The Impact of African Ancestry on Prostate Cancer Disparities in the Era of Precision Medicine
Abstract
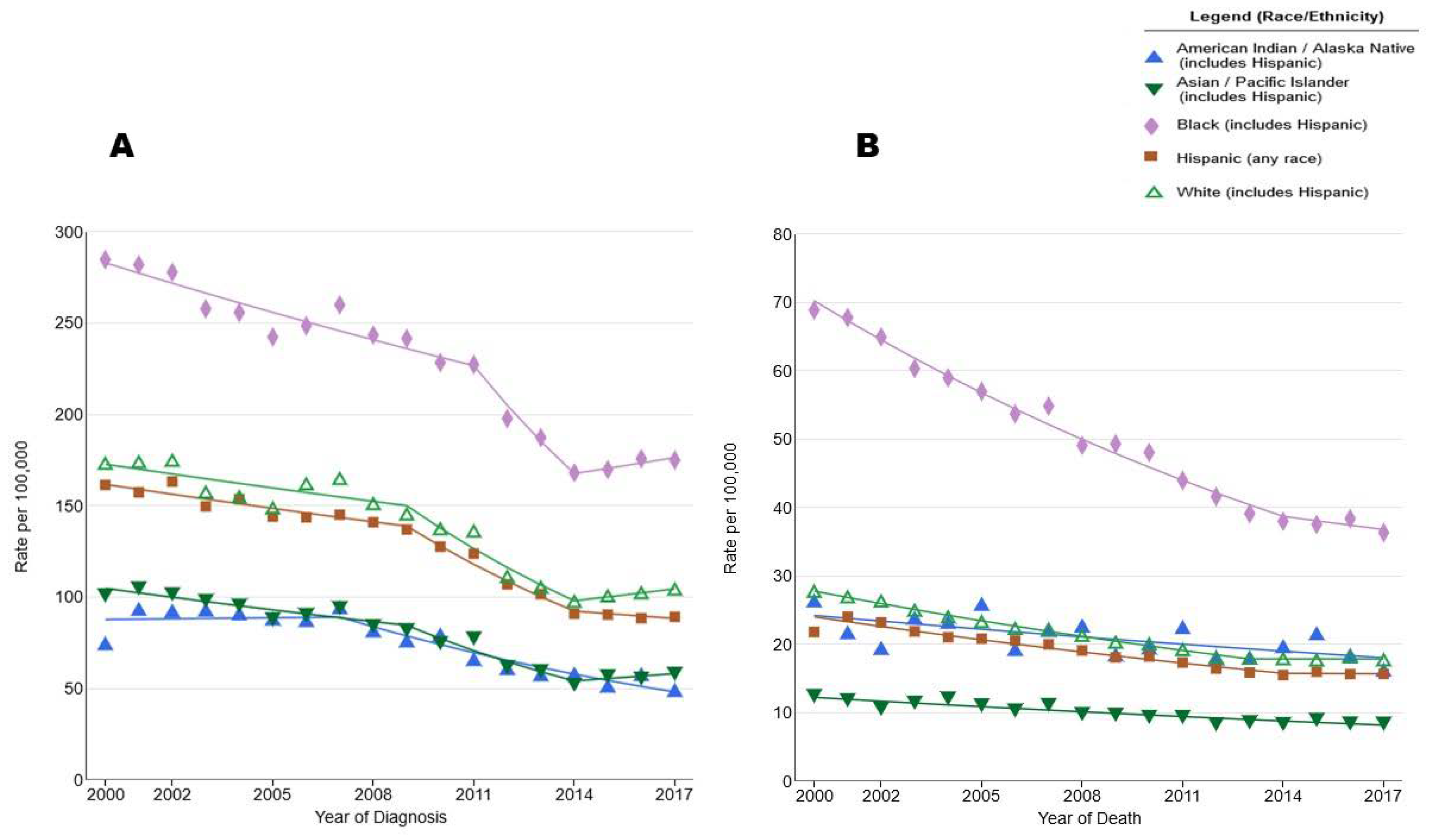
1. Introduction
2. Genetic Influential Factors for Prostate Cancer Risk
2.1. Rare Variants of Moderate to Large Effects
2.2. Association Studies of Common Smaller Effect Variants
2.3. Somatic Mutations and Tumor Biomarkers
3. Diet and Anthropometric Risk Factors for Prostate Cancer
3.1. Diet
3.2. Obesity
4. Social Determinants of Health
5. Lack of Diversity in Clinical Trials and Genetic Studies
6. Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Resnick, M.J.; Canter, D.J.; Guzzo, T.J.; Brucker, B.M.; Bergey, M.; Sonnad, S.S.; Wein, A.J.; Malkowicz, S.B. Does race affect postoperative outcomes in patients with low-risk prostate cancer who undergo radical prostatectomy? Urology 2009, 73, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef]
- Odedina, F.T.; Akinremi, T.O.; Chinegwundoh, F.; Roberts, R.; Yu, D.; Reams, R.R.; Freedman, M.L.; Rivers, B.; Green, B.L.; Kumar, N. Prostate cancer disparities in Black men of African descent: A comparative literature review of prostate cancer burden among Black men in the United States, Caribbean, United Kingdom, and West Africa. Infect. Agent Cancer 2009, 4 (Suppl. 1), S2. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Devesa, S.S.; Chang, B.L.; Bunker, C.H.; Cheng, I.; Cooney, K.; Eeles, R.; Fernandez, P.; Giri, V.N.; Gueye, S.M.; et al. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of african descent. Prostate Cancer 2013, 2013, 560857. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures for African Americans 2019–2021. Available online: https://www.cancer.org/research/cancer-facts-statistics/cancer-facts-figures-for-african-americans.html (accessed on 23 September 2019).
- Schwartz, K.; Powell, I.J.; Underwood, W., 3rd; George, J.; Yee, C.; Banerjee, M. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology 2009, 74, 1296–1302. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Siegel, R.L.; Sauer, A.G.; Miller, K.D.; Fedewa, S.A.; Alcaraz, K.I.; Jemal, A. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J. Clin. 2016, 66, 290–308. [Google Scholar] [CrossRef]
- Fernandez, P.; Salie, M.; du Toit, D.; van der Merwe, A. Analysis of Prostate Cancer Susceptibility Variants in South African Men: Replicating Associations on Chromosomes 8q24 and 10q11. Prostate Cancer 2015, 2015, 465184. [Google Scholar] [CrossRef]
- Mahal, B.A.; Berman, R.A.; Taplin, M.E.; Huang, F.W. Prostate Cancer-Specific Mortality Across Gleason Scores in Black vs Nonblack Men. JAMA 2018, 320, 2479–2481. [Google Scholar] [CrossRef]
- Pietro, G.D.; Chornokur, G.; Kumar, N.B.; Davis, C.; Park, J.Y. Racial Differences in the Diagnosis and Treatment of Prostate Cancer. Int. Neurourol. J. 2016, 20, S112–S119. [Google Scholar] [CrossRef] [PubMed]
- Chornokur, G.; Dalton, K.; Borysova, M.E.; Kumar, N.B. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate 2011, 71, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.A.; Rimm, E.B.; Willett, W.C.; Kantoff, P.W.; Giovannucci, E. Racial variation in prostate cancer incidence and in hormonal system markers among male health professionals. J. Natl. Cancer Inst. 2000, 92, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Surveillance Research Program, National Cancer Institute. Epidemiology and End Results Program. An Interactive Website for SEER Cancer Statistics. Available online: https://seer.cancer.gov/explorer/ (accessed on 23 September 2020).
- GLOBOCAN. Prostate Cancer Fact Sheet 2018; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Zeegers, M.P.; Jellema, A.; Ostrer, H. Empiric risk of prostate carcinoma for relatives of patients with prostate carcinoma: A meta-analysis. Cancer 2003, 97, 1894–1903. [Google Scholar] [CrossRef]
- Kicinski, M.; Vangronsveld, J.; Nawrot, T.S. An epidemiological reappraisal of the familial aggregation of prostate cancer: A meta-analysis. PLoS ONE 2011, 6, e27130. [Google Scholar] [CrossRef]
- Albright, F.; Stephenson, R.A.; Agarwal, N.; Teerlink, C.C.; Lowrance, W.T.; Farnham, J.M.; Albright, L.A. Prostate cancer risk prediction based on complete prostate cancer family history. Prostate 2015, 75, 390–398. [Google Scholar] [CrossRef]
- Kittles, R.A.; Baffoe-Bonnie, A.B.; Moses, T.Y.; Robbins, C.M.; Ahaghotu, C.; Huusko, P.; Pettaway, C.; Vijayakumar, S.; Bennett, J.; Hoke, G.; et al. A common nonsense mutation in EphB2 is associated with prostate cancer risk in African American men with a positive family history. J. Med. Genet. 2006, 43, 507–511. [Google Scholar] [CrossRef][Green Version]
- Hjelmborg, J.B.; Scheike, T.; Holst, K.; Skytthe, A.; Penney, K.L.; Graff, R.E.; Pukkala, E.; Christensen, K.; Adami, H.O.; Holm, N.V.; et al. The heritability of prostate cancer in the Nordic Twin Study of Cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2014, 23, 2303–2310. [Google Scholar] [CrossRef]
- Pilie, P.G.; Giri, V.N.; Cooney, K.A. HOXB13 and other high penetrant genes for prostate cancer. Asian J. Androl. 2016, 18, 530–532. [Google Scholar] [CrossRef]
- Ewing, C.M.; Ray, A.M.; Lange, E.M.; Zuhlke, K.A.; Robbins, C.M.; Tembe, W.D.; Wiley, K.E.; Isaacs, S.D.; Johng, D.; Wang, Y.; et al. Germline mutations in HOXB13 and prostate-cancer risk. N. Engl. J. Med. 2012, 366, 141–149. [Google Scholar] [CrossRef]
- Xu, J.; Lange, E.M.; Lu, L.; Zheng, S.L.; Wang, Z.; Thibodeau, S.N.; Cannon-Albright, L.A.; Teerlink, C.C.; Camp, N.J.; Johnson, A.M.; et al. HOXB13 is a susceptibility gene for prostate cancer: Results from the International Consortium for Prostate Cancer Genetics (ICPCG). Hum. Genet. 2013, 132, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Beebe-Dimmer, J.L.; Hathcock, M.; Yee, C.; Okoth, L.A.; Ewing, C.M.; Isaacs, W.B.; Cooney, K.A.; Thibodeau, S.N. The HOXB13 G84E Mutation Is Associated with an Increased Risk for Prostate Cancer and Other Malignancies. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2015, 24, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Cooney, K.A.; McCarthy, J.D.; Lange, E.; Huang, L.; Miesfeldt, S.; Montie, J.E.; Oesterling, J.E.; Sandler, H.M.; Lange, K. Prostate cancer susceptibility locus on chromosome 1q: A confirmatory study. J. Natl. Cancer Inst. 1997, 89, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.S.; Beaty, T.H.; Steinberg, G.D.; Childs, B.; Walsh, P.C. Mendelian inheritance of familial prostate cancer. Proc. Natl. Acad. Sci. USA 1992, 89, 3367–3371. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.; Schaid, D.J.; Smith, J.R.; French, A.J.; Schroeder, J.J.; McDonnell, S.K.; Peterson, B.J.; Wang, Z.Y.; Carpten, J.D.; Roberts, S.G.; et al. Linkage analyses at the chromosome 1 loci 1q24-25 (HPC1), 1q42.2-43 (PCAP), and 1p36 (CAPB) in families with hereditary prostate cancer. Am. J. Hum. Genet. 2000, 66, 539–546. [Google Scholar] [CrossRef][Green Version]
- Smith, J.R.; Freije, D.; Carpten, J.D.; Gronberg, H.; Xu, J.; Isaacs, S.D.; Brownstein, M.J.; Bova, G.S.; Guo, H.; Bujnovszky, P.; et al. Major susceptibility locus for prostate cancer on chromosome 1 suggested by a genome-wide search. Science 1996, 274, 1371–1374. [Google Scholar] [CrossRef]
- Schleutker, J.; Matikainen, M.; Smith, J.; Koivisto, P.; Baffoe-Bonnie, A.; Kainu, T.; Gillanders, E.; Sankila, R.; Pukkala, E.; Carpten, J.; et al. A genetic epidemiological study of hereditary prostate cancer (HPC) in Finland: Frequent HPCX linkage in families with late-onset disease. Clin. Cancer Res. 2000, 6, 4810–4815. [Google Scholar]
- Berry, R.; Schroeder, J.J.; French, A.J.; McDonnell, S.K.; Peterson, B.J.; Cunningham, J.M.; Thibodeau, S.N.; Schaid, D.J. Evidence for a prostate cancer-susceptibility locus on chromosome 20. Am. J. Hum. Genet. 2000, 67, 82–91. [Google Scholar] [CrossRef]
- Neuhausen, S.L.; Farnham, J.M.; Kort, E.; Tavtigian, S.V.; Skolnick, M.H.; Cannon-Albright, L.A. Prostate cancer susceptibility locus HPC1 in Utah high-risk pedigrees. Hum. Mol. Genet. 1999, 8, 2437–2442. [Google Scholar] [CrossRef][Green Version]
- Xu, J.; Zheng, S.L.; Chang, B.; Smith, J.R.; Carpten, J.D.; Stine, O.C.; Isaacs, S.D.; Wiley, K.E.; Henning, L.; Ewing, C.; et al. Linkage of prostate cancer susceptibility loci to chromosome 1. Hum. Genet. 2001, 108, 335–345. [Google Scholar] [CrossRef]
