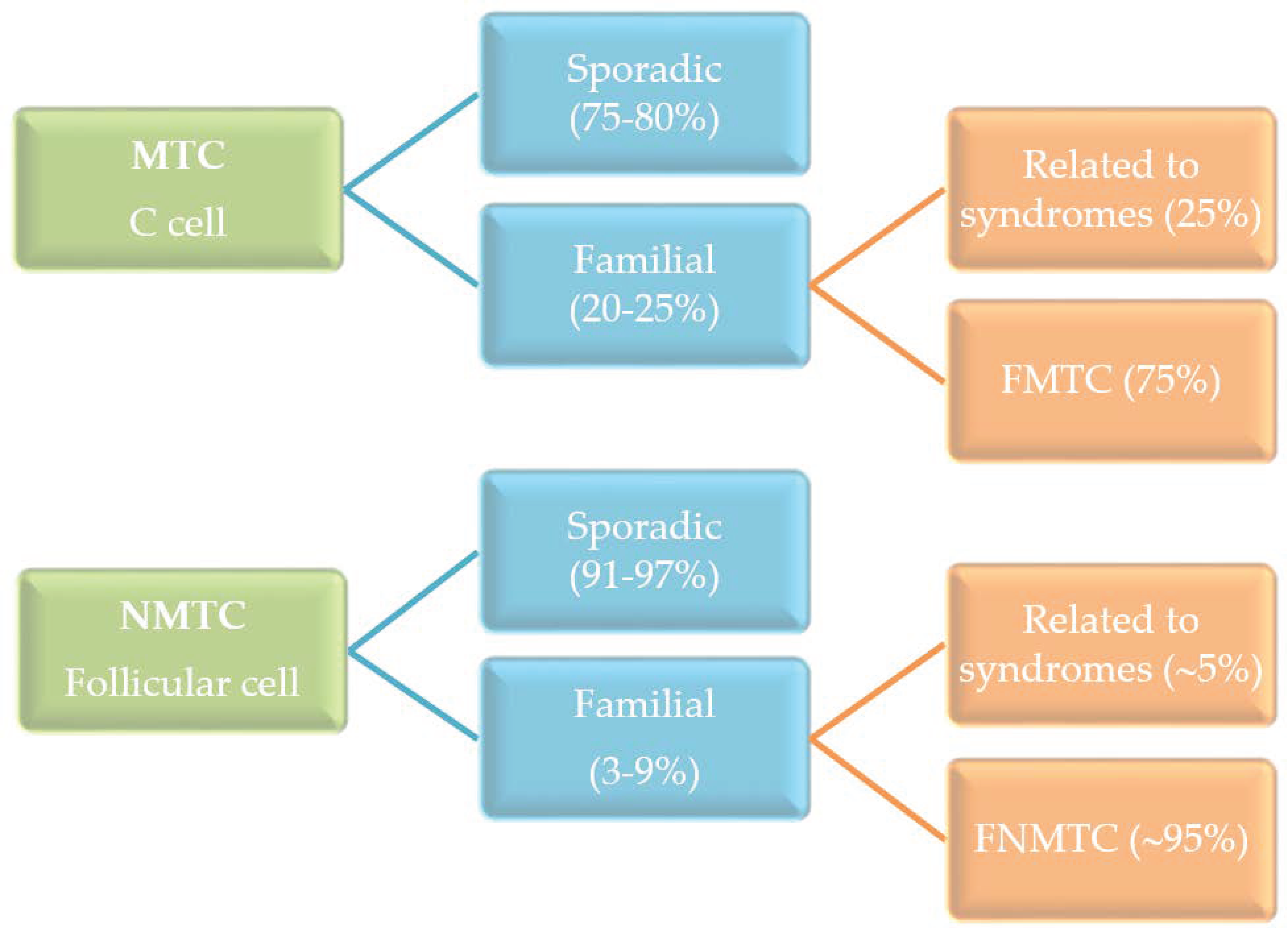
:1. Introduction
2. Syndromic Causes of Non-Medullary Thyroid Cancer
2.1. Cowden Syndrome
2.2. Carney Complex
2.3. Werner Syndrome
2.4. Familial Adenomatous Polyposis
2.5. Ataxia-Telangiectasia Syndrome
2.6. DICER 1 Syndrome and miRNA Processing
2.7. Li-Fraumeni Syndrome
3. Non-Syndromic FNMTC
3.1. Linkage Analysis
3.1.1. TCO Locus (19p13.2)
3.1.2. PRN1 Locus (1q21)
3.1.3. NMTC1 Locus (2q21)
3.1.4. q32 Locus (an Enhancer of Unknown Function)
3.1.5. 6q22 Locus
3.1.6. 8p23.1–p22 Locus
3.1.7. 8q24 Locus, a lncRNA inside the Thyroglobulin (TG) Gene
3.1.8. SRGAP1 (12q14 Locus)
3.1.9. NKX2-1 (14q13.3 Locus)
3.1.10. MNG1 Locus (14q32)-DICER1
3.2. Genome-Wide Linkage Analysis in the Population of PTC Patients
3.2.1. FOXE1/PTCSC2
3.2.2. NKX2-1
3.2.3. NRG1
3.2.4. DIRC3
3.2.5. Polygenic Contribution
3.2.6. Telomere Abnormalities
3.2.7. miRNA
3.3. Whole Exome/Genome Sequence
3.3.1. SRRM2
3.3.2. NOP53
3.3.3. HABP2
3.4. Candidate Variants Associated with FNMTC
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | autosomal dominant |
| AR | autosomal recessive |
| ATC | anaplastic thyroid cancer |
| CMVPTC | cribriform-morular variant of PTC |
| cPTC | classical PTC |
| DDR | DNA damage response |
| DTC | differentiated thyroid cancer |
| FA | follicular adenoma |
| FAP | familial adenomatous polyposis |
| FNMTC | familial nonmedullary thyroid cancer |
| FTC | follicular thyroid cancer |
| FVPTC | follicular variant of PTC |
| GWAS | genome-wide association studies |
| HR | hazard ratio |
| LOD | logarithm of odds |
| MAF | minor allele frequency |
| miRNAs | microRNAs |
| MNG | multinodular goiter |
| MTC | medullary thyroid cancer |
| NGS | next generation sequencing |
| NMTC | nonmedullary thyroid cancer |
| PTC | papillary thyroid cancer |
| SNPs | single nucleotide polymorphisms |
| TCGA | The Cancer Genome Atlas |
| VUS | variant of uncertain significance |
| WES | whole exome sequence |
| WGS | whole genome sequence |
References
- Hincza, K.; Kowalik, A.; Kowalska, A. Current Knowledge of Germline Genetic Risk Factors for the Development of Non-Medullary Thyroid Cancer. Genes 2019, 10, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilmette, J.; Nosé, V. Hereditary and familial thyroid tumours. Histopathology 2017, 72, 70–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firminger, H.I.; Skelton, F.R. Carcinoma of the thyroid: Papillary adenocarcinoma occurring in twins and a case of Hürthle cell carcinoma; tumor conference. J. Kans. Med. Soc. 1953, 54, 427–432. [Google Scholar] [PubMed]
- Sippel, R.S.; Caron, N.R.; Clark, O.H. An evidence-based approach to familial nonmedullary thyroid cancer: Screening, clinical management, and follow-up. World J. Surg. 2007, 31, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Mazeh, H.; Sippel, R.S. Familial nonmedullary thyroid carcinoma. Thyroid 2013, 23, 1049–1056. [Google Scholar] [CrossRef]
- Canzian, F.; Amati, P.; Harach, H.R.; Kraimps, J.-L.; Lesueur, F.; Barbier, J.; Levillain, P.; Romeo, G.; Bonneau, D. A gene predisposing to familial thyroid tumors with cell oxyphilia maps to chromosome 19p13.2. Am. J. Hum. Genet. 1998, 63, 1743–1748. [Google Scholar] [CrossRef] [Green Version]
- Bignell, G.R.; Canzian, F.; Shayeghi, M.; Stark, M.; Shugart, Y.Y.; Biggs, P.; Mangion, J.; Hamoudi, R.; Rosenblatt, J.; Buu, P.; et al. Familial nontoxic multinodular thyroid goiter locus maps to chromosome 14q but does not account for familial nonmedullary thyroid cancer. Am. J. Hum. Genet. 1997, 61, 1123–1130. [Google Scholar] [CrossRef] [Green Version]
- Malchoff, C.D.; Sarfarazi, M.; Tendler, B.; Forouhar, F.; Whalen, G.; Joshi, V.; Arnold, A.; Malchoff, D.M. Papillary thyroid carcinoma associated with papillary renal neoplasia: Genetic linkage analysis of a distinct heritable tumor syndrome. J. Clin. Endocrinol. Metab. 2000, 85, 1758–1764. [Google Scholar] [CrossRef]
- McKay, J.D.; Lesueur, F.; Jonard, L.; Pastore, A.; Williamson, J.; Hoffman, L.; Burgess, J.; Duffield, A.; Papotti, M.; Stark, M.; et al. Localization of a susceptibility gene for familial nonmedullary thyroid carcinoma to chromosome 2q21. Am. J. Hum. Genet. 2001, 69, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Cavaco, B.M.; Batista, P.F.; Sobrinho, L.G.; Leite, V. Mapping a new familial thyroid epithelial neoplasia susceptibility locus to chromosome 8p23.1-p22 by high-density single-nucleotide polymorphism genome-wide linkage analysis. J. Clin. Endocrinol. Metab. 2008, 93, 4426–4430. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liyanarachchi, S.; Miller, K.E.; Nieminen, T.T.; Comiskey, D.F., Jr.; Li, W.; Brock, P.; Symer, D.E.; Akagi, K.; DeLap, K.E.; et al. Identification of Rare Variants Predisposing to Thyroid Cancer. Thyroid 2019, 29, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Saenko, V.A.; Rogounovitch, T.I. Genetic Polymorphism Predisposing to Differentiated Thyroid Cancer: A Review of Major Findings of the Genome-Wide Association Studies. Endocrinol. Metab. 2018, 33, 164–174. [Google Scholar] [CrossRef]
