Atherosclerosis in Different Vascular Locations Unbiasedly Approached with Mouse Genetics
Abstract
:1. Introduction
2. Susceptibility to Atherosclerosis Varies at Different Aortic Locations
2.1. Location-Specific Atherosclerosis in Humans
2.2. Mouse Models of Atherosclerosis
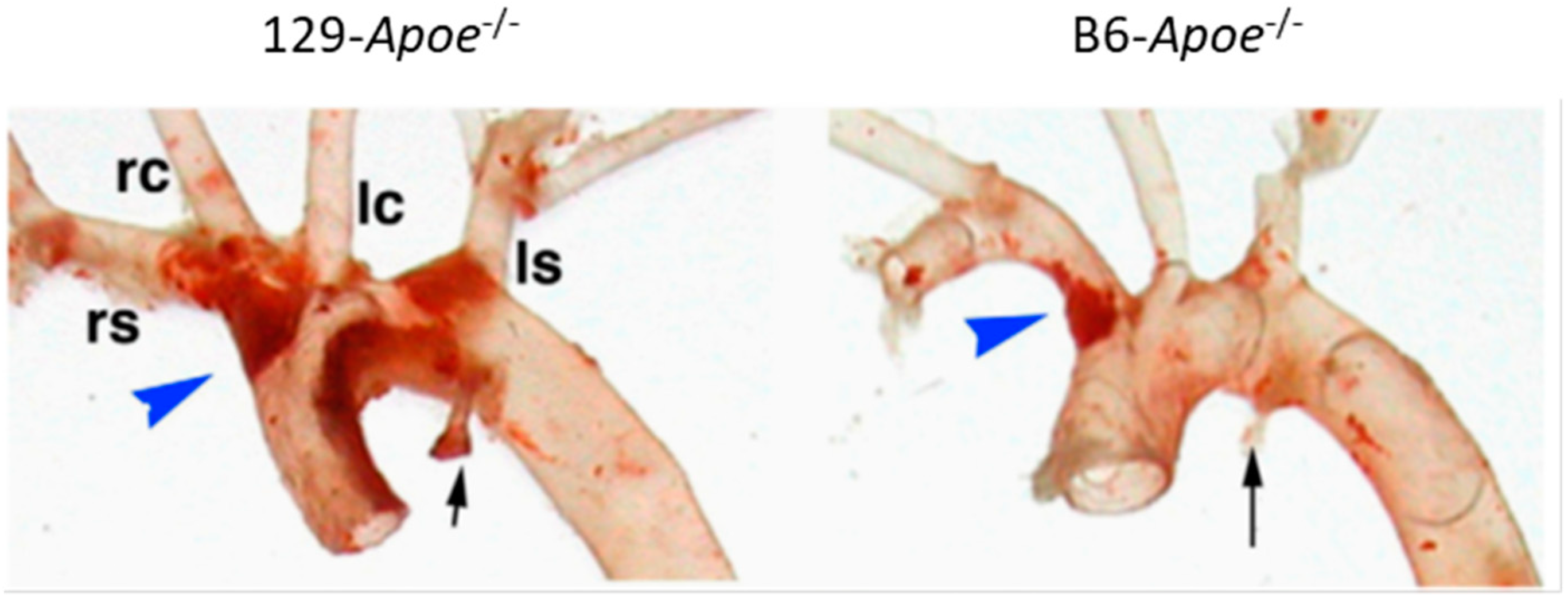
2.3. Strain- and Location-Specific Susceptibility to Atherosclerosis in Mice
3. Genetic Studies Support the Presence of Location-Specific Loci for Atherosclerosis
3.1. Genome-Wide Association Studies (GWAS) in Humans
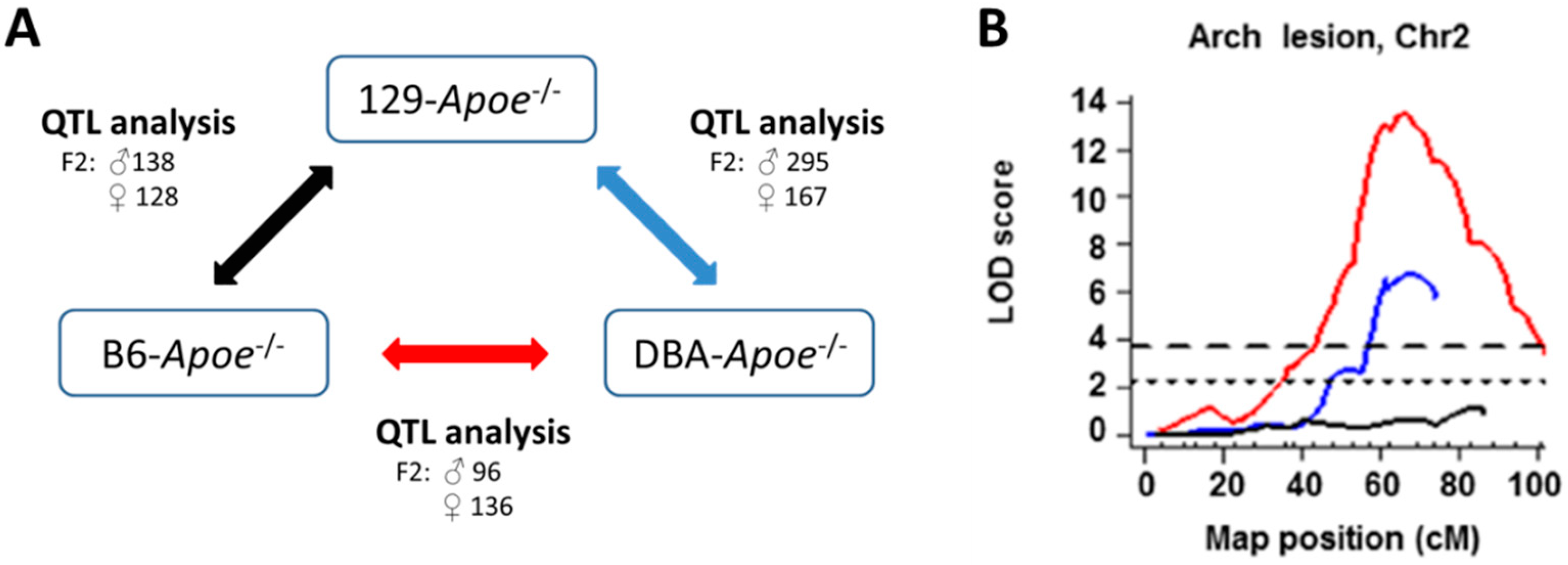
3.2. Quantitative Trait Loci (QTL) Analysis in Mice
3.3. QTLs for the Location-Specific Atherosclerosis
3.4. From QTL to the Gene
3.5. Genetic Analyses of Other Mouse Populations
4. What Causes the Location-Specific Susceptibility to Atherosclerosis?
4.1. Vascular Geometry, Hemodynamics, and Atherosclerosis
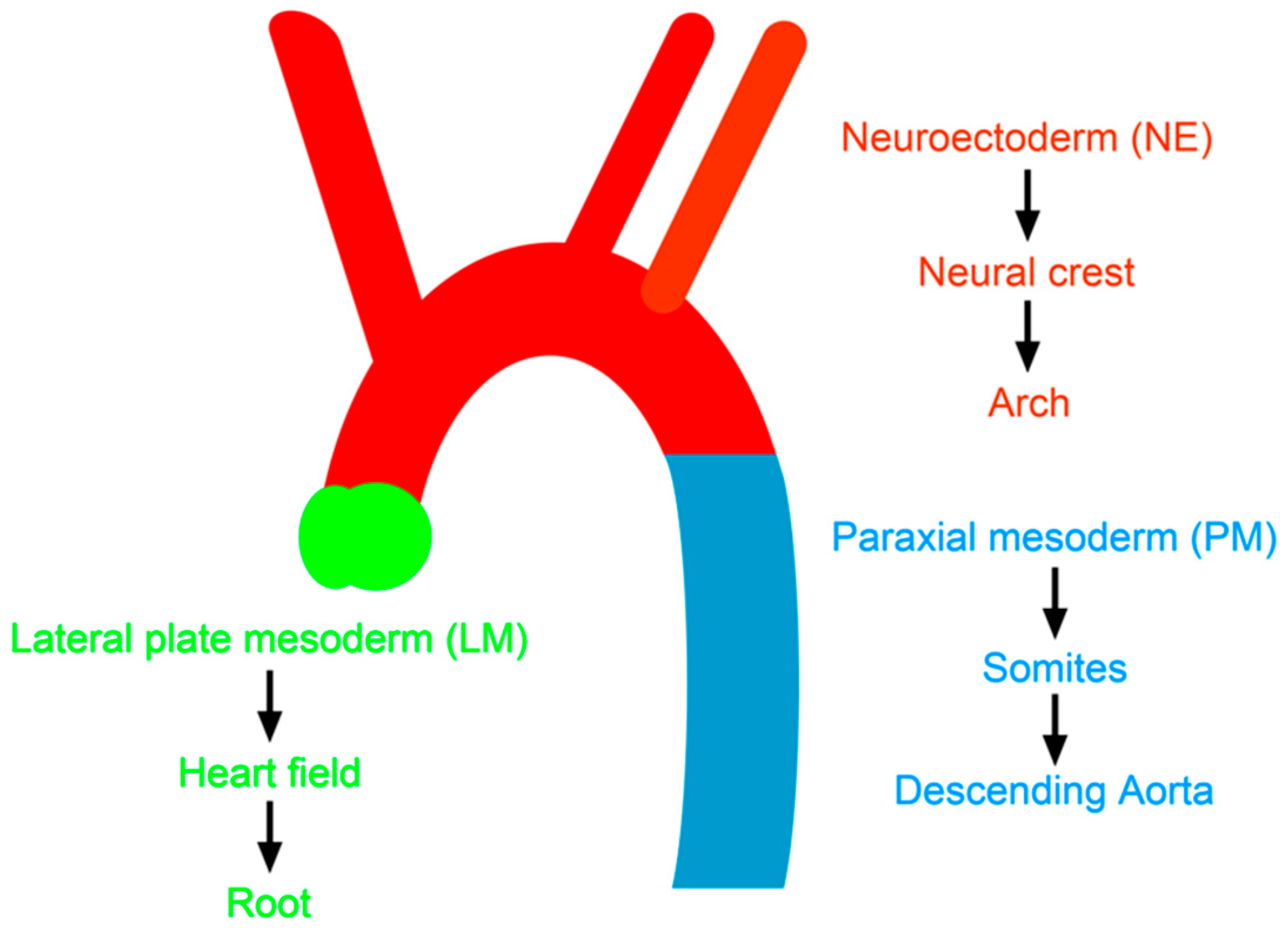
4.2. Developmental Origin of Vascular SMCs and Atherosclerosis
4.3. Intrinsic Heterogeneities of Vascular Cells Revealed by Whole Genome Transcriptome Analyses
4.4. SMC Plasticity and Residential Vascular Progenitor Cells
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McGill, H.C., Jr.; McMahan, C.A.; Herderick, E.E.; Tracy, R.E.; Malcom, G.T.; Zieske, A.W.; Strong, J.P. Effects of coronary heart disease risk factors on atherosclerosis of selected regions of the aorta and right coronary artery. PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 836–845. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari, S.; Jang, E.; Nabi, F.N.; Sanwal, R.; Khosraviani, N.; Wang, C.; Steinberg, B.E.; Goldenberg, N.M.; Ikeda, J.; Lee, W.L. Endothelial HMGB1 Is a Critical Regulator of LDL Transcytosis via an SREBP2-SR-BI Axis. Arterioscler. Thromb. Vasc. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Smithies, O. Many little things: One geneticist’s view of complex diseases. Nat. Rev. Genet. 2005, 6, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999, 282, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.S.; Gotlieb, A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Investig. 2005, 85, 9–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.C.; Moses, C.; Wilkins, R.H. Autopsy Studies in Atherosclerosis.1. Distribution and Severity of Atherosclerosis in Patients Dying without Morphologic Evidence of Atherosclerotic Catastrophe. Circulation 1959, 20, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.C.; Wilkins, R.H.; Moses, C. Autopsy Studies in Atherosclerosis.2. Distribution and Severity of Atherosclerosis in Patients Dying with Morphologic Evidence of Atherosclerotic Catastrophe. Circulation 1959, 20, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Haimovici, H.; Maier, N.; Strauss, L. Fate of aortic homografts in experimental canine atherosclerosis; study of fresh thoracic implants into abdominal aorta. AMA Arch. Surg. 1958, 76, 282–288. [Google Scholar] [CrossRef]