- Berthon, P.; Valeri, A.; Cohen-Akenine, A.; Drelon, E.; Paiss, T.; Wöhr, G.; Latil, A.; Millasseau, P.; Mellah, I.; Cohen, N.; et al. Predisposing gene for early-onset prostate cancer, localized on chromosome 1q42.2-43. Am. J. Hum. Genet. 1998, 62, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.; Dumont, M.; Labuda, D.; Sinnett, D.; Meloche, C.; El-Alfy, M.; Berger, L.; Lees, E.; Labrie, F.; Tavtigian, S.V. Prostate cancer susceptibility genes: Lessons learned and challenges posed. Endocr. Relat. Cancer 2003, 10, 225–259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown, W.M.; Lange, E.M.; Chen, H.; Zheng, S.L.; Chang, B.; Wiley, K.E.; Isaacs, S.D.; Walsh, P.C.; Isaacs, W.B.; Xu, J.; et al. Hereditary prostate cancer in African American families: Linkage analysis using markers that map to five candidate susceptibility loci. Br. J. Cancer 2004, 90, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Ledet, E.M.; Sartor, O.; Rayford, W.; Bailey-Wilson, J.E.; Mandal, D.M. Suggestive evidence of linkage identified at chromosomes 12q24 and 2p16 in African American prostate cancer families from Louisiana. Prostate 2012, 72, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Baffoe-Bonnie, A.B.; Kittles, R.A.; Gillanders, E.; Ou, L.; George, A.; Robbins, C.; Ahaghotu, C.; Bennett, J.; Boykin, W.; Hoke, G.; et al. Genome-wide linkage of 77 families from the African American Hereditary Prostate Cancer study (AAHPC). Prostate 2007, 67, 22–31. [Google Scholar] [CrossRef]
- Alvarez-Cubero, M.J.; Entrala, C.; Fernandez-Rosado, F.; Martinez-Gonzalez, L.J.; Alvarez, J.C.; Suarez, A.; Lorente, J.A.; Cozar, J.M. Predictive value in the analysis of RNASEL genotypes in relation to prostate cancer. Prostate Cancer Prostatic Dis. 2012, 15, 144–149. [Google Scholar] [CrossRef]
- Walsh, P.C. RNASEL Arg462Gln variant is implicated in up to 13% of prostate cancer cases. J. Urol. 2003, 169, 1591. [Google Scholar]
- Li, H.; Tai, B.C. RNASEL gene polymorphisms and the risk of prostate cancer: A meta-analysis. Clin. Cancer Res. 2006, 12, 5713–5719. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Walker, A.H.; Zeigler-Johnson, C.; Weisburg, S.; Martin, A.M.; Nathanson, K.L.; Wein, A.J.; Malkowicz, S.B. Association of HPC2/ELAC2 genotypes and prostate cancer. Am. J. Hum. Genet. 2000, 67, 1014–1019. [Google Scholar] [CrossRef]
- Walsh, P.C. Germline mutations and sequence variants of macrophage scavenger receptor 1 gene are associated with prostate cancer risk. J. Urol. 2003, 169, 1589–1590. [Google Scholar]
- Zhou, A.; Paranjape, J.; Brown, T.L.; Nie, H.; Naik, S.; Dong, B.; Chang, A.; Trapp, B.; Fairchild, R.; Colmenares, C.; et al. Interferon action and apoptosis are defective in mice devoid of 2’,5’-oligoadenylate-dependent RNase L. EMBO J. 1997, 16, 6355–6363. [Google Scholar] [CrossRef]
- Alvarez-Cubero, M.J.; Pascual-Geler, M.; Martinez-Gonzalez, L.J.; Exposito Ruiz, M.; Saiz, M.; Cozar, J.M.; Lorente, J.A. Association between RNASEL, MSR1, and ELAC2 single nucleotide polymorphisms and gene expression in prostate cancer risk. Urol. Oncol. 2016, 34, 431.e1–431.e8. [Google Scholar] [CrossRef] [PubMed]
- Powell, I.J.; Vigneau, F.D.; Bock, C.H.; Ruterbusch, J.; Heilbrun, L.K. Reducing prostate cancer racial disparity: Evidence for aggressive early prostate cancer PSA testing of African American men. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2014, 23, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Grindedal, E.M.; Møller, P.; Eeles, R.; Stormorken, A.T.; Bowitz-Lothe, I.M.; Landrø, S.M.; Clark, N.; Kvåle, R.; Shanley, S.; Maehle, L. Germ-line mutations in mismatch repair genes associated with prostate cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2009, 18, 2460–2467. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, E.K.; Page, E.C.; Castro, E.; Lilja, H.; Vickers, A.; Sjoberg, D.; Assel, M.; Foster, C.S.; Mitchell, G.; Drew, K.; et al. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: Results from the initial screening round of the IMPACT study. Eur. Urol. 2014, 66, 489–499. [Google Scholar] [CrossRef]
- Castro, E.; Goh, C.; Olmos, D.; Saunders, E.; Leongamornlert, D.; Tymrakiewicz, M.; Mahmud, N.; Dadaev, T.; Govindasami, K.; Guy, M.; et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J. Clin. Oncol. 2013, 31, 1748–1757. [Google Scholar] [CrossRef]
- Carter, H.B.; Helfand, B.; Mamawala, M.; Wu, Y.; Landis, P.; Yu, H.; Wiley, K.; Na, R.; Shi, Z.; Petkewicz, J.; et al. Germline Mutations in ATM and BRCA1/2 Are Associated with Grade Reclassification in Men on Active Surveillance for Prostate Cancer. Eur. Urol. 2019, 75, 743–749. [Google Scholar] [CrossRef]
- Na, R.; Zheng, S.L.; Han, M.; Yu, H.; Jiang, D.; Shah, S.; Ewing, C.M.; Zhang, L.; Novakovic, K.; Petkewicz, J.; et al. Germline Mutations in ATM and BRCA1/2 Distinguish Risk for Lethal and Indolent Prostate Cancer and are Associated with Early Age at Death. Eur. Urol. 2017, 71, 740–747. [Google Scholar] [CrossRef]
- Petrovics, G.; Price, D.K.; Lou, H.; Chen, Y.; Garland, L.; Bass, S.; Jones, K.; Kohaar, I.; Ali, A.; Ravindranath, L.; et al. Increased frequency of germline BRCA2 mutations associates with prostate cancer metastasis in a racially diverse patient population. Prostate Cancer Prostatic Dis. 2019, 22, 406–410. [Google Scholar] [CrossRef]
- Beebe-Dimmer, J.L.; Zuhlke, K.A.; Johnson, A.M.; Liesman, D.; Cooney, K.A. Rare germline mutations in African American men diagnosed with early-onset prostate cancer. Prostate 2018, 78, 321–326. [Google Scholar] [CrossRef]
- Mitra, A.; Fisher, C.; Foster, C.S.; Jameson, C.; Barbachanno, Y.; Bartlett, J.; Bancroft, E.; Doherty, R.; Kote-Jarai, Z.; Peock, S.; et al. Prostate cancer in male BRCA1 and BRCA2 mutation carriers has a more aggressive phenotype. Br. J. Cancer 2008, 98, 502–507. [Google Scholar] [CrossRef]
- Taioli, E.; Sears, V.; Watson, A.; Flores-Obando, R.E.; Jackson, M.D.; Ukoli, F.A.; de Syllos Cólus, I.M.; Fernandez, P.; McFarlane-Anderson, N.; Ostrander, E.A.; et al. Polymorphisms in CYP17 and CYP3A4 and prostate cancer in men of African descent. Prostate 2013, 73, 668–676. [Google Scholar] [CrossRef]
- Aiken, W.D. Historical determinants of contemporary attributes of African descendants in the Americas: The androgen receptor holds the key. Med. Hypotheses 2011, 77, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.; Petrovics, G.; McLeod, D.G.; Srivastava, S. Genetic and molecular differences in prostate carcinogenesis between African American and Caucasian American men. Int. J. Mol. Sci. 2013, 14, 15510–15531. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zou, Y.F.; Feng, X.L.; Su, H.; Huang, F. CYP17 gene polymorphisms and prostate cancer risk: A meta-analysis based on 38 independent studies. Prostate 2011, 71, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Ntais, C.; Polycarpou, A.; Ioannidis, J.P. Association of the CYP17 gene polymorphism with the risk of prostate cancer: A meta-analysis. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2003, 12, 120–126. [Google Scholar]
- Sarma, A.V.; Dunn, R.L.; Lange, L.A.; Ray, A.; Wang, Y.; Lange, E.M.; Cooney, K.A. Genetic polymorphisms in CYP17, CYP3A4, CYP19A1, SRD5A2, IGF-1, and IGFBP-3 and prostate cancer risk in African-American men: The Flint Men’s Health Study. Prostate 2008, 68, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Kittles, R.A.; Panguluri, R.K.; Chen, W.; Massac, A.; Ahaghotu, C.; Jackson, A.; Ukoli, F.; Adams-Campbell, L.; Isaacs, W.; Dunston, G.M. Cyp17 promoter variant associated with prostate cancer aggressiveness in African Americans. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2001, 10, 943–947. [Google Scholar]
- Stanford, J.L.; Noonan, E.A.; Iwasaki, L.; Kolb, S.; Chadwick, R.B.; Feng, Z.; Ostrander, E.A. A polymorphism in the CYP17 gene and risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2002, 11, 243–247. [Google Scholar]
- Haiman, C.A.; Stampfer, M.J.; Giovannucci, E.; Ma, J.; Decalo, N.E.; Kantoff, P.W.; Hunter, D.J. The relationship between a polymorphism in CYP17 with plasma hormone levels and prostate cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2001, 10, 743–748. [Google Scholar]
- Yamada, Y.; Watanabe, M.; Murata, M.; Yamanaka, M.; Kubota, Y.; Ito, H.; Katoh, T.; Kawamura, J.; Yatani, R.; Shiraishi, T. Impact of genetic polymorphisms of 17-hydroxylase cytochrome P-450 (CYP17) and steroid 5alpha-reductase type II (SRD5A2) genes on prostate-cancer risk among the Japanese population. Int. J. Cancer 2001, 92, 683–686. [Google Scholar] [CrossRef]
- Sobti, R.C.; Gupta, L.; Thakur, H.; Seth, A.; Singh, S.K.; Kaur, P. CYP17 gene polymorphism and its association in north Indian prostate cancer patients. Anticancer Res. 2009, 29, 1659–1663. [Google Scholar] [PubMed]