- Carbone, M.; Arron, S.T.; Beutler, B.; Bononi, A.; Cavenee, W.; Cleaver, J.E.; Croce, C.M.; D’Andrea, A.; Foulkes, W.D.; Gaudino, G.; et al. Tumour predisposition and cancer syndromes as models to study gene-environment interactions. Nat. Rev. Cancer 2020, 20, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Van Os, N.J.; Roeleveld, N.; Weemaes, C.M.R.; Jongmans, M.C.J.; Janssens, G.O.; Taylor, A.M.R.; Hoogerbrugge, N.; Willemsen, M.A.A.P. Health risks for ataxia-telangiectasia mutated heterozygotes: A systematic review, meta-analysis and evidence-based guideline. Clin. Genet. 2016, 90, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy-Perez, B.; Janin, N.; Ossian, K.; Laugé, A.; Croquette, M.F.; Griscelli, C.; Debré, M.; Bressac-de-Paillerets, B.; Aurias, A.; Stoppa-Lyonnet, D.; et al. Cancer risk in heterozygotes for ataxia-telangiectasia. Int. J. Cancer 2001, 93, 288–293. [Google Scholar] [CrossRef]
- Kamilaris, C.D.C.; Faucz, F.R.; Voutetakis, A.; Stratakis, C.A. Carney Complex. Exp. Clin. Endocrinol. Diabetes 2019, 127, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Gammon, A.; Jasperson, K.; Champine, M. Genetic basis of Cowden syndrome and its implications for clinical practice and risk management. Appl. Clin. Genet. 2016, 9, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Yehia, L.; Keel, E.; Eng, C. The Clinical Spectrum of PTEN Mutations. Annu. Rev. Med. 2020, 71, 103–116. [Google Scholar] [CrossRef] [Green Version]
- Frio, T.R.; Bahubeshi, A.; Kanellopoulou, C.; Hamel, N.; Niedziela, M.; Sabbaghian, N.; Pouchet, C.; Gilbert, L.; O’Brien, P.K.; Serfas, K.; et al. DICER1 Mutations in Familial Multinodular Goiter With and Without Ovarian Sertoli-Leydig Cell Tumors. JAMA 2011, 305, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.A.; Ivanovich, J.; Priest, J.R.; Gurnett, C.A.; Dehner, L.P.; Desruisseau, D.; Jarzembowski, J.A.; Wikenheiser-Brokamp, K.A.; Suarez, B.K.; Whelan, A.J.; et al. DICER1 Mutations in Familial Pleuropulmonary Blastoma. Science 2009, 325, 965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomoda, C.; Miyauchi, A.; Uruno, T.; Takamura, Y.; Ito, Y.; Miya, A.; Kobayashi, K.; Matsuzuka, F.; Kuma, S.; Kuma, K.; et al. Cribriform-morular variant of papillary thyroid carcinoma: Clue to early detection of familial adenomatous polyposis-associated colon cancer. World J. Surg. 2004, 28, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, T.T.; Walker, C.J.; Olkinuora, A.; Genutis, L.K.; O’Malley, M.; Wakely, P.E.; LaGuardia, L.; Koskenvuo, L.; Arola, J.; Lepistö, A.H.; et al. Thyroid Carcinomas That Occur in Familial Adenomatous Polyposis Patients Recurrently Harbor Somatic Variants in APC, BRAF, and KTM2D. Thyroid 2020, 30, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Formiga, M.N.D.C.; De Andrade, K.C.; Kowalski, L.P.; Achatz, M.I. Frequency of Thyroid Carcinoma in Brazilian TP53 p.R337H Carriers with Li Fraumeni Syndrome. JAMA Oncol. 2017, 3, 1400–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshima, J.; Sidorova, J.M.; Monnat, R.J., Jr. Werner syndrome: Clinical features, pathogenesis and potential therapeutic interventions. Ageing Res. Rev. 2017, 33, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereda, C.M.; Lesueur, F.; Pertesi, M.; Robinot, N.; Lence-Anta, J.J.; Turcios, S.; Velasco, M.; Chappe, M.; Infante, I.; Bustillo, M.; et al. Common variants at the 9q22.33, 14q13.3 and ATM loci, and risk of differentiated thyroid cancer in the Cuban population. BMC Genet. 2015, 16, 22. [Google Scholar] [CrossRef] [Green Version]
- Maillard, S.; Damiola, F.; Clero, E.; Pertesi, M.; Robinot, N.; Rachédi, F.; Boissin, J.-L.; Sebbag, J.; Shan, L.; Bost-Bezeaud, F.; et al. Common variants at 9q22.33, 14q13.3, and ATM loci, and risk of differentiated thyroid cancer in the French Polynesian population. PLoS ONE 2015, 10, e0123700. [Google Scholar] [CrossRef]
- Tan, M.-H.; Mester, J.; Peterson, C.; Yang, Y.; Chen, J.-L.; Rybicki, L.A.; Milas, K.; Pederson, H.; Remzi, B.; Orloff, M.S.; et al. A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am. J. Hum. Genet. 2011, 88, 42–56. [Google Scholar] [CrossRef] [Green Version]
- Schultz, K.A.P.; Rednam, S.P.; Kamihara, J.; Doros, L.; Achatz, M.I.; Wasserman, J.D.; Diller, L.R.; Brugières, L.; Druker, H.; Schneider, K.A.; et al. PTEN, DICER1, FH, and Their Associated Tumor Susceptibility Syndromes: Clinical Features, Genetics, and Surveillance Recommendations in Childhood. Clin. Cancer Res. 2017, 23, e76–e82. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Kiyotani, K.; Yew, P.Y.; Kato, T.; Tamura, K.; Yap, K.L.; Nielsen, S.M.; Mester, J.L.; Eng, C.; Nakamura, Y.; et al. Germline PARP4 mutations in patients with primary thyroid and breast cancers. Endocr. Relat. Cancer 2016, 23, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Yehia, L.; Ni, Y.; Sesock, K.; Niazi, F.; Fletcher, B.; Chen, H.J.L.; LaFramboise, T.; Eng, C. Unexpected cancer-predisposition gene variants in Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome patients without underlying germline PTEN mutations. PLoS Genet. 2018, 14, e1007352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngeow, J.; Ni, Y.; Tohme, R.; Chen, F.S.; Bebek, G.; Eng, C. Germline alterations in RASAL1 in Cowden syndrome patients presenting with follicular thyroid cancer and in individuals with apparently sporadic epithelial thyroid cancer. J. Clin. Endocrinol. Metab. 