- Haimovici, H.; Maier, N.; Strauss, L. Fate of aortic homografts in experimental canine atherosclerosis. II. Study of fresh abdominal aortic implants into abdominal aorta. AMA Arch. Surg. 1959, 78, 239–245. [Google Scholar] [CrossRef]
- Haimovici, H.; Maier, N. Fate of Aortic Homografts in Canine Atherosclerosis. 3. Study of Fresh Abdominal and Thoracic Aortic Implants into Thoracic Aorta: Role of Tissue Susceptibility in Atherogenesis. Arch. Surg. 1964, 89, 961–969. [Google Scholar] [CrossRef] [PubMed]
- McGill, H.C.J.; McMahan, C.A.; Zieske, A.W.; Sloop, G.D.; Walcott, J.V.; Troxclair, D.A.; Malcom, G.T.; Tracy, R.E.; Oalmann, M.C.; Strong, J.P. Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth. The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1998–2004. [Google Scholar] [CrossRef] [Green Version]
- Danese, C.; Vestri, A.R.; D’Alfonso, V.; Deriu, G.; Dispensa, S.; Baldini, R.; Ambrosino, M.; Colotto, M. Do hypertension and diabetes mellitus influence the site of atherosclerotic plaques? Clin. Ter. 2006, 157, 9–13. [Google Scholar] [PubMed]
- Suzuki, T.; Nozawa, T.; Fujii, N.; Sobajima, M.; Ohori, T.; Shida, T.; Matsuki, A.; Kameyama, T.; Inoue, H. Plaque regression in one artery is not necessarily associated with parallel changes in other vascular beds. Heart Vessels 2011, 26, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.W.; Reddick, R.L.; Jennette, J.C.; Shesely, E.G.; Smithies, O.; Maeda, N. Enhanced atherosclerosis and kidney dysfunction in eNOS(-/-)Apoe(-/-) mice are ameliorated by enalapril treatment. J. Clin. Investig. 2000, 105, 451–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.H.; Hagaman, J.; Kim, S.; Reddick, R.L.; Maeda, N. Aortic constriction exacerbates atherosclerosis and induces cardiac dysfunction in mice lacking apolipoprotein E. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Rattazzi, M.; Bennett, B.J.; Bea, F.; Kirk, E.A.; Ricks, J.L.; Speer, M.; Schwartz, S.M.; Giachelli, C.M.; Rosenfeld, M.E. Calcification of advanced atherosclerotic lesions in the innominate arteries of ApoE-deficient mice: Potential role of chondrocyte-like cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1420–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awan, Z.; Denis, M.; Bailey, D.; Giaid, A.; Prat, A.; Goltzman, D.; Seidah, N.G.; Genest, J. The LDLR deficient mouse as a model for aortic calcification and quantification by micro-computed tomography. Atherosclerosis 2011, 219, 455–462. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, I.K.; Jeon, J.H. Vascular Calcification-New Insights Into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M.R.; Owens, G.K. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu. Rev. Physiol. 2012, 74, 13–40. [Google Scholar] [CrossRef]