- Souiden, Y.; Mahdouani, M.; Chaieb, K.; Elkamel, R.; Mahdouani, K. CYP17 gene polymorphism and prostate cancer susceptibility in a Tunisian population. Cancer Epidemiol. 2011, 35, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Powell, I.J.; Bock, C.H.; Ruterbusch, J.J.; Sakr, W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J. Urol. 2010, 183, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Powell, I.J. Epidemiology and pathophysiology of prostate cancer in African-American men. J. Urol. 2007, 177, 444–449. [Google Scholar] [CrossRef]
- Wallace, T.A.; Martin, D.N.; Ambs, S. Interactions among genes, tumor biology and the environment in cancer health disparities: Examining the evidence on a national and global scale. Carcinogenesis 2011, 32, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Freeman, V.L.; Leszczak, J.; Cooper, R.S. Race and the histologic grade of prostate cancer. Prostate 1997, 30, 79–84. [Google Scholar] [CrossRef]
- Sartor, O.; Zheng, Q.; Eastham, J.A. Androgen receptor gene CAG repeat length varies in a race-specific fashion in men without prostate cancer. Urology 1999, 53, 378–380. [Google Scholar] [CrossRef]
- Coetzee, G.A.; Ross, R.K. Re: Prostate cancer and the androgen receptor. J. Natl. Cancer Inst. 1994, 86, 872–873. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.L.; Price, D.K.; Kim, S.; Liu, D.; Jovanovic, B.D.; Nathan, D.; Johnson, M.E.; Montgomery, J.S.; Cude, K.; Brockbank, J.C.; et al. Racial variation in CAG repeat lengths within the androgen receptor gene among prostate cancer patients of lower socioeconomic status. J. Clin. Oncol. 2002, 20, 3599–3604. [Google Scholar] [CrossRef]
- Kittles, R.A.; Young, D.; Weinrich, S.; Hudson, J.; Argyropoulos, G.; Ukoli, F.; Adams-Campbell, L.; Dunston, G.M. Extent of linkage disequilibrium between the androgen receptor gene CAG and GGC repeats in human populations: Implications for prostate cancer risk. Hum. Genet. 2001, 109, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, N.L.; Driver, E.D.; Miesfeld, R.L. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994, 22, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Ingles, S.A.; Ross, R.K.; Yu, M.C.; Irvine, R.A.; La Pera, G.; Haile, R.W.; Coetzee, G.A. Association of prostate cancer risk with genetic polymorphisms in vitamin D receptor and androgen receptor. J. Natl. Cancer Inst. 1997, 89, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Stampfer, M.J.; Krithivas, K.; Brown, M.; Dahl, D.; Brufsky, A.; Talcott, J.; Hennekens, C.H.; Kantoff, P.W. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc. Natl. Acad. Sci. USA 1997, 94, 3320–3323. [Google Scholar] [CrossRef] [PubMed]
- Price, D.K.; Chau, C.H.; Till, C.; Goodman, P.J.; Baum, C.E.; Ockers, S.B.; English, B.C.; Minasian, L.; Parnes, H.L.; Hsing, A.W.; et al. Androgen receptor CAG repeat length and association with prostate cancer risk: Results from the prostate cancer prevention trial. J. Urol. 2010, 184, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, T.; Manola, J.; Sartor, O.; Weinrich, S.P.; Moul, J.W.; Kantoff, P.W. Absence of a correlation of androgen receptor gene CAG repeat length and prostate cancer risk in an African-American population. Clin. Prostate Cancer 2004, 3, 98–103. [Google Scholar] [CrossRef]
- Freedman, M.L.; Pearce, C.L.; Penney, K.L.; Hirschhorn, J.N.; Kolonel, L.N.; Henderson, B.E.; Altshuler, D. Systematic evaluation of genetic variation at the androgen receptor locus and risk of prostate cancer in a multiethnic cohort study. Am. J. Hum. Genet. 2005, 76, 82–90. [Google Scholar] [CrossRef]
- Lange, E.M.; Sarma, A.V.; Ray, A.; Wang, Y.; Ho, L.A.; Anderson, S.A.; Cunningham, J.M.; Cooney, K.A. The androgen receptor CAG and GGN repeat polymorphisms and prostate cancer susceptibility in African-American men: Results from the Flint Men’s Health Study. J. Hum. Genet. 2008, 53, 220–226. [Google Scholar] [CrossRef]
- Benafif, S.; Kote-Jarai, Z.; Eeles, R.A. A Review of Prostate Cancer Genome-Wide Association Studies (GWAS). Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2018, 27, 845–857. [Google Scholar] [CrossRef]
- Eeles, R.A.; Kote-Jarai, Z.; Giles, G.G.; Olama, A.A.; Guy, M.; Jugurnauth, S.K.; Mulholland, S.; Leongamornlert, D.A.; Edwards, S.M.; Morrison, J.; et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nat. Genet. 2008, 40, 316–321. [Google Scholar] [CrossRef]
- Gudmundsson, J.; Sulem, P.; Rafnar, T.; Bergthorsson, J.T.; Manolescu, A.; Gudbjartsson, D.; Agnarsson, B.A.; Sigurdsson, A.; Benediktsdottir, K.R.; Blondal, T.; et al. Common sequence variants on 2p15 and Xp11.22 confer susceptibility to prostate cancer. Nat. Genet. 2008, 40, 281–283. [Google Scholar] [CrossRef]
- Duggan, D.; Zheng, S.L.; Knowlton, M.; Benitez, D.; Dimitrov, L.; Wiklund, F.; Robbins, C.; Isaacs, S.D.; Cheng, Y.; Li, G.; et al. Two genome-wide association studies of aggressive prostate cancer implicate putative prostate tumor suppressor gene DAB2IP. J. Natl. Cancer Inst. 2007, 99, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, J.; Sulem, P.; Steinthorsdottir, V.; Bergthorsson, J.T.; Thorleifsson, G.; Manolescu, A.; Rafnar, T.; Gudbjartsson, D.; Agnarsson, B.A.; Baker, A.; et al. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat. Genet. 2007, 39, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Yeager, M.; Orr, N.; Hayes, R.B.; Jacobs, K.B.; Kraft, P.; Wacholder, S.; Minichiello, M.J.; Fearnhead, P.; Yu, K.; Chatterjee, N.; et al. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat. Genet. 2007, 39, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Haiman, C.A.; Patterson, N.; Freedman, M.L.; Myers, S.R.; Pike, M.C.; Waliszewska, A.; Neubauer, J.; Tandon, A.; Schirmer, C.; McDonald, G.J.; et al. Multiple regions within 8q24 independently affect risk for prostate cancer. Nat. Genet. 2007, 39, 638–644. [Google Scholar] [CrossRef]
- Haiman, C.A.; Chen, G.K.; Blot, W.J.; Strom, S.S.; Berndt, S.I.; Kittles, R.A.; Rybicki, B.A.; Isaacs, W.B.; Ingles, S.A.; Stanford, J.L.; et al. Characterizing genetic risk at known prostate cancer susceptibility loci in African Americans. PLoS Genet. 2011, 7, e1001387. [Google Scholar] [CrossRef]
- Haiman, C.A.; Chen, G.K.; Blot, W.J.; Strom, S.S.; Berndt, S.I.; Kittles, R.A.; Rybicki, B.A.; Isaacs, W.B.; Ingles, S.A.; Stanford, J.L.; et al. Genome-wide association study of prostate cancer in men of African ancestry identifies a susceptibility locus at 17q21. Nat. Genet. 2011, 43, 570–573. [Google Scholar] [CrossRef]
- Amundadottir, L.T.; Sulem, P.; Gudmundsson, J.; Helgason, A.; Baker, A.; Agnarsson, B.A.; Sigurdsson, A.; Benediktsdottir, K.R.; Cazier, J.B.; Sainz, J.; et al. A common variant associated with prostate cancer in European and African populations. Nat. Genet. 2006, 38, 652–658. [Google Scholar] [CrossRef]
- Freedman, M.L.; Haiman, C.A.; Patterson, N.; McDonald, G.J.; Tandon, A.; Waliszewska, A.; Penney, K.; Steen, R.G.; Ardlie, K.; John, E.M.; et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc. Natl. Acad. Sci. USA 2006, 103, 14068–14073. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.; Torres, J.B.; Hooker, S.; Bonilla, C.; Hernandez, W.; Candreva, A.; Ahaghotu, C.; Kittles, R.; Carpten, J. Confirmation study of prostate cancer risk variants at 8q24 in African Americans identifies a novel risk locus. Genome Res. 2007, 17, 1717–1722. [Google Scholar] [CrossRef]
- Han, Y.; Signorello, L.B.; Strom, S.S.; Kittles, R.A.; Rybicki, B.A.; Stanford, J.L.; Goodman, P.J.; Berndt, S.I.; Carpten, J.; Casey, G.; et al. Generalizability of established prostate cancer risk variants in men of African ancestry. Int. J. Cancer 2015, 136, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Rand, K.A.; Hazelett, D.J.; Ingles, S.A.; Kittles, R.A.; Strom, S.S.; Rybicki, B.A.; Nemesure, B.; Isaacs, W.B.; Stanford, J.L.; et al. Prostate Cancer Susceptibility in Men of African Ancestry at 8q24. J. Natl. Cancer Inst. 2016, 108, djv431. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.B.; Ukoli, F.; Freeman, V.; Bennett, F.; Aiken, W.; Tulloch, T.; Coard, K.; Angwafo, F.; Kittles, R.A. 8q24 risk alleles in West African and Caribbean men. Prostate 2012, 72, 1366–1373. [Google Scholar] [CrossRef]
- Cropp, C.D.; Robbins, C.M.; Sheng, X.; Hennis, A.J.; Carpten, J.D.; Waterman, L.; Worrell, R.; Schwantes-An, T.H.; Trent, J.M.; Haiman, C.A.; et al. 8q24 risk alleles and prostate cancer in African-Barbadian men. Prostate 2014, 74, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Eeles, R.A.; Olama, A.A.; Benlloch, S.; Saunders, E.J.; Leongamornlert, D.A.; Tymrakiewicz, M.; Ghoussaini, M.; Luccarini, C.; Dennis, J.; Jugurnauth-Little, S.; et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet. 2013, 45, 385–391. [Google Scholar] [CrossRef]