2014, 99, E1316–E1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naderali, E.; Khaki, A.A.; Rad, J.S.; Ali-Hemmati, A.; Rahmati, M.; Charoudeh, H.N. Regulation and modulation of PTEN activity. Mol. Biol. Rep. 2018, 45, 2869–2881. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.A.; Leitner, M.G.; Sos, M.L.; Mavrantoni, A.; Rychkova, A.; Johnson, J.R.; Newton, B.W.; Yee, M.C.; De La Vega, F.M.; Ford, J.M.; et al. Discovery and functional characterization of a neomorphic PTEN mutation. Proc. Natl. Acad. Sci. USA 2015, 112, 13976–13981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milella, M.; Falcone, I.; Conciatori, F.; Cesta Incani, U.; Del Curatolo, A.; Inzerilli, N.; Nuzzo, C.M.; Vaccaro, V.; Vari, S.; Cognetti, F.; et al. PTEN: Multiple Functions in Human Malignant Tumors. Front. Oncol. 2015, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Ringel, M.D.; Hayre, N.; Saito, J.; Saunier, B.; Schuppert, F.; Burch, H.; Bernet, V.; Burman, K.D.; Kohn, L.D.; Saji, M. Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res. 2001, 61, 6105–6111. [Google Scholar]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef]
- Liu, D.; Yang, C.; Bojdani, E.; Murugan, A.K.; Xing, M. Identification of RASAL1 as a major tumor suppressor gene in thyroid cancer. J. Natl. Cancer Inst. 2013, 105, 1617–1627. [Google Scholar] [CrossRef] [Green Version]
- Pepe, S.; Korbonits, M.; Iacovazzo, D. Germline and mosaic mutations causing pituitary tumours: Genetic and molecular aspects. J. Endocrinol. 2019, 240, R21–R45. [Google Scholar] [CrossRef] [Green Version]
- Griffin, K.J.; Kirschner, L.S.; Matyakhina, L.; Stergiopoulos, S.; Robinson-White, A.; Lenherr, S.; Weinberg, F.D.; Claflin, E.; Meoli, E.; Cho-Chung, Y.S.; et al. Down-regulation of regulatory subunit type 1A of protein kinase A leads to endocrine and other tumors. Cancer Res. 2004, 64, 8811–8815. [Google Scholar] [CrossRef] [Green Version]
- Sandrini, F.; Matyakhina, L.; Sarlis, N.J.; Kirschner, L.S.; Farmakidis, C.; Gimm, O.; Stratakis, C.A. Regulatory subunit type I-α of protein kinase A (PRKAR1A): A tumor-suppressor gene for sporadic thyroid cancer. Genes Chromosomes Cancer 2002, 35, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Pringle, D.R.; Yin, Z.; Lee, A.A.; Manchanda, P.K.; Yu, L.; Parlow, A.F.; Jarjoura, D.; La Perle, K.M.D.; Kirschner, L.S. Thyroid-specific ablation of the Carney complex gene, PRKAR1A, results in hyperthyroidism and follicular thyroid cancer. Endocrine-Related Cancer 2012, 19, 435–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kari, S.; Vasko, V.V.; Priya, S.; Kirschner, L.S. PKA Activates AMPK Through LKB1 Signaling in Follicular Thyroid Cancer. Front. Endocrinol. 2019, 10, 769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, M.; Miller, R.W.; Ishikawa, Y.; Sugano, H. Excess of rare cancers in Werner syndrome (adult progeria). Cancer Epidemiol. Biomarkers Prev. 1996, 5, 239–246. [Google Scholar] [PubMed]
- Muftuoglu, M.; Oshima, J.; von Kobbe, C.; Cheng, W.H.; Leistritz, D.F.; Bohr, V.A. The clinical characteristics of Werner syndrome: Molecular and biochemical diagnosis. Hum. Genet. 2008, 124, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Shamanna, R.A.; Lu, H.; de Freitas, J.K.; Tian, J.; Croteau, D.L.; Bohr, V.A. WRN regulates pathway choice between classical and alternative non-homologous end joining. Nat. Commun. 2016, 7, 13785. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, Y.; Sugano, H.; Matsumoto, T.; Furuichi, Y.; Miller, R.W.; Goto, M. Unusual features of thyroid carcinomas in Japanese patients with Werner syndrome and possible genotype-phenotype relations to cell type and race. Cancer 1999, 85, 1345–1352. [Google Scholar] [CrossRef]
- Uchino, S.; Noguchi, S.; Yamashita, H.; Yamashita, H.; Watanabe, S.; Ogawa, T.; Tsuno, A.; Murakami, A.; Miyauchi, A. Mutational analysis of the APC gene in cribriform-morula variant of papillary thyroid carcinoma. World J. Surg. 2006, 30, 775–779. [Google Scholar] [CrossRef]
- De Herreros, A.G.; Duñach, M. Intracellular Signals Activated by Canonical Wnt Ligands Independent of GSK3 Inhibition and β-Catenin Stabilization. Cells 2019, 8, 1148. [Google Scholar] [CrossRef] [Green Version]
- Heinen, C.D. Genotype to phenotype: Analyzing the effects of inherited mutations in colorectal cancer families. Mutat. Res. 2010, 693, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Giannelli, S.M.; McPhaul, L.; Nakamoto, J.; Gianoukakis, A.G. Familial adenomatous polyposis-associated, cribriform morular variant of papillary thyroid carcinoma harboring a K-RAS mutation: Case presentation and review of molecular mechanisms. Thyroid 2014, 24, 1184–1189. [Google Scholar] [CrossRef]
- Iwama, T.; Konishi, M.; Iijima, T.; Yoshinaga, K.; Tominaga, T.; Koike, M.; Miyaki, M. Somatic mutation of the APC gene in thyroid carcinoma associated with familial adenomatous polyposis. Jpn. J. Cancer Res. 1999, 90, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Miyaki, M.; Iijima, T.; Ishii, R.; Hishima, T.; Mori, T.; Yoshinaga, K.; Takami, H.; Kuroki, T.; Iwama, T. Molecular Evidence for Multicentric Development of Thyroid Carcinomas in Patients with Familial Adenomatous Polyposis. Am. J. Pathol. 2000, 157, 1825–1827. [Google Scholar] [CrossRef] [Green Version]