- Maeda, N.; Givens, R.C.; Reddick, R.L. Cardiovascular Disease: Mouse Models of Atherosclerosis. In The Mouse in Biomedical Research, 2nd ed.; Academic Press: Cambridge, MA, USA, 2007; Volume III, pp. 535–563. [Google Scholar]
- Daeichin, V.; Sluimer, J.C.; van der Heiden, K.; Skachkov, I.; Kooiman, K.; Janssen, A.; Janssen, B.; Bosch, J.G.; de Jong, N.; Daemen, M.J.; et al. Live Observation of Atherosclerotic Plaque Disruption in Apolipoprotein E-Deficient Mouse. Ultrasound Int. Open 2015, 1, E67–E71. [Google Scholar] [CrossRef] [Green Version]
- van der Heiden, K.; Hoogendoorn, A.; Daemen, M.J.; Gijsen, F.J. Animal models for plaque rupture: A biomechanical assessment. Thromb Haemost 2016, 115, 501–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.D.; James, D.; Dansky, H.M.; Wittkowski, K.M.; Moore, K.J.; Breslow, J.L. In silico quantitative trait locus map for atherosclerosis susceptibility in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Paigen, B.; Morrow, A.; Brandon, C.; Mitchell, D.; Holmes, P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis 1985, 57, 65–73. [Google Scholar] [CrossRef]
- Maeda, N.; Johnson, L.; Kim, S.; Hagaman, J.; Friedman, M.; Reddick, R. Anatomical differences and atherosclerosis in apolipoprotein E-deficient mice with 129/SvEv and C57BL/6 genetic backgrounds. Atherosclerosis 2007, 195, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Tomita, H.; Zhilicheva, S.; Kim, S.; Maeda, N. Aortic arch curvature and atherosclerosis have overlapping quantitative trait loci in a cross between 129S6/SvEvTac and C57BL/6J apolipoprotein E-null mice. Circ. Res. 2010, 106, 1052–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayashima, Y.; Makhanova, N.A.; Matsuki, K.; Tomita, H.; Bennett, B.J.; Maeda, N. Identification of aortic arch-specific quantitative trait loci for atherosclerosis by an intercross of DBA/2J and 129S6 apolipoprotein E-deficient mice. PLoS ONE 2015, 10, e0117478. [Google Scholar] [CrossRef] [Green Version]
- Makhanova, N.; Morgan, A.P.; Kayashima, Y.; Makhanov, A.; Hiller, S.; Zhilicheva, S.; Xu, L.; Pardo-Manuel de Villena, F.; Maeda, N. Genetic architecture of atherosclerosis dissected by QTL analyses in three F2 intercrosses of apolipoprotein E-null mice on C57BL6/J, DBA/2J and 129S6/SvEvTac backgrounds. PLoS ONE 2017, 12, e0182882. [Google Scholar] [CrossRef] [Green Version]
- Teslovich, T.M.; Musunuru, K.; Smith, A.V.; Edmondson, A.C.; Stylianou, I.M.; Koseki, M.; Pirruccello, J.P.; Ripatti, S.; Chasman, D.I.; Willer, C.J.; et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010, 466, 707–713. [Google Scholar] [CrossRef]
- Erdmann, J.; Kessler, T.; Munoz Venegas, L.; Schunkert, H. A decade of genome-wide association studies for coronary artery disease: The challenges ahead. Cardiovasc. Res. 2018, 114, 1241–1257. [Google Scholar] [CrossRef]