- Cropp, C.D.; Simpson, C.L.; Wahlfors, T.; Ha, N.; George, A.; Jones, M.S.; Harper, U.; Ponciano-Jackson, D.; Green, T.A.; Tammela, T.L.; et al. Genome-wide linkage scan for prostate cancer susceptibility in Finland: Evidence for a novel locus on 2q37.3 and confirmation of signal on 17q21-q22. Int. J. Cancer 2011, 129, 2400–2407. [Google Scholar] [CrossRef]
- Conti, D.V.; Wang, K.; Sheng, X.; Bensen, J.T.; Hazelett, D.J.; Cook, M.B.; Ingles, S.A.; Kittles, R.A.; Strom, S.S.; Rybicki, B.A.; et al. Two Novel Susceptibility Loci for Prostate Cancer in Men of African Ancestry. J. Natl. Cancer Inst. 2017, 109, djx084. [Google Scholar] [CrossRef]
- Du, Z.; Lubmawa, A.; Gundell, S.; Wan, P.; Nalukenge, C.; Muwanga, P.; Lutalo, M.; Nansereko, D.; Ndaruhutse, O.; Katuku, M.; et al. Genetic risk of prostate cancer in Ugandan men. Prostate 2018, 78, 370–376. [Google Scholar] [CrossRef]
- Cook, M.B.; Wang, Z.; Yeboah, E.D.; Tettey, Y.; Biritwum, R.B.; Adjei, A.A.; Tay, E.; Truelove, A.; Niwa, S.; Chung, C.C.; et al. A genome-wide association study of prostate cancer in West African men. Hum. Genet. 2014, 133, 509–521. [Google Scholar] [CrossRef]
- Xu, J.; Kibel, A.S.; Hu, J.J.; Turner, A.R.; Pruett, K.; Zheng, S.L.; Sun, J.; Isaacs, S.D.; Wiley, K.E.; Kim, S.T.; et al. Prostate cancer risk associated loci in African Americans. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2009, 18, 2145–2149. [Google Scholar] [CrossRef]
- Waters, K.M.; Le Marchand, L.; Kolonel, L.N.; Monroe, K.R.; Stram, D.O.; Henderson, B.E.; Haiman, C.A. Generalizability of associations from prostate cancer genome-wide association studies in multiple populations. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2009, 18, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Hooker, S.; Hernandez, W.; Chen, H.; Robbins, C.; Torres, J.B.; Ahaghotu, C.; Carpten, J.; Kittles, R.A. Replication of prostate cancer risk loci on 8q24, 11q13, 17q12, 19q33, and Xp11 in African Americans. Prostate 2010, 70, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.L.; Spangler, E.; Gallagher, S.; Haiman, C.A.; Henderson, B.; Isaacs, W.; Benford, M.L.; Kidd, L.R.; Cooney, K.; Strom, S.; et al. Validation of genome-wide prostate cancer associations in men of African descent. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2011, 20, 23–32. [Google Scholar] [CrossRef]
- Rebbeck, T.R. Prostate Cancer Genetics: Variation by Race, Ethnicity, and Geography. Semin. Radiat. Oncol. 2017, 27, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Spratt, D.E.; Chan, T.; Waldron, L.; Speers, C.; Feng, F.Y.; Ogunwobi, O.O.; Osborne, J.R. Racial/Ethnic Disparities in Genomic Sequencing. JAMA Oncol. 2016, 2, 1070–1074. [Google Scholar] [CrossRef]
- Clark, J.P.; Cooper, C.S. ETS gene fusions in prostate cancer. Nat. Rev. Urol. 2009, 6, 429–439. [Google Scholar] [CrossRef]
- Perner, S.; Demichelis, F.; Beroukhim, R.; Schmidt, F.H.; Mosquera, J.M.; Setlur, S.; Tchinda, J.; Tomlins, S.A.; Hofer, M.D.; Pienta, K.G.; et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 2006, 66, 8337–8341. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.W.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R.; et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005, 310, 644–648. [Google Scholar] [CrossRef]
- Blackburn, J.; Vecchiarelli, S.; Heyer, E.E.; Patrick, S.M.; Lyons, R.J.; Jaratlerdsiri, W.; van Zyl, S.; Bornman, M.S.R.; Mercer, T.R.; Hayes, V.M. TMPRSS2-ERG fusions linked to prostate cancer racial health disparities: A focus on Africa. Prostate 2019, 79, 1191–1196. [Google Scholar] [CrossRef]
- McGinley, K.F.; Tay, K.J.; Moul, J.W. Prostate cancer in men of African origin. Nat. Rev. Urol. 2016, 13, 99–107. [Google Scholar] [CrossRef]
- Goh, L.K.; Liem, N.; Vijayaraghavan, A.; Chen, G.; Lim, P.L.; Tay, K.J.; Chang, M.; Low, J.S.; Joshi, A.; Huang, H.H.; et al. Diagnostic and prognostic utility of a DNA hypermethylated gene signature in prostate cancer. PLoS ONE 2014, 9, e91666. [Google Scholar] [CrossRef] [PubMed]
- Kwabi-Addo, B.; Wang, S.; Chung, W.; Jelinek, J.; Patierno, S.R.; Wang, B.D.; Andrawis, R.; Lee, N.H.; Apprey, V.; Issa, J.P.; et al. Identification of differentially methylated genes in normal prostate tissues from African American and Caucasian men. Clin. Cancer Res. 2010, 16, 3539–3547. [Google Scholar] [CrossRef] [PubMed]
- Khani, F.; Mosquera, J.M.; Park, K.; Blattner, M.; O’Reilly, C.; MacDonald, T.Y.; Chen, Z.; Srivastava, A.; Tewari, A.K.; Barbieri, C.E.; et al. Evidence for molecular differences in prostate cancer between African American and Caucasian men. Clin. Cancer Res. 2014, 20, 4925–4934. [Google Scholar] [CrossRef] [PubMed]
- Petrovics, G.; Li, H.; Stumpel, T.; Tan, S.H.; Young, D.; Katta, S.; Li, Q.; Ying, K.; Klocke, B.; Ravindranath, L.; et al. A novel genomic alteration of LSAMP associates with aggressive prostate cancer in African American men. EBioMedicine 2015, 2, 1957–1964. [Google Scholar] [CrossRef]
- Tosoian, J.J.; Almutairi, F.; Morais, C.L.; Glavaris, S.; Hicks, J.; Sundi, D.; Humphreys, E.; Han, M.; De Marzo, A.M.; Ross, A.E.; et al. Prevalence and Prognostic Significance of PTEN Loss in African-American and European-American Men Undergoing Radical Prostatectomy. Eur. Urol. 2017, 71, 697–700. [Google Scholar] [CrossRef]
- Lindquist, K.J.; Paris, P.L.; Hoffmann, T.J.; Cardin, N.J.; Kazma, R.; Mefford, J.A.; Simko, J.P.; Ngo, V.; Chen, Y.; Levin, A.M.; et al. Mutational Landscape of Aggressive Prostate Tumors in African American Men. Cancer Res. 2016, 76, 1860–1868. [Google Scholar] [CrossRef]
- Huang, F.W.; Mosquera, J.M.; Garofalo, A.; Oh, C.; Baco, M.; Amin-Mansour, A.; Rabasha, B.; Bahl, S.; Mullane, S.A.; Robinson, B.D.; et al. Exome Sequencing of African-American Prostate Cancer Reveals Loss-of-Function ERF Mutations. Cancer Discov. 2017, 7, 973–983. [Google Scholar] [CrossRef]
- Blattner, M.; Lee, D.J.; O’Reilly, C.; Park, K.; MacDonald, T.Y.; Khani, F.; Turner, K.R.; Chiu, Y.L.; Wild, P.J.; Dolgalev, I.; et al. SPOP mutations in prostate cancer across demographically diverse patient cohorts. Neoplasia 2014, 16, 14–20. [Google Scholar] [CrossRef]
- Faisal, F.A.; Sundi, D.; Tosoian, J.J.; Choeurng, V.; Alshalalfa, M.; Ross, A.E.; Klein, E.; Den, R.; Dicker, A.; Erho, N.; et al. Racial Variations in Prostate Cancer Molecular Subtypes and Androgen Receptor Signaling Reflect Anatomic Tumor Location. Eur. Urol. 2016, 70, 14–17. [Google Scholar] [CrossRef]
- Yamoah, K.; Johnson, M.H.; Choeurng, V.; Faisal, F.A.; Yousefi, K.; Haddad, Z.; Ross, A.E.; Alshalafa, M.; Den, R.; Lal, P.; et al. Novel Biomarker Signature That May Predict Aggressive Disease in African American Men with Prostate Cancer. J. Clin. Oncol. 2015, 33, 2789–2796. [Google Scholar] [CrossRef]
- Yuan, J.; Kensler, K.H.; Hu, Z.; Zhang, Y.; Zhang, T.; Jiang, J.; Xu, M.; Pan, Y.; Long, M.; Montone, K.T.; et al. Integrative comparison of the genomic and transcriptomic landscape between prostate cancer patients of predominantly African or European genetic ancestry. PLoS Genet. 2020, 16, e1008641. [Google Scholar] [CrossRef] [PubMed]
- Barnabas, N.; Xu, L.; Savera, A.; Hou, Z.; Barrack, E.R. Chromosome 8 markers of metastatic prostate cancer in African American men: Gain of the MIR151 gene and loss of the NKX3-1 gene. Prostate 2011, 71, 857–871. [Google Scholar] [CrossRef]
- Haenszel, W.; Kurihara, M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J. Natl. Cancer Inst. 1968, 40, 43–68. [Google Scholar] [PubMed]
- Staszewski, J.; Haenszel, W. Cancer mortality among the Polish-born in the United States. J. Natl. Cancer Inst. 1965, 35, 291–297. [Google Scholar] [PubMed]
- Maskarinec, G.; Noh, J.J. The effect of migration on cancer incidence among Japanese in Hawaii. Ethn. Dis. 2004, 14, 431–439. [Google Scholar]
- Tillin, T.; Forouhi, N.; Johnston, D.G.; McKeigue, P.M.; Chaturvedi, N.; Godsland, I.F. Metabolic syndrome and coronary heart disease in South Asians, African-Caribbeans and white Europeans: A UK population-based cross-sectional study. Diabetologia 2005, 48, 649–656. [Google Scholar] [CrossRef]
- Fischbacher, C.M.; Bhopal, R.; Povey, C.; Steiner, M.; Chalmers, J.; Mueller, G.; Jamieson, J.; Knowles, D. Record linked retrospective cohort study of 4.6 million people exploring ethnic variations in disease: Myocardial infarction in South Asians. BMC Public Health 2007, 7, 142. [Google Scholar] [CrossRef]
- Hosper, K.; Nierkens, V.; Nicolaou, M.; Stronks, K. Behavioural risk factors in two generations of non-Western migrants: Do trends converge towards the host population? Eur. J. Epidemiol. 2007, 22, 163–172. [Google Scholar] [CrossRef]