- Syngal, S.; Brand, R.E.; Church, J.M.; Giardiello, F.M.; Hampel, H.L.; Burt, R.W. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am. J. Gastroenterol. 2015, 110, 223–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achatz, M.I.; Porter, C.C.; Brugières, L.; Druker, H.; Frebourg, T.; Foulkes, W.D.; Kratz, C.P.; Kuiper, R.P.; Hansford, J.R.; Hernandez, H.S.; et al. Cancer Screening Recommendations and Clinical Management of Inherited Gastrointestinal Cancer Syndromes in Childhood. Clin. Cancer Res. 2017, 23, e107–e114. [Google Scholar] [CrossRef] [Green Version]
- Dombernowsky, S.L.; Weischer, M.; Allin, K.H.; Bojesen, S.E.; Tybjirg-Hansen, A.; Nordestgaard, B.G. Risk of cancer by ATM missense mutations in the general population. J. Clin. Oncol. 2008, 26, 3057–3062. [Google Scholar] [CrossRef]
- Shiloh, Y. ATM: Expanding roles as a chief guardian of genome stability. Exp. Cell Res. 2014, 329, 154–161. [Google Scholar] [CrossRef]
- Ribezzo, F.; Shiloh, Y.; Schumacher, B. Systemic DNA damage responses in aging and diseases. Semin. Cancer Biol. 2016, 37–38, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Akulevich, N.M.; Saenko, V.A.; Rogounovitch, T.I.; Drozd, V.M.; Lushnikov, E.F.; Ivanov, V.K.; Mitsutake, N.; Kominami, R.; Yamashita, S. Polymorphisms of DNA damage response genes in radiation-related and sporadic papillary thyroid carcinoma. Endocr. Relat. Cancer 2009, 16, 491–503. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Morari, E.C.; Wei, Q.; Sturgis, E.M.; Ward, L.S. Functional variations in the ATM gene and susceptibility to differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2012, 97, 1913–1921. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Shi, J.; Qiu, S.; Qiao, Y.; Zhang, X.; Cheng, Y.; Liu, Y. Association between ATM rs1801516 polymorphism and cancer susceptibility: A meta-analysis involving 12,879 cases and 18,054 controls. BMC Cancer 2018, 18, 1060. [Google Scholar] [CrossRef] [Green Version]
- Song, C.M.; Kwon, T.K.; Park, B.L.; Ji, Y.B.; Tae, K. Single nucleotide polymorphisms of ataxia telangiectasia mutated and the risk of papillary thyroid carcinoma. Environ. Mol. Mutagen. 2015, 56, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Wójcicka, A.; Czetwertyńska, M.; Świerniak, M.; Długosińska, J.; Maciąg, M.; Czajka, A.; Dymecka, K.; Kubiak, A.; Kot, A.; Płoski, R.; et al. Variants in the ATM-CHEK2-BRCA1 axis determine genetic predisposition and clinical presentation of papillary thyroid carcinoma. Genes Chromosomes Cancer 2014, 53, 516–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitahara, C.M.; Farkas, D.K.R.; Jørgensen, J.O.L.; Cronin-Fenton, D.; Sørensen, H.T. Benign Thyroid Diseases and Risk of Thyroid Cancer: A Nationwide Cohort Study. J. Clin. Endocrinol. Metab. 2018, 103, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.J.; Chen, X.; Schneider, D.F.; Broome, J.T.; Sippel, R.S.; Chen, H.; Solórzano, C.C. Cancer after Thyroidectomy: A Multi-Institutional Experience with 1,523 Patients. J. Am. Coll. Surg. 2013, 216, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.E.; Bauer, A.J.; Schultz, K.A.P.; Doros, L.; DeCastro, R.M.; Ling, A.; Lodish, M.B.; Harney, L.A.; Kase, R.G.; Carr, A.G.; et al. Quantification of Thyroid Cancer and Multinodular Goiter Risk in the DICER1 Syndrome: A Family-Based Cohort Study. J. Clin. Endocrinol. Metab. 2017, 102, 1614–1622. [Google Scholar] [CrossRef]
- Lin, S.; Gregory, R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 2015, 15, 321–333. [Google Scholar] [CrossRef]
- Poma, A.M.; Condello, V.; Denaro, M.; Torregrossa, L.; Elisei, R.; Vitti, P.; Basolo, F. DICER1 somatic mutations strongly impair miRNA processing even in benign thyroid lesions. Oncotarget 2019, 10, 1785–1797. [Google Scholar] [CrossRef]
- Fuziwara, C.S.; Kimura, E.T. MicroRNAs in thyroid development, function and tumorigenesis. Mol. Cell. Endocrinol. 2017, 456, 44–50. [Google Scholar] [CrossRef]
- Rodriguez, W.; Jin, L.; Janssens, V.; Pierreux, C.; Hick, A.-C.; Urizar, E.; Costagliola, S. Deletion of the RNaseIII Enzyme Dicer in Thyroid Follicular Cells Causes Hypothyroidism with Signs of Neoplastic Alterations. PLoS ONE 2012, 7, e29929. [Google Scholar] [CrossRef]
- Frezzetti, D.; Reale, C.; Calì, G.; Nitsch, L.; Fagman, H.; Nilsson, O.; Scarfò, M.; De Vita, G.; Di Lauro, R. The microRNA-Processing Enzyme Dicer Is Essential for Thyroid Function. PLoS ONE 2011, 6, e27648. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.A.P.; Williams, G.M.; Kamihara, J.; Stewart, D.R.; Harris, A.K.; Bauer, A.J.; Turner, J.; Shah, R.; Schneider, K.; Schneider, K.W.; et al. DICER1 and Associated Conditions: Identification of At-risk Individuals and Recommended Surveillance Strategies. Clin. Cancer Res. 2018, 24, 2251–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutter, M.M.; Jha, P.; Schultz, K.A.P.; Sheil, A.; Harris, A.K.; Bauer, A.J.; Field, A.L.; Geller, J.; Hill, D.A. DICER1Mutations and Differentiated Thyroid Carcinoma: Evidence of a Direct Association. J. Clin. Endocrinol. Metab. 2016, 101, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data: Figure 1. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
- cBioPortal for Cancer Genomics. Available online: https://www.cbioportal.org (accessed on 16 August 2020).