- Jarinova, O.; Stewart, A.F.; Roberts, R.; Wells, G.; Lau, P.; Naing, T.; Buerki, C.; McLean, B.W.; Cook, R.C.; Parker, J.S.; et al. Functional analysis of the chromosome 9p21.3 coronary artery disease risk locus. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1671–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bis, J.C.; Kavousi, M.; Franceschini, N.; Isaacs, A.; Abecasis, G.R.; Schminke, U.; Post, W.S.; Smith, A.V.; Cupples, L.A.; Markus, H.S.; et al. Meta-analysis of genome-wide association studies from the CHARGE consortium identifies common variants associated with carotid intima media thickness and plaque. Nat. Genet. 2011, 43, 940–947. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ria, M.; Kelmenson, P.M.; Eriksson, P.; Higgins, D.C.; Samnegard, A.; Petros, C.; Rollins, J.; Bennet, A.M.; Wiman, B.; et al. Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nat. Genet. 2005, 37, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Manichaikul, A.; Wang, Q.; Shi, Y.L.; Zhang, Z.; Leitinger, N.; Shi, W. Characterization of Ath29, a major mouse atherosclerosis susceptibility locus, and identification of Rcn2 as a novel regulator of cytokine expression. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1056–H1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idel, S.; Dansky, H.M.; Breslow, J.L. A20, a regulator of NFkappaB, maps to an atherosclerosis locus and differs between parental sensitive C57BL/6J and resistant FVB/N strains. Proc. Natl. Acad. Sci. USA 2003, 100, 14235–14240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holdt, L.M.; Thiery, J.; Breslow, J.L.; Teupser, D. Increased ADAM17 mRNA expression and activity is associated with atherosclerosis resistance in LDL-receptor deficient mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1097–1103. [Google Scholar] [CrossRef] [Green Version]
- Stylianou, I.M.; Bauer, R.C.; Reilly, M.P.; Rader, D.J. Genetic basis of atherosclerosis: Insights from mice and humans. Circ. Res. 2012, 110, 337–355. [Google Scholar] [CrossRef] [Green Version]
- Rapp, J.P.; Joe, B. Dissecting Epistatic QTL for Blood Pressure in Rats: Congenic Strains versus Heterogeneous Stocks, a Reality Check. Compr. Physiol. 2019, 9, 1305–1337. [Google Scholar] [CrossRef]
- Kayashima, Y.; Tomita, H.; Zhilicheva, S.; Kim, S.; Kim, H.S.; Bennett, B.J.; Maeda, N. Quantitative trait loci affecting atherosclerosis at the aortic root identified in an intercross between DBA2J and 129S6 apolipoprotein E-null mice. PLoS ONE 2014, 9, e88274. [Google Scholar] [CrossRef] [Green Version]
- Seidelmann, S.B.; De Luca, C.; Leibel, R.L.; Breslow, J.L.; Tall, A.R.; Welch, C.L. Quantitative trait locus mapping of genetic modifiers of metabolic syndrome and atherosclerosis in low-density lipoprotein receptor-deficient mice: Identification of a locus for metabolic syndrome and increased atherosclerosis on chromosome 4. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 204–210. [Google Scholar] [CrossRef]