- Bylsma, L.C.; Alexander, D.D. A review and meta-analysis of prospective studies of red and processed meat, meat cooking methods, heme iron, heterocyclic amines and prostate cancer. Nutr. J. 2015, 14, 125. [Google Scholar] [CrossRef]
- Hans-Olov, A.; Hunter, D.; Trichopoulos, D. Textbook of Cancer Epidemiology, 2nd ed.; Oxford University Press: New York, NY, USA, 2008. [Google Scholar]
- Park, S.Y.; Murphy, S.P.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. Fat and meat intake and prostate cancer risk: The multiethnic cohort study. Int. J. Cancer 2007, 121, 1339–1345. [Google Scholar] [CrossRef]
- Rohrmann, S.; Platz, E.A.; Kavanaugh, C.J.; Thuita, L.; Hoffman, S.C.; Helzlsouer, K.J. Meat and dairy consumption and subsequent risk of prostate cancer in a US cohort study. Cancer Causes Control 2007, 18, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, A.G.; van den Brandt, P.A.; Dorant, E.; Goldbohm, R.A. Animal products, calcium and protein and prostate cancer risk in The Netherlands Cohort Study. Br. J. Cancer 1999, 80, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; McCullough, M.L.; Mondul, A.M.; Jacobs, E.J.; Chao, A.; Patel, A.V.; Thun, M.J.; Calle, E.E. Meat consumption among Black and White men and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2006, 15, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Augustsson, K.; Rimm, E.B.; Stampfer, M.J.; Willet, W.C.; Giovannucci, E. A prospective study on intake of animal products and risk of prostate cancer. Cancer Causes Control 2001, 12, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Augustsson, K.; Michaud, D.S.; Rimm, E.B.; Leitzmann, M.F.; Stampfer, M.J.; Willett, W.C.; Giovannucci, E. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2003, 12, 64–67. [Google Scholar]
- Chavarro, J.E.; Stampfer, M.J.; Hall, M.N.; Sesso, H.D.; Ma, J. A 22-y prospective study of fish intake in relation to prostate cancer incidence and mortality. Am. J. Clin. Nutr. 2008, 88, 1297–1303. [Google Scholar] [CrossRef]
- Hayes, R.B.; Ziegler, R.G.; Gridley, G.; Swanson, C.; Greenberg, R.S.; Swanson, G.M.; Schoenberg, J.B.; Silverman, D.T.; Brown, L.M.; Pottern, L.M.; et al. Dietary factors and risks for prostate cancer among blacks and whites in the United States. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 1999, 8, 25–34. [Google Scholar]
- Kumar, N.B.; Yu, D.; Akinremi, T.O.; Odedina, F.T. Comparing dietary and other lifestyle factors among immigrant Nigerian men living in the US and indigenous men from Nigeria: Potential implications for prostate cancer risk reduction. J. Immigr. Minor. Health 2009, 11, 391–399. [Google Scholar] [CrossRef]
- National Cancer Institute. Prostate Cancer Prevention (PDQ®)–Patient Version. Available online: https://www.cancer.gov/types/prostate/patient/prostate-prevention-pdq#section/all (accessed on 30 October 2020).
- Wilson, K.M.; Mucci, L.A.; Drake, B.F.; Preston, M.A.; Stampfer, M.J.; Giovannucci, E.; Kibel, A.S. Meat, Fish, Poultry, and Egg Intake at Diagnosis and Risk of Prostate Cancer Progression. Cancer Prev. Res. (Phila.) 2016, 9, 933–941. [Google Scholar] [CrossRef]
- Ross, R.K.; Shimizu, H.; Paganini-Hill, A.; Honda, G.; Henderson, B.E. Case-control studies of prostate cancer in blacks and whites in southern California. J. Natl. Cancer Inst. 1987, 78, 869–874. [Google Scholar]
- Epstein, M.M.; Kasperzyk, J.L.; Mucci, L.A.; Giovannucci, E.; Price, A.; Wolk, A.; Håkansson, N.; Fall, K.; Andersson, S.O.; Andrén, O. Dietary fatty acid intake and prostate cancer survival in Örebro County, Sweden. Am. J. Epidemiol. 2012, 176, 240–252. [Google Scholar] [CrossRef]
- Bidoli, E.; Talamini, R.; Bosetti, C.; Negri, E.; Maruzzi, D.; Montella, M.; Franceschi, S.; La Vecchia, C. Macronutrients, fatty acids, cholesterol and prostate cancer risk. Ann. Oncol. 2005, 16, 152–157. [Google Scholar] [CrossRef]
- Crowe, F.L.; Key, T.J.; Appleby, P.N.; Travis, R.C.; Overvad, K.; Jakobsen, M.U.; Johnsen, N.F.; Tjønneland, A.; Linseisen, J.; Rohrmann, S.; et al. Dietary fat intake and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2008, 87, 1405–1413. [Google Scholar] [CrossRef]
- Fleshner, N.; Bagnell, P.S.; Klotz, L.; Venkateswaran, V. Dietary fat and prostate cancer. J. Urol. 2004, 171, S19–S24. [Google Scholar] [CrossRef]
- Wiseman, M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Proc. Nutr. Soc. 2008, 67, 253–256. [Google Scholar] [CrossRef]
- Dennis, L.K.; Snetselaar, L.G.; Smith, B.J.; Stewart, R.E.; Robbins, M.E. Problems with the assessment of dietary fat in prostate cancer studies. Am. J. Epidemiol. 2004, 160, 436–444. [Google Scholar] [CrossRef]
- Liu, Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006, 9, 230–234. [Google Scholar] [CrossRef]
- Chang, S.N.; Han, J.; Abdelkader, T.S.; Kim, T.H.; Lee, J.M.; Song, J.; Kim, K.S.; Park, J.H.; Park, J.H. High animal fat intake enhances prostate cancer progression and reduces glutathione peroxidase 3 expression in early stages of TRAMP mice. Prostate 2014, 74, 1266–1277. [Google Scholar] [CrossRef]
- McCarty, M.F. Mortality from Western cancers rose dramatically among African-Americans during the 20th century: Are dietary animal products to blame? Med. Hypotheses 2001, 57, 169–174. [Google Scholar] [CrossRef]
- Ohwaki, K.; Endo, F.; Kachi, Y.; Hattori, K.; Muraishi, O.; Nishikitani, M.; Yano, E. Relationship between dietary factors and prostate-specific antigen in healthy men. Urol. Int. 2012, 89, 270–274. [Google Scholar] [CrossRef]
- Zhou, X.; Mei, H.; Agee, J.; Brown, T.; Mao, J. Racial differences in distribution of fatty acids in prostate cancer and benign prostatic tissues. Lipids Health Dis. 2019, 18, 189. [Google Scholar] [CrossRef]
- Figiel, S.; Pinault, M.; Domingo, I.; Guimaraes, C.; Guibon, R.; Besson, P.; Tavernier, E.; Blanchet, P.; Multigner, L.; Bruyère, F.; et al. Fatty acid profile in peri-prostatic adipose tissue and prostate cancer aggressiveness in African-Caribbean and Caucasian patients. Eur. J. Cancer 2018, 91, 107–115. [Google Scholar] [CrossRef]
- Park, S.Y.; Wilkens, L.R.; Henning, S.M.; Le Marchand, L.; Gao, K.; Goodman, M.T.; Murphy, S.P.; Henderson, B.E.; Kolonel, L.N. Circulating fatty acids and prostate cancer risk in a nested case-control study: The Multiethnic Cohort. Cancer Causes Control 2009, 20, 211–223. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chiang, C.I.; Lin, R.S.; Pu, Y.S.; Lai, M.K.; Sung, F.C. Diet, vegetarian food and prostate carcinoma among men in Taiwan. Br. J. Cancer 2005, 93, 1057–1061. [Google Scholar] [CrossRef]
- Kristal, A.R.; Lampe, J.W. Brassica vegetables and prostate cancer risk: A review of the epidemiological evidence. Nutr. Cancer 2002, 42, 1–9. [Google Scholar] [CrossRef]
- Howie, B.J.; Shultz, T.D. Dietary and hormonal interrelationships among vegetarian Seventh-Day Adventists and nonvegetarian men. Am. J. Clin. Nutr. 1985, 42, 127–134. [Google Scholar] [CrossRef]
- McCann, S.E.; Ambrosone, C.B.; Moysich, K.B.; Brasure, J.; Marshall, J.R.; Freudenheim, J.L.; Wilkinson, G.S.; Graham, S. Intakes of selected nutrients, foods, and phytochemicals and prostate cancer risk in western New York. Nutr. Cancer 2005, 53, 33–41. [Google Scholar] [CrossRef]
- Etminan, M.; Takkouche, B.; Caamaño-Isorna, F. The role of tomato products and lycopene in the prevention of prostate cancer: A meta-analysis of observational studies. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2004, 13, 340–345. [Google Scholar]
- Tantamango-Bartley, Y.; Knutsen, S.F.; Knutsen, R.; Jacobsen, B.K.; Fan, J.; Beeson, W.L.; Sabate, J.; Hadley, D.; Jaceldo-Siegl, K.; Penniecook, J.; et al. Are strict vegetarians protected against prostate cancer? Am. J. Clin. Nutr. 2016, 103, 153–160. [Google Scholar] [CrossRef]
- Warner, D.F.; Hayward, M.D. Early-life origins of the race gap in men’s mortality. J. Health Soc. Behav. 2006, 47, 209–226. [Google Scholar] [CrossRef]
- Barrington, W.E.; Schenk, J.M.; Etzioni, R.; Arnold, K.B.; Neuhouser, M.L.; Thompson, I.M., Jr.; Lucia, M.S.; Kristal, A.R. Difference in Association of Obesity with Prostate Cancer Risk Between US African American and Non-Hispanic White Men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA Oncol. 2015, 1, 342–349. [Google Scholar] [CrossRef]
- Amling, C.L.; Riffenburgh, R.H.; Sun, L.; Moul, J.W.; Lance, R.S.; Kusuda, L.; Sexton, W.J.; Soderdahl, D.W.; Donahue, T.F.; Foley, J.P.; et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J. Clin. Oncol. 2004, 22, 439–445. [Google Scholar] [CrossRef]
- Hales, C.; Carroll, M.; Fryar, C.; Ogden, C. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018. Available online: https://www.cdc.gov/nchs/products/databriefs/db360.htm (accessed on 23 August 2020).