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [Green Version]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [Green Version]
- Chernock, R.D.; Rivera, B.; Borrelli, N.; Hill, D.A.; Fahiminiya, S.; Shah, T.; Chong, A.-S.; Aqil, B.; Mehrad, M.; Giordano, T.J.; et al. Poorly differentiated thyroid carcinoma of childhood and adolescence: A distinct entity characterized by DICER1 mutations. Mod. Pathol. 2020, 33, 1264–1274. [Google Scholar] [CrossRef]
- Wasserman, J.D.; Sabbaghian, N.; Fahiminiya, S.; Chami, R.; Mete, O.; Acker, M.; Wu, M.K.; Shlien, A.; De Kock, L.; Foulkes, W.D. DICER1 Mutations Are Frequent in Adolescent-Onset Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2018, 103, 2009–2015. [Google Scholar] [CrossRef] [Green Version]
- Canberk, S.; Ferreira, J.C.; Pereira, L.; Batısta, R.; Vieira, A.F.; Soares, P.; Simões, M.S.; Máximo, V. Analyzing the Role of DICER1 Germline Variations in Papillary Thyroid Carcinoma. Eur. Thyroid J. 2020, 1–8. [Google Scholar] [CrossRef]
- Rivera, B.; Nadaf, J.; Fahiminiya, S.; Apellaniz-Ruiz, M.; Saskin, A.; Chong, A.-S.; Sharma, S.; Wagener, R.; Revil, T.; Condello, V.; et al. DGCR8 microprocessor defect characterizes familial multinodular goiter with schwannomatosis. J. Clin. Investig. 2020, 130, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldgar, D.E.; Easton, D.F.; Cannon-Albright, L.A.; Skolnick, M.H. Systematic Population-Based Assessment of Cancer Risk in First-Degree Relatives of Cancer Probands. J. Natl. Cancer Inst. 1994, 86, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Eng, C.; Chen, B. Familial Risks for Nonmedullary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2005, 90, 5747–5753. [Google Scholar] [CrossRef] [Green Version]
- Bauer, A.J. Clinical Behavior and Genetics of Nonsyndromic, Familial Nonmedullary Thyroid Cancer. Front. Horm. Res. 2013, 41, 141–148. [Google Scholar] [CrossRef]
- Capezzone, M.; Marchisotta, S.; Cantara, S.; Busonero, G.; Brilli, L.; Pazaitou-Panayiotou, K.; Carli, A.F.; Caruso, G.; Toti, P.; Capitani, S.; et al. Familial non-medullary thyroid carcinoma displays the features of clinical anticipation suggestive of a distinct biological entity. Endocr. Relat. Cancer 2008, 15, 1075–1081. [Google Scholar] [CrossRef]
- He, H.; Bronisz, A.; Liyanarachchi, S.; Nagy, R.; Li, W.; Huang, Y.; Akagi, K.; Saji, M.; Kula, D.; Wojcicka, A.; et al. SRGAP1 Is a Candidate Gene for Papillary Thyroid Carcinoma Susceptibility. J. Clin. Endocrinol. Metab. 2013, 98, E973–E980. [Google Scholar] [CrossRef] [Green Version]
- Suh, I.; Filetti, S.; Vriens, M.R.; Guerrero, M.A.; Tumino, S.; Wong, M.; Shen, W.T.; Kebebew, E.; Duh, Q.-Y.; Clark, O.H. Distinct loci on chromosome 1q21 and 6q22 predispose to familial nonmedullary thyroid cancer: A SNP array-based linkage analysis of 38 families. Surgery 2009, 146, 1073–1080. [Google Scholar] [CrossRef]
- He, H.; Nagy, R.; Liyanarachchi, S.; Jiao, H.; Li, W.; Suster, S.; Kere, J.; De La Chapelle, A. A Susceptibility Locus for Papillary Thyroid Carcinoma on Chromosome 8q24. Cancer Res. 2009, 69, 625–631. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Li, W.; Wu, D.; Nagy, R.; Liyanarachchi, S.; Akagi, K.; Jendrzejewski, J.; Jiao, H.; Hoag, K.; Wen, B.; et al. Ultra-Rare Mutation in Long-Range Enhancer Predisposes to Thyroid Carcinoma with High Penetrance. PLoS ONE 2013, 8, e61920. [Google Scholar] [CrossRef]
- Ngan, E.S.W.; Lang, B.H.H.; Liu, T.; Shum, C.K.Y.; So, M.-T.; Lau, D.K.C.; Leon, T.Y.Y.; Cherny, S.S.; Tsai, S.Y.; Lo, C.-Y.; et al. A Germline Mutation (A339V) in Thyroid Transcription Factor-1 (TITF-1/NKX2.1) in Patients with Multinodular Goiter and Papillary Thyroid Carcinoma. J. Natl. Cancer Inst. 2009, 101, 162–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, J.D.; Thompson, D.B.; Lesueur, F.; Stankov, K.; Pastore, A.; Watfah, C.; Strolz, S.; Riccabona, G.; Moncayo, R.C.; Romeo, G.; et al. Evidence for interaction between the TCO and NMTC1 loci in familial non-medullary thyroid cancer. J. Med. Genet. 2004, 41, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Medapati, M.R.; Dahlmann, M.; Ghavami, S.; Pathak, K.A.; Lucman, L.; Klonisch, T.; Hoang-Vu, C.; Stein, U.; Hombach-Klonisch, S. RAGE Mediates the Pro-Migratory Response of Extracellular S100A4 in Human Thyroid Cancer Cells. Thyroid 2015, 25, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.R.; Duffield, A.; Wilkinson, S.J.; Ware, R.; Greenaway, T.M.; Percival, J.; Hoffman, L. Two Families with an Autosomal Dominant Inheritance Pattern for Papillary Carcinoma of the Thyroid. J. Clin. Endocrinol. Metab. 1997, 82, 345–348. [Google Scholar] [CrossRef]
- Bakhsh, A.; Kirov, G.; Gregory, J.W.; Williams, E.D.; Ludgate, M. A new form of familial multi-nodular goitre with progression to differentiated thyroid cancer. Endocr.-Relat. Cancer 2006, 13, 475–483. [Google Scholar] [CrossRef]
- Franceschi, S.; Preston-Martin, S.; Maso, L.D.; Negri, E.; La Vecchia, C.; Mack, W.J.; McTiernan, A.; Kolonel, L.; Mark, S.D.; Mabuchi, K.; et al. A pooled analysis of case—Control studies of thyroid cancer. IV. Benign thyroid diseases. Cancer Causes Control. 1999, 10, 583–595. [Google Scholar] [CrossRef]