- Thorp, E.; Cui, D.; Schrijvers, D.M.; Kuriakose, G.; Tabas, I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe-/-mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1421–1428. [Google Scholar] [CrossRef] [Green Version]
- Ait-Oufella, H.; Pouresmail, V.; Simon, T.; Blanc-Brude, O.; Kinugawa, K.; Merval, R.; Offenstadt, G.; Leseche, G.; Cohen, P.L.; Tedgui, A.; et al. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1429–1431. [Google Scholar] [CrossRef] [Green Version]
- Kayashima, Y.; Makhanova, N.; Maeda, N. DBA/2J Haplotype on Distal Chromosome 2 Reduces Mertk Expression, Restricts Efferocytosis, and Increases Susceptibility to Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e82–e91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda-Smithies, N.; Hiller, S.; Dong, S.; Kim, H.S.; Bennett, B.J.; Kayashima, Y. Ectopic expression of the Stabilin2 gene triggered by an intracisternal A particle (IAP) element in DBA/2J strain of mice. Mamm. Genome 2020, 31, 2–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferns, G.A.; Konneh, M.; Rutherford, C.; Woolaghan, E.; Anggard, E.E. Hyaluronan (HYAL-BV 5200) inhibits neo-intimal macrophage influx after balloon-catheter induced injury in the cholesterol-fed rabbit. Atherosclerosis 1995, 114, 157–164. [Google Scholar] [CrossRef]
- Chajara, A.; Raoudi, M.; Delpech, B.; Levesque, H. Inhibition of arterial cells proliferation in vivo in injured arteries by hyaluronan fragments. Atherosclerosis 2003, 171, 15–19. [Google Scholar] [CrossRef]
- Fischer, J.W. Role of hyaluronan in atherosclerosis: Current knowledge and open questions. Matrix Biol. 2019, 78–79, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Grainger, A.T.; Jones, M.B.; Chen, M.H.; Shi, W. Polygenic Control of Carotid Atherosclerosis in a BALB/cJ × SM/J Intercross and a Combined Cross Involving Multiple Mouse Strains. G3 (Bethesda) 2017, 7, 731–739. [Google Scholar] [CrossRef] [Green Version]
- Aylor, D.L.; Valdar, W.; Foulds-Mathes, W.; Buus, R.J.; Verdugo, R.A.; Baric, R.S.; Ferris, M.T.; Frelinger, J.A.; Heise, M.; Frieman, M.B.; et al. Genetic analysis of complex traits in the emerging Collaborative Cross. Genome Res. 2011, 21, 1213–1222. [Google Scholar] [CrossRef] [Green Version]
- Churchill, G.A.; Gatti, D.M.; Munger, S.C.; Svenson, K.L. The Diversity Outbred mouse population. Mamm. Genome 2012, 23, 713–718. [Google Scholar] [CrossRef] [Green Version]
- Saul, M.C.; Philip, V.M.; Reinholdt, L.G.; Center for Systems Neurogenetics of, A.; Chesler, E.J. High-Diversity Mouse Populations for Complex Traits. Trends Genet. 