- Satia-Abouta, J.; Patterson, R.E.; Schiller, R.N.; Kristal, A.R. Energy from fat is associated with obesity in U.S. men: Results from the Prostate Cancer Prevention Trial. Prev. Med. 2002, 34, 493–501. [Google Scholar] [CrossRef]
- Pasquali, R.; Casimirri, F.; Cantobelli, S.; Melchionda, N.; Morselli Labate, A.M.; Fabbri, R.; Capelli, M.; Bortoluzzi, L. Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism 1991, 40, 101–104. [Google Scholar] [CrossRef]
- Basen-Engquist, K.; Chang, M. Obesity and cancer risk: Recent review and evidence. Curr. Oncol. Rep. 2011, 13, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Vainio, H.; Kaaks, R.; Bianchini, F. Weight control and physical activity in cancer prevention: International evaluation of the evidence. Eur. J. Cancer Prev. 2002, 11 (Suppl. 2), S94–S100. [Google Scholar]
- West, D.W.; Slattery, M.L.; Robison, L.M.; French, T.K.; Mahoney, A.W. Adult dietary intake and prostate cancer risk in Utah: A case-control study with special emphasis on aggressive tumors. Cancer Causes Control 1991, 2, 85–94. [Google Scholar] [CrossRef]
- Su, L.J.; Arab, L.; Steck, S.E.; Fontham, E.T.; Schroeder, J.C.; Bensen, J.T.; Mohler, J.L. Obesity and prostate cancer aggressiveness among African and Caucasian Americans in a population-based study. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2011, 20, 844–853. [Google Scholar] [CrossRef]
- Severson, R.K.; Grove, J.S.; Nomura, A.M.; Stemmermann, G.N. Body mass and prostatic cancer: A prospective study. BMJ 1988, 297, 713–715. [Google Scholar] [CrossRef]
- Gallina, A.; Karakiewicz, P.I.; Hutterer, G.C.; Chun, F.K.; Briganti, A.; Walz, J.; Antebi, E.; Shariat, S.F.; Suardi, N.; Graefen, M.; et al. Obesity does not predispose to more aggressive prostate cancer either at biopsy or radical prostatectomy in European men. Int. J. Cancer 2007, 121, 791–795. [Google Scholar] [CrossRef]
- Beebe-Dimmer, J.L.; Nock, N.L.; Neslund-Dudas, C.; Rundle, A.; Bock, C.H.; Tang, D.; Jankowski, M.; Rybicki, B.A. Racial differences in risk of prostate cancer associated with metabolic syndrome. Urology 2009, 74, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, J.; Bañez, L.L.; Aronson, W.J.; Terris, M.K.; Presti, J.C., Jr.; Amling, C.L.; Kane, C.J.; Freedland, S.J. Obesity as a predictor of adverse outcome across black and white race: Results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer 2009, 115, 5263–5271. [Google Scholar] [CrossRef]
- Cockerham, W.C.; Bauldry, S.; Hamby, B.W.; Shikany, J.M.; Bae, S. A Comparison of Black and White Racial Differences in Health Lifestyles and Cardiovascular Disease. Am. J. Prev. Med. 2017, 52, S56–S62. [Google Scholar] [CrossRef]
- Ellis, L.; Canchola, A.J.; Spiegel, D.; Ladabaum, U.; Haile, R.; Gomez, S.L. Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics. J. Clin. Oncol. 2018, 36, 25–33. [Google Scholar] [CrossRef]
- Ellison, G.L.; Coker, A.L.; Hebert, J.R.; Sanderson, S.M.; Royal, C.D.; Weinrich, S.P. Psychosocial stress and prostate cancer: A theoretical model. Ethn. Dis. 2001, 11, 484–495. [Google Scholar]
- Du, X.L.; Fang, S.; Coker, A.L.; Sanderson, M.; Aragaki, C.; Cormier, J.N.; Xing, Y.; Gor, B.J.; Chan, W. Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma: Findings from a large community-based cohort. Cancer 2006, 106, 1276–1285. [Google Scholar] [CrossRef]
- Moses, K.A.; Orom, H.; Brasel, A.; Gaddy, J.; Underwood, W., 3rd. Racial/Ethnic Disparity in Treatment for Prostate Cancer: Does Cancer Severity Matter? Urology 2017, 99, 76–83. [Google Scholar] [CrossRef]
- Fletcher, S.A.; Marchese, M.; Cole, A.P.; Mahal, B.A.; Friedlander, D.F.; Krimphove, M.; Kilbridge, K.L.; Lipsitz, S.R.; Nguyen, P.L.; Choueiri, T.K.; et al. Geographic Distribution of Racial Differences in Prostate Cancer Mortality. JAMA Netw. Open 2020, 3, e201839. [Google Scholar] [CrossRef]
- Williams, D.R.; Priest, N.; Anderson, N.B. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol. 2016, 35, 407–411. [Google Scholar] [CrossRef]
- Cheng, I.; Witte, J.S.; McClure, L.A.; Shema, S.J.; Cockburn, M.G.; John, E.M.; Clarke, C.A. Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California. Cancer Causes Control 2009, 20, 1431–1440. [Google Scholar] [CrossRef]
- Kilpeläinen, T.P.; Talala, K.; Raitanen, J.; Taari, K.; Kujala, P.; Tammela, T.L.J.; Auvinen, A. Prostate Cancer and Socioeconomic Status in the Finnish Randomized Study of Screening for Prostate Cancer. Am. J. Epidemiol. 2016, 184, 720–731. [Google Scholar] [CrossRef]
- Clegg, L.X.; Reichman, M.E.; Miller, B.A.; Hankey, B.F.; Singh, G.K.; Lin, Y.D.; Goodman, M.T.; Lynch, C.F.; Schwartz, S.M.; Chen, V.W.; et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: Selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control 2009, 20, 417–435. [Google Scholar] [CrossRef]
- Liu, L.; Cozen, W.; Bernstein, L.; Ross, R.K.; Deapen, D. Changing relationship between socioeconomic status and prostate cancer incidence. J. Natl. Cancer Inst. 2001, 93, 705–709. [Google Scholar] [CrossRef]
- Richardus, J.H.; Kunst, A.E. Black-white differences in infectious disease mortality in the United States. Am. J. Public Health 2001, 91, 1251–1253. [Google Scholar] [CrossRef]
- Sorlie, P.; Rogot, E.; Anderson, R.; Johnson, N.J.; Backlund, E. Black-white mortality differences by family income. Lancet 1992, 340, 346–350. [Google Scholar] [CrossRef]
- Davey Smith, G.; Neaton, J.D.; Wentworth, D.; Stamler, R.; Stamler, J. Mortality differences between black and white men in the USA: Contribution of income and other risk factors among men screened for the MRFIT. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Lancet 1998, 351, 934–939. [Google Scholar] [CrossRef]
- United States Census Bureau 2016: American Community Survey. Available online: https://data.census.gov/cedsci/table?tid=ACSDT1Y2016.B02001&q=B02001 (accessed on 24 July 2020).
- Center for American Progress. Center for American Progress Project. 2020. Available online: https://talkpoverty.org/basics/ (accessed on 24 July 2020).
- Percheski, C.; Gibson-Davis, C. A Penny on the Dollar: Racial Inequalities in Wealth among Households with Children. SAGE J. 2020, 6, 1–17. [Google Scholar] [CrossRef]
- Dickman, S.L.; Himmelstein, D.U.; Woolhandler, S. Inequality and the health-care system in the USA. Lancet 2017, 389, 1431–1441. [Google Scholar] [CrossRef]
- Braveman, P.; Egerter, S.; Williams, D.R. The social determinants of health: Coming of age. Annu. Rev. Public Health 2011, 32, 381–398. [Google Scholar] [CrossRef]
- Walker, R.E.; Keane, C.R.; Burke, J.G. Disparities and access to healthy food in the United States: A review of food deserts literature. Health Place 2010, 16, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.D.; Angel, R.J. Neighborhood disorder, psychological distress, and heavy drinking. Soc. Sci. Med. 2005, 61, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Geronimus, A.T.; Hicken, M.; Keene, D.; Bound, J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am. J. Public Health 2006, 96, 826–833. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef]
- Zeigler-Johnson, C.; Weber, A.; Glanz, K.; Spangler, E.; Rebbeck, T.R. Gender- and ethnic-specific associations with obesity: Individual and neighborhood-level factors. J. Natl. Med. Assoc. 2013, 105, 173–182. [Google Scholar] [CrossRef]
- Carpenter, W.R.; Howard, D.L.; Taylor, Y.J.; Ross, L.E.; Wobker, S.E.; Godley, P.A. Racial differences in PSA screening interval and stage at diagnosis. Cancer Causes Control 2010, 21, 1071–1080. [Google Scholar] [CrossRef]
- Lynch, S.M.; Sorice, K.; Tagai, E.K.; Handorf, E.A. Use of empiric methods to inform prostate cancer health disparities: Comparison of neighborhood-wide association study “hits” in black and white men. Cancer 2020, 126, 1949–1957. [Google Scholar] [CrossRef]
- Friedlander, D.F.; Trinh, Q.D.; Krasnova, A.; Lipsitz, S.R.; Sun, M.; Nguyen, P.L.; Kibel, A.S.; Choueiri, T.K.; Weissman, J.S.; Menon, M.; et al. Racial Disparity in Delivering Definitive Therapy for Intermediate/High-risk Localized Prostate Cancer: The Impact of Facility Features and Socioeconomic Characteristics. Eur. Urol. 2018, 73, 445–451. [Google Scholar] [CrossRef]
- Krimphove, M.J.; Fletcher, S.A.; Cole, A.P.; Berg, S.; Sun, M.; Lipsitz, S.R.; Mahal, B.A.; Nguyen, P.L.; Choueiri, T.K.; Kibel, A.S.; et al. Quality of Care in the Treatment of Localized Intermediate and High Risk Prostate Cancer at Minority Serving Hospitals. J. Urol. 2019, 201, 735–741. [Google Scholar] [CrossRef]
- Underwood, W.; De Monner, S.; Ubel, P.; Fagerlin, A.; Sanda, M.G.; Wei, J.T. Racial/ethnic disparities in the treatment of localized/regional prostate cancer. J. Urol. 2004, 171, 1504–1507. [Google Scholar] [CrossRef]