- Gudmundsson, J.; Sulem, P.; Gudbjartsson, D.F.; Jonasson, J.G.; Sigurdsson, A.; Bergthorsson, J.T.; He, H.; Blondal, T.; Geller, F.; Jakobsdottir, M.; et al. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat. Genet. 2009, 41, 460–464. [Google Scholar] [CrossRef]
- Gudmundsson, J.; Sulem, P.; Gudbjartsson, D.F.; Jonasson, J.G.; Masson, G.; He, H.; Jonasdottir, A.; Sigurdsson, A.; Stacey, S.N.; Johannsdottir, H.; et al. Discovery of common variants associated with low TSH levels and thyroid cancer risk. Nat. Genet. 2012, 44, 319–322. [Google Scholar] [CrossRef]
- Takahashi, M.; Saenko, V.A.; Rogounovitch, T.I.; Kawaguchi, T.; Drozd, V.M.; Takigawa-Imamura, H.; Akulevich, N.M.; Ratanajaraya, C.; Mitsutake, N.; Takamura, N.; et al. The FOXE1 locus is a major genetic determinant for radiation-related thyroid carcinoma in Chernobyl. Hum. Mol. Genet. 2010, 19, 2516–2523. [Google Scholar] [CrossRef] [Green Version]
- Köhler, A.; Chen, B.; Gemignani, F.; Elisei, R.; Romei, C.; Figlioli, G.; Cipollini, M.; Cristaudo, A.; Bambi, F.; Hoffmann, P.; et al. Genome-Wide Association Study on Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2013, 98, E1674–E1681. [Google Scholar] [CrossRef] [Green Version]
- Son, H.-Y.; Hwangbo, Y.; Yoo, S.-K.; Im, S.-W.; Yang, S.D.; Kwak, S.-J.; Park, M.S.; Kwak, S.H.; Cho, S.W.; Ryu, J.S.; et al. Genome-wide association and expression quantitative trait loci studies identify multiple susceptibility loci for thyroid cancer. Nat. Commun. 2017, 8, 15966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudmundsson, J.; Thorleifsson, G.; Sigurdsson, J.K.; Stefansdottir, L.; Jonasson, J.G.; Gudjonsson, S.A.; Gudbjartsson, D.F.; Masson, G.; Johannsdottir, H.; Halldorsson, G.H.; et al. A genome-wide association study yields five novel thyroid cancer risk loci. Nat. Commun. 2017, 8, 14517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikitski, A.V.; Rogounovitch, T.I.; Bychkov, A.; Takahashi, M.; Yoshiura, K.-I.; Mitsutake, N.; Kawaguchi, T.; Matsuse, M.; Drozd, V.M.; Demidchik, Y.; et al. Genotype Analyses in the Japanese and Belarusian Populations Reveal Independent Effects of rs965513 and rs1867277 but Do Not Support the Role of FOXE1 Polyalanine Tract Length in Conferring Risk for Papillary Thyroid Carcinoma. Thyroid 2017, 27, 224–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landa, I.; Ruiz-Llorente, S.; Montero-Conde, C.; Inglada-Pérez, L.; Schiavi, F.; Leskelä, S.; Pita, G.; Milne, R.; Maravall, J.; Ramos, I.; et al. The Variant rs1867277 in FOXE1 Gene Confers Thyroid Cancer Susceptibility through the Recruitment of USF1/USF2 Transcription Factors. PLoS Genet. 2009, 5, e1000637. [Google Scholar] [CrossRef] [Green Version]
- Nikitski, A.; Saenko, V.; Shimamura, M.; Nakashima, M.; Matsuse, M.; Suzuki, K.; Rogounovitch, T.I.; Bogdanova, T.; Shibusawa, N.; Yamada, M.; et al. Targeted Foxe1 Overexpression in Mouse Thyroid Causes the Development of Multinodular Goiter But Does Not Promote Carcinogenesis. Endocrinology 2016, 157, 2182–2195. [Google Scholar] [CrossRef]
- Wang, Y.; He, H.; Li, W.; Phay, J.; Shen, R.; Yu, L.; Hancioglu, B.; De La Chapelle, A. MYH9 binds to lncRNA genePTCSC2and regulates FOXE1 in the 9q22 thyroid cancer risk locus. Proc. Natl. Acad. Sci. USA 2017, 114, 474–479. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.M.; Howarth, K.M.; Martin, L.; Gorman, M.; Mihai, R.; Moss, L.; Auton, A.; Lemon, C.; Mehanna, H.; Mohan, H.; et al. Thyroid cancer susceptibility polymorphisms: Confirmation of loci on chromosomes 9q22 and 14q13, validation of a recessive 8q24 locus and failure to replicate a locus on 5q24. J. Med. Genet. 2012, 49, 158–163. [Google Scholar] [CrossRef]
- Jendrzejewski, J.; He, H.; Radomska, H.S.; Li, W.; Tomsic, J.; Liyanarachchi, S.; Davuluri, R.V.; Nagy, R.; De La Chapelle, A. The polymorphism rs944289 predisposes to papillary thyroid carcinoma through a large intergenic noncoding RNA gene of tumor suppressor type. Proc. Natl. Acad. Sci. USA 2012, 109, 8646–8651. [Google Scholar] [CrossRef] [Green Version]
- Goedert, L.; Plaça, J.R.; Fuziwara, C.S.; Machado, M.C.R.; Plaça, D.R.; Almeida, P.P.; Sanches, T.P.; Dos Santos, J.F.; Corveloni, A.C.; Pereira, I.E.G.; et al. Identification of Long Noncoding RNAs Deregulated in Papillary Thyroid Cancer and Correlated with BRAFV600E Mutation by Bioinformatics Integrative Analysis. Sci. Rep. 2017, 7, 1662. [Google Scholar] [CrossRef] [Green Version]
- Jendrzejewski, J.; Thomas, A.; Liyanarachchi, S.; Eiterman, A.; Tomsic, J.; He, H.; Radomska, H.S.; Li, W.; Nagy, R.; Sworczak, K.; et al. PTCSC3 Is Involved in Papillary Thyroid Carcinoma Development by Modulating S100A4 Gene Expression. J. Clin. Endocrinol. Metab. 2015, 100, E1370–E1377. [Google Scholar] [CrossRef] [Green Version]