2019, 35, 501–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, B.J.; Farber, C.R.; Orozco, L.; Kang, H.M.; Ghazalpour, A.; Siemers, N.; Neubauer, M.; Neuhaus, I.; Yordanova, R.; Guan, B.; et al. A high-resolution association mapping panel for the dissection of complex traits in mice. Genome Res. 2010, 20, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lusis, A.J.; Seldin, M.M.; Allayee, H.; Bennett, B.J.; Civelek, M.; Davis, R.C.; Eskin, E.; Farber, C.R.; Hui, S.; Mehrabian, M.; et al. The Hybrid Mouse Diversity Panel: A resource for systems genetics analyses of metabolic and cardiovascular traits. J. Lipid Res. 2016, 57, 925–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, B.J.; Davis, R.C.; Civelek, M.; Orozco, L.; Wu, J.; Qi, H.; Pan, C.; Packard, R.R.; Eskin, E.; Yan, M.; et al. Genetic Architecture of Atherosclerosis in Mice: A Systems Genetics Analysis of Common Inbred Strains. PLoS Genet. 2015, 11, e1005711. [Google Scholar] [CrossRef] [Green Version]
- Smallwood, T.L.; Gatti, D.M.; Quizon, P.; Weinstock, G.M.; Jung, K.C.; Zhao, L.; Hua, K.; Pomp, D.; Bennett, B.J. High-resolution genetic mapping in the diversity outbred mouse population identifies Apobec1 as a candidate gene for atherosclerosis. G3 (Bethesda) 2014, 4, 2353–2363. [Google Scholar] [CrossRef] [Green Version]
- Friedman, M.H.; Deters, O.J.; Mark, F.F.; Bargeron, C.B.; Hutchins, G.M. Arterial geometry affects hemodynamics. A potential risk factor for athersoclerosis. Atherosclerosis 1983, 46, 225–231. [Google Scholar] [CrossRef]
- Black, M.M.; Hose, D.R.; Lawford, P.V. The origin and significance of secondary flows in the aortic arch. J. Med. Eng. Technol. 1995, 19, 192–197. [Google Scholar] [CrossRef]
- Tse, K.M.; Chang, R.; Lee, H.P.; Lim, S.P.; Venkatesh, S.K.; Ho, P. A computational fluid dynamics study on geometrical influence of the aorta on haemodynamics. Eur. J. Cardiothorac. Surg. 2013, 43, 829–838. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, J.; Shih, J.; Lopez-Bertoni, F.; Hagaman, J.R.; Maeda, N.; Friedman, M.H. Differences in aortic arch geometry, hemodynamics, and plaque patterns between C57BL/6 and 129/SvEv mice. J. Biomech. Eng. 2009, 131, 121005. [Google Scholar] [CrossRef]
- Tomita, H.; Hagaman, J.; Friedman, M.H.; Maeda, N. Relationship between hemodynamics and atherosclerosis in aortic arches of apolipoprotein E-null mice on 129S6/SvEvTac and C57BL/6J genetic backgrounds. Atherosclerosis 2012, 220, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Dinsmore, C.; Reiter, J.F. Endothelial primary cilia inhibit atherosclerosis. EMBO Rep. 2016, 17, 156–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, C.J.; Chang, C.H.; Ibrahim, R.B.; Lin, I.H.; Wang, C.H.; Wang, W.J.; Tsai, J.W. Gli2 modulates cell cycle re-entry through autophagy-mediated regulation of the length of primary cilia. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passman, J.N.; Dong, X.R.; Wu, S.P.; Maguire, C.T.; Hogan, K.A.; Bautch, V.L.; Majesky, M.W. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc. Natl. Acad. Sci. USA 2008, 105, 9349–9354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aravani, D.; Morris, G.E.; Jones, P.D.; Tattersall, H.K.; Karamanavi, E.; Kaiser, M.A.; Kostogrys, R.B.; Ghaderi Najafabadi, M.; Andrews, S.L.; Nath, M.; et al. HHIPL1, a Gene at the 14q32 Coronary Artery Disease Locus, Positively Regulates Hedgehog Signaling and Promotes Atherosclerosis. Circulation 2019, 140, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, C.M.; Weissberg, P.L. Smooth muscle cell heterogeneity: Patterns of gene expression in vascular smooth muscle cells in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 333–338. [Google Scholar] [CrossRef] [Green Version]
- VanderLaan, P.A.; Reardon, C.A.; Getz, G.S. Site specificity of atherosclerosis: Site-selective responses to atherosclerotic modulators. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Majesky, M.W. Developmental basis of vascular smooth muscle diversity. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1248–1258. [Google Scholar] [CrossRef] [Green Version]
- Topouzis, S.; Majesky, M.W. Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-beta. Dev. Biol. 1996, 178, 430–445. [Google Scholar] [CrossRef]
- Sinha, S.; Santoro, M.M. New models to study vascular mural cell embryonic origin: Implications in vascular diseases. Cardiovasc Res. 2018, 114, 481–491. [Google Scholar] [CrossRef]
- Cheung, C.; Bernardo, A.S.; Trotter, M.W.; Pedersen, R.A.; Sinha, S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat. Biotechnol. 2012, 30, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.K.; Xu, T.; Assoian, R.K.; Rader, D.J. Mining the Stiffness-Sensitive Transcriptome in Human Vascular Smooth Muscle Cells Identifies Long Noncoding RNA Stiffness Regulators. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Trigueros-Motos, L.; Gonzalez-Granado, J.M.; Cheung, C.; Fernandez, P.; Sanchez-Cabo, F.; Dopazo, A.; Sinha, S.; Andres, V. Embryological-origin-dependent differences in homeobox expression in adult aorta: Role in regional phenotypic variability and regulation of NF-kappaB activity. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1248–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, H.; Carvalho, J.; Looso, M.; Singh, P.; Chennupati, R.; Preussner, J.; Gunther, S.; Albarran-Juarez, J.; Tischner, D.; Classen, S.; et al. Single-cell profiling reveals heterogeneity and functional patterning of GPCR expression in the vascular system. Nat. Commun. 2017, 8, 15700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobnikar, L.; Taylor, A.L.; Chappell, J.; Oldach, P.; Harman, J.L.; Oerton, E.; Dzierzak, E.; Bennett, M.R.; Spivakov, M.; Jorgensen, H.F. Disease-relevant transcriptional signatures identified in individual smooth muscle cells from healthy mouse vessels. Nat. Commun. 2018, 9, 4567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Zhang, Z.; Torsney, E.; Afzal, A.R.; Davison, F.; Metzler, B.; Xu, Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J. Clin. Investig. 