- Hoffman, R.M.; Harlan, L.C.; Klabunde, C.N.; Gilliland, F.D.; Stephenson, R.A.; Hunt, W.C.; Potosky, A.L. Racial differences in initial treatment for clinically localized prostate cancer. Results from the prostate cancer outcomes study. J. Gen. Intern. Med. 2003, 18, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Lairson, D.R.; Swartz, M.D.; Du, X.L. Racial, Socioeconomic, and Geographic Disparities in the Receipt, Timing to Initiation, and Duration of Adjuvant Androgen Deprivation Therapy in Men with Prostate Cancer. J. Racial. Ethn. Health Disparities 2019, 6, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Mahal, B.A.; Chen, Y.W.; Muralidhar, V.; Mahal, A.R.; Choueiri, T.K.; Hoffman, K.E.; Hu, J.C.; Sweeney, C.J.; Yu, J.B.; Feng, F.Y.; et al. Racial disparities in prostate cancer outcome among prostate-specific antigen screening eligible populations in the United States. Ann. Oncol. 2017, 28, 1098–1104. [Google Scholar] [CrossRef]
- Wang, E.H.; Yu, J.B.; Abouassally, R.; Meropol, N.J.; Cooper, G.; Shah, N.D.; Williams, S.B.; Gonzalez, C.; Smaldone, M.C.; Kutikov, A.; et al. Disparities in Treatment of Patients With High-risk Prostate Cancer: Results From a Population-based Cohort. Urology 2016, 95, 88–94. [Google Scholar] [CrossRef]
- Lillie-Blanton, M.; Hoffman, C. The role of health insurance coverage in reducing racial/ethnic disparities in health care. Health Aff. (Millwood) 2005, 24, 398–408. [Google Scholar] [CrossRef]
- Kirby, J.B.; Taliaferro, G.; Zuvekas, S.H. Explaining racial and ethnic disparities in health care. Med. Care 2006, 44, I64–I72. [Google Scholar] [CrossRef]
- Buchmueller, T.C.; Levinson, Z.M.; Levy, H.G.; Wolfe, B.L. Effect of the Affordable Care Act on Racial and Ethnic Disparities in Health Insurance Coverage. Am. J. Public Health 2016, 106, 1416–1421. [Google Scholar] [CrossRef]
- Mahal, A.R.; Mahal, B.A.; Nguyen, P.L.; Yu, J.B. Prostate cancer outcomes for men aged younger than 65 years with Medicaid versus private insurance. Cancer 2018, 124, 752–759. [Google Scholar] [CrossRef]
- Riviere, P.; Luterstein, E.; Kumar, A.; Vitzthum, L.K.; Deka, R.; Sarkar, R.R.; Bryant, A.K.; Bruggeman, A.; Einck, J.P.; Murphy, J.D.; et al. Survival of African American and non-Hispanic white men with prostate cancer in an equal-access health care system. Cancer 2020, 126, 1683–1690. [Google Scholar] [CrossRef]
- Hanson, H.A.; Martin, C.; O’Neil, B.; Leiser, C.L.; Mayer, E.N.; Smith, K.R.; Lowrance, W.T. The Relative Importance of Race Compared to Health Care and Social Factors in Predicting Prostate Cancer Mortality: A Random Forest Approach. J. Urol. 2019, 202, 1209–1216. [Google Scholar] [CrossRef]
- Dash, A.; Lee, P.; Zhou, Q.; Jean-Gilles, J.; Taneja, S.; Satagopan, J.; Reuter, V.; Gerald, W.; Eastham, J.; Osman, I. Impact of socioeconomic factors on prostate cancer outcomes in black patients treated with surgery. Urology 2008, 72, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Powell, I.J.; Heilbrun, L.K.; Sakr, W.; Grignon, D.; Montie, J.; Novallo, M.; Smith, D.; Pontes, J.E. The predictive value of race as a clinical prognostic factor among patients with clinically localized prostate cancer: A multivariate analysis of positive surgical margins. Urology 1997, 49, 726–731. [Google Scholar] [CrossRef]
- Powell, I.J.; Heilbrun, L.; Littrup, P.L.; Franklin, A.; Parzuchowski, J.; Gelfand, D.; Sakr, W. Outcome of African American men screened for prostate cancer: The Detroit Education and Early Detection Study. J. Urol. 1997, 158, 146–149. [Google Scholar] [CrossRef]
- Harris, Y.; Gorelick, P.B.; Samuels, P.; Bempong, I. Why African Americans may not be participating in clinical trials. J. Natl. Med. Assoc. 1996, 88, 630–634. [Google Scholar] [PubMed]
- Wissing, M.D.; Kluetz, P.G.; Ning, Y.M.; Bull, J.; Merenda, C.; Murgo, A.J.; Pazdur, R. Under-representation of racial minorities in prostate cancer studies submitted to the US Food and Drug Administration to support potential marketing approval, 1993–2013. Cancer 2014, 120, 3025–3032. [Google Scholar] [CrossRef]
- Branson, R.D.; Davis, K., Jr.; Butler, K.L. African Americans’ participation in clinical research: Importance, barriers, and solutions. Am. J. Surg. 2007, 193, 32–39. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Harris, Y.; Burnett, B.; Bonecutter, F.J. The recruitment triangle: Reasons why African Americans enroll, refuse to enroll, or voluntarily withdraw from a clinical trial. An interim report from the African-American Antiplatelet Stroke Prevention Study (AAASPS). J. Natl. Med. Assoc. 1998, 90, 141–145. [Google Scholar]
- National Institue of Health. National Institute of Health Revitalization Act of 1993. Available online: https://grants.nih.gov/grants/guide/notice-files/not94-100.html (accessed on 21 August 2020).
- Institute of Medicine; Board on Health Sciences Policy; Committee on the Review and Assessment of the NIH’s Strategic Research Plan and Budget to Reduce and Ultimately Eliminate Health Disparities. The National Academies Collection: Reports funded by National Institutes of Health. In Examining the Health Disparities Research Plan of the National Institutes of Health: Unfinished Business; Thomson, G.E., Mitchell, F., Williams, M.B., Eds.; National Academies Press; National Academy of Sciences: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Murthy, V.H.; Krumholz, H.M.; Gross, C.P. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 2004, 291, 2720–2726. [Google Scholar] [CrossRef]
- Oren, O.; Beach, D.F. Representation of African-American patients in clinical studies of prostate cancer. J. Clin. Oncol. 2016, 34. [Google Scholar] [CrossRef]
- Spratt, D.E.; Osborne, J.R. Disparities in castration-resistant prostate cancer trials. J. Clin. Oncol. 2015, 33, 1101–1103. [Google Scholar] [CrossRef]
- Nightingale, L.; Dumas, M.; Ogagarue, R.; Odedina, F.T.; Warlick, C.; Dahm, P. Participation of black men with prostate cancer: A longitudinal assessment of 25 years (1991–2015) of randomized controlled trials. J. Urol. 2017, 197, e195–e196. [Google Scholar] [CrossRef]
- Wilt, T.J.; Brawer, M.K.; Jones, K.M.; Barry, M.J.; Aronson, W.J.; Fox, S.; Gingrich, J.R.; Wei, J.T.; Gilhooly, P.; Grob, B.M.; et al. Radical prostatectomy versus observation for localized prostate cancer. N. Engl. J. Med. 2012, 367, 203–213. [Google Scholar] [CrossRef] [PubMed]
- George, D.J.; Heath, E.I.; Sartor, A.O.; Sonpavde, G.; Berry, W.R.; Healy, P.; Winters, C.; Riggan, C.; Anand, M.; Kephart, J.; et al. Abi Race: A prospective, multicenter study of black (B) and white (W) patients (pts) with metastatic castrate resistant prostate cancer (mCRPC) treated with abiraterone acetate and prednisone (AAP). J. Clin. Oncol. 2018, 36, LBA5009. [Google Scholar] [CrossRef]
- Schmotzer, G.L. Barriers and facilitators to participation of minorities in clinical trials. Ethn. Dis. 2012, 22, 226–230. [Google Scholar] [PubMed]
- George, S.; Duran, N.; Norris, K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am. J. Public Health 2014, 104, e16–e31. [Google Scholar] [CrossRef]
- Swanson, G.M.; Ward, A.J. Recruiting minorities into clinical trials: Toward a participant-friendly system. J. Natl. Cancer Inst. 1995, 87, 1747–1759. [Google Scholar] [CrossRef]
- Symonds, R.P.; Lord, K.; Mitchell, A.J.; Raghavan, D. Recruitment of ethnic minorities into cancer clinical trials: Experience from the front lines. Br. J. Cancer 2012, 107, 1017–1021. [Google Scholar] [CrossRef]
- Brandon, D.T.; Isaac, L.A.; LaVeist, T.A. The legacy of Tuskegee and trust in medical care: Is Tuskegee responsible for race differences in mistrust of medical care? J. Natl. Med. Assoc. 2005, 97, 951–956. [Google Scholar]
- Gamble, V.N. Under the shadow of Tuskegee: African Americans and health care. Am. J. Public Health 1997, 87, 1773–1778. [Google Scholar] [CrossRef]
- Seto, B. History of medical ethics and perspectives on disparities in minority recruitment and involvement in health research. Am. J. Med. Sci. 2001, 322, 248–252. [Google Scholar] [CrossRef]
- Borad, M.J.; LoRusso, P.M. Twenty-First Century Precision Medicine in Oncology: Genomic Profiling in Patients With Cancer. Mayo Clin. Proc. 2017, 92, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Corbie-Smith, G.; Thomas, S.B.; Williams, M.V.; Moody-Ayers, S. Attitudes and beliefs of African Americans toward participation in medical research. J. Gen. Intern. Med. 1999, 14, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Skloot, R. The Immortal Life of Henrietta Lacks; Crown Publishing Group: New York, NY, USA, 2010. [Google Scholar]
- Caplan, A. NIH Finally Makes Good with Henrietta Lacks’ Family—And It’s about Time, Ethicist Says. Available online: https://www.nbcnews.com/healthmain/nih-finally-makes-good-henrietta-lacks-family-its-about-time-6C10867941 (accessed on 22 September 2020).