- Cantara, S.; Capuano, S.; Formichi, C.; Pisu, M.; Capezzone, M.; Pacini, F. Lack of germline A339V mutation in thyroid transcription factor-1 (TITF-1/NKX2.1) gene in familial papillary thyroid cancer. Thyroid Res. 2010, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Li, W.; Liyanarachchi, S.; Wang, Y.; Yu, L.; Genutis, L.K.; Maharry, S.; Phay, J.E.; Shen, R.; Brock, P.; et al. The Role of NRG1 in the Predisposition to Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2018, 103, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Coe, E.A.; Tan, J.Y.; Shapiro, M.; Louphrasitthiphol, P.; Bassett, A.R.; Marques, A.C.; Goding, C.R.; Vance, K.W. The MITF-SOX10 regulated long non-coding RNA DIRC3 is a melanoma tumour suppressor. PLoS Genet. 2019, 15, e1008501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Świerniak, M.; Wójcicka, A.; Czetwertyńska, M.; Długosińska, J.; Stachlewska, E.; Gierlikowski, W.; Kot, A.; Górnicka, B.; Koperski, Ł.; Bogdańska, M.; et al. Association between GWAS-Derived rs966423 Genetic Variant and Overall Mortality in Patients with Differentiated Thyroid Cancer. Clin. Cancer Res. 2016, 22, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Hińcza, K.; Kowalik, A.; Pałyga, I.; Walczyk, A.; Gąsior-Perczak, D.; Mikina, E.; Trybek, T.; Szymonek, M.; Gadawska-Juszczyk, K.; Zajkowska, K.; et al. Does the TT Variant of the rs966423 Polymorphism in DIRC3 Affect the Stage and Clinical Course of Papillary Thyroid Cancer? Cancers 2020, 12, 423. [Google Scholar] [CrossRef] [Green Version]
- Liyanarachchi, S.; Gudmundsson, J.; Ferkingstad, E.; He, H.; Jonasson, J.G.; Tragante, V.; Asselbergs, F.W.; Xu, L.; Kiemeney, L.A.; Netea-Maier, R.T.; et al. Assessing thyroid cancer risk using polygenic risk scores. Proc. Natl. Acad. Sci. USA 2020, 117, 5997–6002. [Google Scholar] [CrossRef]
- Capezzone, M.; Cantara, S.; Marchisotta, S.; Filetti, S.; De Santi, M.M.; Rossi, B.; Ronga, G.; Durante, C.; Pacini, F. Short Telomeres, Telomerase Reverse Transcriptase Gene Amplification, and Increased Telomerase Activity in the Blood of Familial Papillary Thyroid Cancer Patients. J. Clin. Endocrinol. Metab. 2008, 93, 3950–3957. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Bian, B.; Gesuwan, K.; Gulati, N.; Zhang, L.; Nilubol, N.; Kebebew, E. Telomere Length Is Shorter in Affected Members of Families with Familial Nonmedullary Thyroid Cancer. Thyroid 2013, 23, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Cantara, S.; Capuano, S.; Capezzone, M.; Benigni, M.; Pisu, M.; Marchisotta, S.; Pacini, F. Lack of Mutations of the Telomerase RNA Component in Familial Papillary Thyroid Cancer with Short Telomeres. Thyroid 2012, 22, 363–368. [Google Scholar] [CrossRef]
- He, H.; Li, W.; Comiskey, D.F.; Liyanarachchi, S.; Nieminen, T.T.; Wang, Y.; DeLap, K.E.; Brock, P.; De La Chapelle, A. A Truncating Germline Mutation of TINF2 in Individuals with Thyroid Cancer or Melanoma Results in Longer Telomeres. Thyroid 2020, 30, 204–213. [Google Scholar] [CrossRef]
- Srivastava, A.; Miao, B.; Skopelitou, D.; Kumar, V.; Kumar, A.; Paramasivam, N.; Bonora, E.; Hemminki, K.; Foersti, A.; Bandapalli, O.R. A Germline Mutation in the POT1 Gene Is a Candidate for Familial Non-Medullary Thyroid Cancer. Cancers 2020, 12, 1441. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.L.-S.; Hattangady, N.; Lerario, A.M.; Williams, C.; Koeppe, E.; Quinonez, S.; Osborne, J.; Cha, K.B.; Else, T. A new POT1 germline mutation—expanding the spectrum of POT1-associated cancers. Fam. Cancer 2017, 16, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.A.; Lupo, P.J.; Morton, L.M.; Yasui, Y.A.; Sapkota, Y.A.; Arnold, M.A.; Aubert, G.; Neglia, J.P.; Turcotte, L.M.; Leisenring, W.M.; et al. Genetic variation in POT1 and risk of thyroid subsequent malignant neoplasm: A report from the Childhood Cancer Survivor Study. PLoS ONE 2020, 15, e0228887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomsic, J.; Fultz, R.; Liyanarachchi, S.; Genutis, L.K.; Wang, Y.; Li, W.; Volinia, S.; Jazdzewski, K.; He, H.; Wakely, P.E., Jr.; et al. Variants in microRNA genes in familial papillary thyroid carcinoma. Oncotarget 2017, 8, 6475–6482. [Google Scholar] [CrossRef] [Green Version]
- Tomsic, J.; He, H.; Akagi, K.; Liyanarachchi, S.; Pan, Q.; Bertani, B.; Nagy, R.; Symer, D.E.; Blencowe, B.J.; De La Chapelle, A. A germline mutation in SRRM2, a splicing factor gene, is implicated in papillary thyroid carcinoma predisposition. Sci. Rep. 2015, 5, 10566. [Google Scholar] [CrossRef]
- Orois, A.; Gara, S.K.; Mora, M.; Halperin, I.; Martínez, S.; Alfayate, R.; Kebebew, E.; Oriola, J. NOP53 as A Candidate Modifier Locus for Familial Non-Medullary Thyroid Cancer. Genes 2019, 10, 899. [Google Scholar] [CrossRef] [Green Version]
- Gara, S.K.; Jia, L.; Merino, M.J.; Agarwal, S.K.; Zhang, L.; Cam, M.; Patel, D.; Kebebew, E. Germline HABP2 Mutation Causing Familial Nonmedullary Thyroid Cancer. N. Engl. J. Med. 2015, 373, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Tomsic, J.; He, H.; de la Chapelle, A. HABP2 Mutation and Nonmedullary Thyroid Cancer. N. Engl. J. Med. 2015, 373, 2086. [Google Scholar] [CrossRef]
- Tomsic, J.; Fultz, R.; Liyanarachchi, S.; He, H.; Senter, L.; De La Chapelle, A. HABP2 G534E Variant in Papillary Thyroid Carcinoma. PLoS ONE 2016, 11, e0146315. [Google Scholar] [CrossRef] [Green Version]
- Kowalik, A.; Gąsior-Perczak, D.; Gromek, M.; Siołek, M.; Walczyk, A.; Pałyga, I.; Chłopek, M.; Kopczyński, J.; Mężyk, R.; Kowalska, A.; et al. The p.G534E variant of HABP2 is not associated with sporadic papillary thyroid carcinoma in a Polish population. Oncotarget 2017, 8, 58304–58308. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Wu, K.; Lin, Z.; Bai, S.; Wu, J.; Li, P.; Xue, H.; Du, J.; Shen, B.; Wang, H.; et al. Identification of susceptibility gene mutations associated with the pathogenesis of familial nonmedullary thyroid cancer. Mol. Genet. Genom. Med. 2019, 7, e1015. [Google Scholar] [CrossRef] [PubMed]