2004, 113, 1258–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sainz, J.; Al Haj Zen, A.; Caligiuri, G.; Demerens, C.; Urbain, D.; Lemitre, M.; Lafont, A. Isolation of “side population” progenitor cells from healthy arteries of adult mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zengin, E.; Chalajour, F.; Gehling, U.M.; Ito, W.D.; Treede, H.; Lauke, H.; Weil, J.; Reichenspurner, H.; Kilic, N.; Ergun, S. Vascular wall resident progenitor cells: A source for postnatal vasculogenesis. Development 2006, 133, 1543–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majesky, M.W.; Horita, H.; Ostriker, A.; Lu, S.; Regan, J.N.; Bagchi, A.; Dong, X.R.; Poczobutt, J.; Nemenoff, R.A.; Weiser-Evans, M.C. Differentiated Smooth Muscle Cells Generate a Subpopulation of Resident Vascular Progenitor Cells in the Adventitia Regulated by Klf4. Circ. Res. 2017, 120, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Chappell, J.; Harman, J.L.; Narasimhan, V.M.; Yu, H.; Foote, K.; Simons, B.D.; Bennett, M.R.; Jorgensen, H.F. Extensive Proliferation of a Subset of Differentiated, yet Plastic, Medial Vascular Smooth Muscle Cells Contributes to Neointimal Formation in Mouse Injury and Atherosclerosis Models. Circ. Res. 2016, 119, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, K.; Lund, M.B.; Shim, J.; Gunnersen, S.; Fuchtbauer, E.M.; Kjolby, M.; Carramolino, L.; Bentzon, J.F. Diverse cellular architecture of atherosclerotic plaque derives from clonal expansion of a few medial SMCs. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
| Phenotype | QTL | Chr | Peak Mb (CI) | B6 × 129 | DBA × 129 | B6 × DBA | Consistency |
|---|---|---|---|---|---|---|---|
| Arch lesion size | Aath1 | 1 | 109 (85–135) | 129 > B6 | no | ||
| Arch lesion size | Aath2 | 1 | 163 (151–173) | 129 > B6 | no | ||
| Arch lesion size | Aath4 | 2 | 136 (124–147) | DBA > 129 | DBA > B6 | 129 = B6 ≠ DBA | |
| Arch lesion size | Aath5 | 10 | 60 (38–100) | 129 > DBA | B6 > DBA | 129 = B6 ≠ DBA | |
| Arch lesion size | Aath3 | 15 | 91.8 (80–102) | 129 > B6 | no | ||
| Root lesion size | Ath44 | 1 | 158 (153–168) | DBA > 129 | no | ||
| Root lesion size | Ath45 | 2 | 162 (154–165) | B6 > 129 | DBA > 129 | DBA > B6 | multiple |
| Root lesion size | Ath29 | 9 | 61 (47–71) | B6 > 129 | no | ||
| Root lesion size | Ath31 | 7 | 78 (55–84) | 129 > DBA | B6 > DBA | 129 = B6 ≠ DBA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayashima, Y.; Maeda-Smithies, N. Atherosclerosis in Different Vascular Locations Unbiasedly Approached with Mouse Genetics. Genes 2020, 11, 1427. https://doi.org/10.3390/genes11121427
Kayashima Y, Maeda-Smithies N. Atherosclerosis in Different Vascular Locations Unbiasedly Approached with Mouse Genetics. Genes. 2020; 11(12):1427. https://doi.org/10.3390/genes11121427
Chicago/Turabian StyleKayashima, Yukako, and Nobuyo Maeda-Smithies. 2020. "Atherosclerosis in Different Vascular Locations Unbiasedly Approached with Mouse Genetics" Genes 11, no. 12: 1427. https://doi.org/10.3390/genes11121427