- Rogers, C.R.; Rovito, M.J.; Hussein, M.; Obidike, O.J.; Pratt, R.; Alexander, M.; Berge, J.M.; Dall’Era, M.; Nix, J.W.; Warlick, C. Attitudes toward Genomic Testing and Prostate Cancer Research among Black Men. Am. J. Prev. Med. 2018, 55, S103–S111. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.R.; Hodgson, N.A.; Gitlin, L.N. It’s a Matter of Trust: Older African Americans Speak about Their Health Care Encounters. J. Appl. Gerontol. 2016, 35, 1058–1076. [Google Scholar] [CrossRef] [PubMed]
- Quinn, S.C.; Kass, N.E.; Thomas, S.B. Building trust for engagement of minorities in human subjects research: Is the glass half full, half empty, or the wrong size? Am. J. Public Health 2013, 103, 2119–2121. [Google Scholar] [CrossRef]
- Bilodeau, R.; Gilmore, J.; Jones, L.; Palmisano, G.; Banks, T.; Tinney, B.; Lucas, G.I. Putting the “community” into community-based participatory research. A commentary. Am. J. Prev. Med. 2009, 37, S192–S194. [Google Scholar] [CrossRef]
- Jones, R.A.; Sleeves, R.; Williams, I. Strategies for recruiting African American men into prostate cancer screening studies. Nurs. Res. 2009, 58, 452–456. [Google Scholar] [CrossRef]
- Ford, A.F.; Reddick, K.; Browne, M.C.; Robins, A.; Thomas, S.B.; Crouse Quinn, S. Beyond the cathedral: Building trust to engage the African American community in health promotion and disease prevention. Health Promot. Pract. 2009, 10, 485–489. [Google Scholar] [CrossRef]
- Horowitz, C.R.; Brenner, B.L.; Lachapelle, S.; Amara, D.A.; Arniella, G. Effective recruitment of minority populations through community-led strategies. Am. J. Prev. Med. 2009, 37, S195–S200. [Google Scholar] [CrossRef]
- Oren, O.; Oren, M.; Beach, D. On the generalizability of prostate cancer studies: Why race matters. Ann. Oncol. 2016, 27, 2146–2148. [Google Scholar] [CrossRef] [PubMed]
- Woods, V.D.; Montgomery, S.B.; Herring, R.P. Recruiting Black/African American men for research on prostate cancer prevention. Cancer 2004, 100, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Ahaghotu, C.; Tyler, R.; Sartor, O. African American Participation in Oncology Clinical Trials—Focus on Prostate Cancer: Implications, Barriers, and Potential Solutions. Clin. Genitourin Cancer 2016, 14, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Toms, C.; Cahill, F.; George, G.; Van Hemelrijck, M. Research engagement among black men with prostate cancer. Ecancermedicalscience 2016, 10, 695. [Google Scholar] [CrossRef] [PubMed]
- Fracasso, P.M.; Goodner, S.A.; Creekmore, A.N.; Morgan, H.P.; Foster, D.M.; Hardmon, A.A.; Engel, S.J.; Springer, B.C.; Mathews, K.J.; Fisher, E.B.; et al. Coaching intervention as a strategy for minority recruitment to cancer clinical trials. J. Oncol. Pract. 2013, 9, 294–299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haiman, C.A.; Carpten, J.; Conti, D.; Lotan, T.; Sfanos, K.; Huang, F.; DeRouen, M.; Shariff-Marco, S.; Chanock, S.; Modjesk, D.; et al. Research on Prostate Cancer in Men of African Ancestry: Defining the Roles of Genetics, Immunity and Stress (RESPOND). National Cancer Institute, National Institute of Minority Health and Health Disparities of the National Institutes of Health; University Of Southern California: Los Angeles, CA, USA, 2018. [Google Scholar]
- Popejoy, A.B.; Fullerton, S.M. Genomics is failing on diversity. Nature 2016, 538, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Welter, D.; Bowler, E.H.; Cerezo, M.; Harris, L.W.; McMahon, A.C.; Hall, P.; Junkins, H.A.; Milano, A.; Hastings, E.; et al. A standardized framework for representation of ancestry data in genomics studies, with application to the NHGRI-EBI GWAS Catalog. Genome Biol. 2018, 19, 21. [Google Scholar] [CrossRef]
- MADCaP Network Men of African Descent and Carcinoma of the Prostate. Available online: https://www.madcapnetwork.org/ (accessed on 23 July 2020).
- Cooper, R.S. Race in biological and biomedical research. Cold Spring Harb. Perspect. Med. 2013, 3, a008573. [Google Scholar] [CrossRef]
- Sankar, P.; Cho, M.K. Genetics. Toward a new vocabulary of human genetic variation. Science 2002, 298, 1337–1338. [Google Scholar] [CrossRef]
- Hoggart, C.J.; Parra, E.J.; Shriver, M.D.; Bonilla, C.; Kittles, R.A.; Clayton, D.G.; McKeigue, P.M. Control of confounding of genetic associations in stratified populations. Am. J. Hum. Genet. 2003, 72, 1492–1504. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Rosenberg, N.A.; Donnelly, P. Association mapping in structured populations. Am. J. Hum. Genet. 2000, 67, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Nassir, R.; Kosoy, R.; Tian, C.; White, P.A.; Butler, L.M.; Silva, G.; Kittles, R.; Alarcon-Riquelme, M.E.; Gregersen, P.K.; Belmont, J.W.; et al. An ancestry informative marker set for determining continental origin: Validation and extension using human genome diversity panels. BMC Genet. 2009, 10, 39. [Google Scholar] [CrossRef]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef]
- Alexander, D.H.; Lange, K. Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinform. 2011, 12, 246. [Google Scholar] [CrossRef]
- Yudell, M.; Roberts, D.; DeSalle, R.; Tishkoff, S. SCIENCE AND SOCIETY. Taking race out of human genetics. Science 2016, 351, 564–565. [Google Scholar] [CrossRef]
- Caulfield, T.; Fullerton, S.M.; Ali-Khan, S.E.; Arbour, L.; Burchard, E.G.; Cooper, R.S.; Hardy, B.J.; Harry, S.; Hyde-Lay, R.; Kahn, J.; et al. Race and ancestry in biomedical research: Exploring the challenges. Genome Med. 2009, 1, 8. [Google Scholar] [CrossRef]
- Marshall, E. DNA studies challenge the meaning of race. Science 1998, 282, 654–655. [Google Scholar] [CrossRef]
- Burchard, E.G.; Ziv, E.; Coyle, N.; Gomez, S.L.; Tang, H.; Karter, A.J.; Mountain, J.L.; Pérez-Stable, E.J.; Sheppard, D.; Risch, N. The importance of race and ethnic background in biomedical research and clinical practice. N. Engl. J. Med. 2003, 348, 1170–1175. [Google Scholar] [CrossRef]
- Tang, H.; Quertermous, T.; Rodriguez, B.; Kardia, S.L.; Zhu, X.; Brown, A.; Pankow, J.S.; Province, M.A.; Hunt, S.C.; Boerwinkle, E.; et al. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. Am. J. Hum. Genet. 2005, 76, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Banda, Y.; Kvale, M.N.; Hoffmann, T.J.; Hesselson, S.E.; Ranatunga, D.; Tang, H.; Sabatti, C.; Croen, L.A.; Dispensa, B.P.; Henderson, M.; et al. Characterizing Race/Ethnicity and Genetic Ancestry for 100,000 Subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) Cohort. Genetics 2015, 200, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Sucheston, L.E.; Bensen, J.T.; Xu, Z.; Singh, P.K.; Preus, L.; Mohler, J.L.; Su, L.J.; Fontham, E.T.; Ruiz, B.; Smith, G.J.; et al. Genetic ancestry, self-reported race and ethnicity in African Americans and European Americans in the PCaP cohort. PLoS ONE 2012, 7, e30950. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.L.; Teitelbaum, S.; Wolff, M.S.; Wetmur, J.G.; Chen, J. Comparing genetic ancestry and self-reported race/ethnicity in a multiethnic population in New York City. J. Genet. 2010, 89, 417–423. [Google Scholar] [CrossRef]
- Hollenbach, J.A.; Saperstein, A.; Albrecht, M.; Vierra-Green, C.; Parham, P.; Norman, P.J.; Maiers, M. Race, Ethnicity and Ancestry in Unrelated Transplant Matching for the National Marrow Donor Program: A Comparison of Multiple Forms of Self-Identification with Genetics. PLoS ONE 2015, 10, e0135960. [Google Scholar] [CrossRef]
- Wendler, D.; Kington, R.; Madans, J.; Van Wye, G.; Christ-Schmidt, H.; Pratt, L.A.; Brawley, O.W.; Gross, C.P.; Emanuel, E. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006, 3, e19. [Google Scholar] [CrossRef]
- Dovidio, J.F.; Fiske, S.T. Under the radar: How unexamined biases in decision-making processes in clinical interactions can contribute to health care disparities. Am. J. Public Health 2012, 102, 945–952. [Google Scholar] [CrossRef]
- Carnethon, M.R.; Kershaw, K.N.; Kandula, N.R. Disparities Research, Disparities Researchers, and Health Equity. JAMA 2019, 323, 211–212. [Google Scholar] [CrossRef]
- Hoppe, T.A.; Litovitz, A.; Willis, K.A.; Meseroll, R.A.; Perkins, M.J.; Hutchins, B.I.; Davis, A.F.; Lauer, M.S.; Valantine, H.A.; Anderson, J.M.; et al. Topic choice contributes to the lower rate of NIH awards to African-American/black scientists. Sci. Adv. 2019, 5, eaaw7238. [Google Scholar] [CrossRef]
- Ginther, D.K.; Schaffer, W.T.; Schnell, J.; Masimore, B.; Liu, F.; Haak, L.L.; Kington, R. Race, ethnicity, and NIH research awards. Science 2011, 333, 1015–1019. [Google Scholar] [CrossRef]


| Loci | Method | Population(s) | Gene/Markers | References (population) |
|---|---|---|---|---|
| 1p36 | Linkage | European | CAPB | [33] |
| 1q24-25 | Linkage/GWAS | European African American | HPC1 | [26] (Afr Amer) [28] (Eur) [29] (Eur) |
| 1q42-43 | Linkage | French and German European African American | PCAP | [34] (Fra & Germ) [28] (Eur) [36] (Afr Amer) |
| 2p16 | Linkage | African American | rs980481 and rs71527 | [37] |
| 2p21 | Linkage | African American | D2S2259 | [38] |
| 11q22 | Linkage | African American | D11S908 | [38] |
| 12q24 | Linkage | African American | rs11067228 | [37] |
| 17p11 | Linkage | African American | D17S1852 | [38] |
| 17q21 | Linkage/GWAS | European | HOXB13 (G84E) rs138213197 | [23] |
| 20q13 | Linkage | European African American | HPC20 | [28] (Eur) [36] (Afr Amer) |
| Xq21 | Linkage | African American | DXS986 | [38] |
| Xq27-28 | Linkage | Finnish African American | HPCX | [30] (Fin) [36] (Afr Amer) |
| ERLEADA® (Apalutamide) | NUBEQA® (Darolutamide) | AXUMIN® (Fluciclovine) | |
|---|---|---|---|
| FDA approval date | 17 September 2019 | 30 July 2019 | 27 May 2016 |
| Purpose of drug | Treatment of prostate cancer that has not spread to other parts of the body (non-metastatic) and no longer responds to a medical or surgical treatment that lowers testosterone (castration-resistant) | Drug for detection of prostate cancer recurrence in men who have been treated for prostate cancer but have persistently high prostate specific antigen (PSA) in their blood. | |
| Total participants | 1207 | 1509 | 596 |
| Total black participants | 68 | 52 | 26 |
| Total black participants, % | 6% | 3% | 4% |
| Total white participants | 800 | 1194 | 186 |
| Total white participants, % | 66% | 79% | 31% |
| Percentage not reported | 16% | 4% | 64% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, D.D.; Cropp, C.D. The Impact of African Ancestry on Prostate Cancer Disparities in the Era of Precision Medicine. Genes 2020, 11, 1471. https://doi.org/10.3390/genes11121471
Lewis DD, Cropp CD. The Impact of African Ancestry on Prostate Cancer Disparities in the Era of Precision Medicine. Genes. 2020; 11(12):1471. https://doi.org/10.3390/genes11121471
Chicago/Turabian StyleLewis, Deyana D., and Cheryl D. Cropp. 2020. "The Impact of African Ancestry on Prostate Cancer Disparities in the Era of Precision Medicine" Genes 11, no. 12: 1471. https://doi.org/10.3390/genes11121471
APA StyleLewis, D. D., & Cropp, C. D. (2020). The Impact of African Ancestry on Prostate Cancer Disparities in the Era of Precision Medicine. Genes, 11(12), 1471. https://doi.org/10.3390/genes11121471