- Sarquis, M.; Moraes, D.C.; Bastos-Rodrigues, L.; Azevedo, P.G.; Ramos, A.V.; Reis, F.V.; Dande, P.V.; Paim, I.; Friedman, E.; De Marco, L. Germline Mutations in Familial Papillary Thyroid Cancer. Endocr. Pathol. 2020, 31, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Siołek, M.; Cybulski, C.; Gąsior-Perczak, D.; Kowalik, A.; Kozak-Klonowska, B.; Kowalska, A.; Chłopek, M.; Kluźniak, W.; Wokołorczyk, D.; Pałyga, I.; et al. CHEK2mutations and the risk of papillary thyroid cancer. Int. J. Cancer 2015, 137, 548–552. [Google Scholar] [CrossRef] [PubMed]
| Syndrome | Gene | Inheritance Pattern | Other Malignant Tumors | Prevalent Types of Thyroid Tumors | Benign Manifestations | Reference |
|---|---|---|---|---|---|---|
| Ataxia-telangiectasia syndrome * | ATM | AR * | Lymphocytic leukemia, lymphoma, stomach adenocarcinoma, medulloblastoma, glioma | FTC, PTC • | degenerative cerebellar atrophy, telangiectasias, immune defects | [15] |
| ATM | AD | breast cancer, digestive tract cancer, lymphoma, leukemia | [15,16] | |||
| Carney complex | PRKAR1A | AD | - | Follicular hyperplasia, nodular hyperplasia, FA, cystic changes, PTC, FTC | Spotty skin pigmentation (lips, conjunctiva, vaginal, and penile mucosa), cutaneous and mucosal myxoma, cardiac myxoma, breast myxomatosis, primary pigmented nodular adrenocortical disease, GH-producing adenoma, large cell calcifying Sertoli cell tumors, psammomatous melanotic schwannomas | [17] |
| Cowden syndrome | PTEN, SDHB-D, SEC23B, KLLN, PARP4, AKT1, PIK3CA, USF3, TTN, RASAL1 | AD | FTC, breast cancer, epithelial endometrial cancer, colon cancer, renal cell carcinoma melanoma | MNG, Hashimoto thyroiditis, FA, FTC, cPTC, FVPTC, C-cell hyperplasia | Macrocephaly | [18,19] |
| DICER1 syndrome | DICER1 | AD | Pleuropulmonary blastoma, ovarian Sertoli-Leydig cell tumor, genitourinary and cerebral sarcomas | MNG, PTC, FA | MNG, cystic nephroma | [20,21] |
| Familial adenomatous polyposis | APC | AD | Digestive tract cancers, fibrosarcomas | CMVPTC, PTC | Intestinal polyps, osteomas, fibromas, desmoid tumors, dental abnormalities, leiomyomas, congenital hypertrophy of the retinal pigment epithelium | [22,23] |
| Li-Fraumeni syndrome | TP53 | AD | Breast, brain, and adreno cortical cancers and sarcomas | cPTC, FVPTC | [13,24] | |
| Werner syndrome | WRN | AR | Atypical melanoma, bone, or soft tissue sarcomas | FTC, PTC, ATC | Aging, bilateral cataract, type 2 diabetes mellitus, hypogonadism, meningioma | [2,25] |
| Loci/Gene | Localization | Characteristics | Reference |
|---|---|---|---|
| Linkage analysis | |||
| TCO | 19p13.2 | Oxyphilic PTC | [6] |
| NMTC1 | 2q21 | [9] | |
| PRN1 | 1q21 | Papillary renal cancer | [8] |
| MNG1/DICER1 | 14q32 | MNG | [7] |
| Linkage analysis and NGS | |||
| SRGAP1 | 12q14 | [88] | |
| 8p23.1–p22 | [10] | ||
| 6q22 | [89] | ||
| lncRNA inside TG | 8q24 | Melanoma in 1 family | [90] |
| Enhancer associated with POU2F1 and YY1 | 4q32 | [91] | |
| Other methodology | |||
| NKX2-1 | 14q13.3 | [92] | |
| Locus | Nearest Gene | Population | Reference |
|---|---|---|---|
| 9q22.33 | FOXE1, PTCSC2 | Belarus, Iceland, Italy, Korea, Netherlands Poland, Spain, USA | [98,99,100] |
| 14q13.3 | PTCSC3, NKX2-1, MBIP1 | Iceland, Italy, Korea, Netherlands, Poland, Spain, USA | [98,99] |
| 2q35 | DIRC3 | Iceland, Italy, Korea, Netherlands, Poland, Spain, UK, USA | [99,101] |
| 8p12 | NRG1 | Iceland, Korea, Netherlands, Spain, USA | [99,102] |
| 1q42.2 | PCNXL2 | Iceland, Korea, Netherlands, Spain, USA | [102,103] |
| European Only | |||
| 3q26.2 | LRRC34 | Iceland, Netherlands, Spain, USA | [103] |
| 5p15.33 | TERT | Iceland, Netherlands, Spain, USA | [103] |
| 5q22.1 | EPB41L4A | Iceland, Netherlands, Spain, USA | [103] |
| 10q24.33 | OBFC1 | Iceland, Netherlands, Spain, USA | [103] |
| 15q22.33 | SMAD3 | Iceland, Netherlands, Spain, USA | [103] |
| Korean Only | |||
| 12q14.3 | MSRB3 | Korea | [102] |
| 1p13.3 | VAV3 | Korea | [102] |
| 4q21.1 | SEPT11 | Korea | [102] |
| 3p14.2 | FHIT | Korea | [102] |
| 19p13.2 | INSR | Korea | [102] |
| 12q24.13 | SLC8B1 | Korea | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miasaki, F.Y.; Fuziwara, C.S.; Carvalho, G.A.d.; Kimura, E.T. Genetic Mutations and Variants in the Susceptibility of Familial Non-Medullary Thyroid Cancer. Genes 2020, 11, 1364. https://doi.org/10.3390/genes11111364
Miasaki FY, Fuziwara CS, Carvalho GAd, Kimura ET. Genetic Mutations and Variants in the Susceptibility of Familial Non-Medullary Thyroid Cancer. Genes. 2020; 11(11):1364. https://doi.org/10.3390/genes11111364
Chicago/Turabian StyleMiasaki, Fabíola Yukiko, Cesar Seigi Fuziwara, Gisah Amaral de Carvalho, and Edna Teruko Kimura. 2020. "Genetic Mutations and Variants in the Susceptibility of Familial Non-Medullary Thyroid Cancer" Genes 11, no. 11: 1364. https://doi.org/10.3390/genes11111364
APA StyleMiasaki, F. Y., Fuziwara, C. S., Carvalho, G. A. d., & Kimura, E. T. (2020). Genetic Mutations and Variants in the Susceptibility of Familial Non-Medullary Thyroid Cancer. Genes, 11(11), 1364. https://doi.org/10.3390/genes11111364






