Genome-Wide Novel Genic Microsatellite Marker Resource Development and Validation for Genetic Diversity and Population Structure Analysis of Banana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Collection and DNA Extraction
2.2. Data Processing, SSR Mining, Marker Development, In-Silico Characterization, Physical Mapping and Functional Annotation
2.3. In-Silico Comparative Genome Mapping
2.4. Evaluation of PCR Amplification and Genetic Marker Potential
2.5. Data Collection and Statistical Analysis
2.6. Population Structure
2.7. Functional SSR Marker Database
3. Results and Discussion
3.1. FRSM Marker Development, In-Silico Characterization, Chromosomal Distribution, Physical Mapping and Genome Coverage
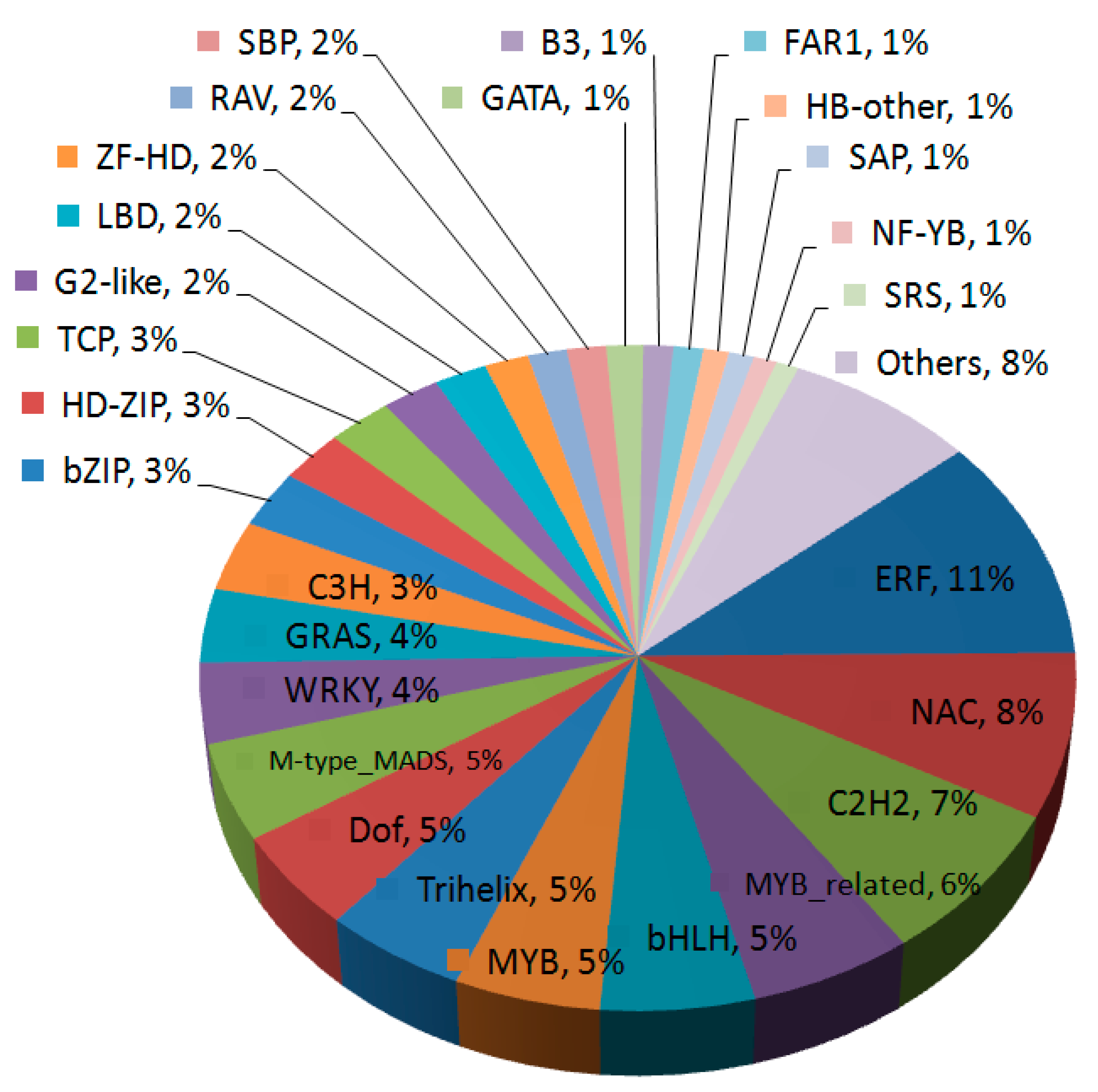
3.2. Functional Annotation and Association of Transcription Factor
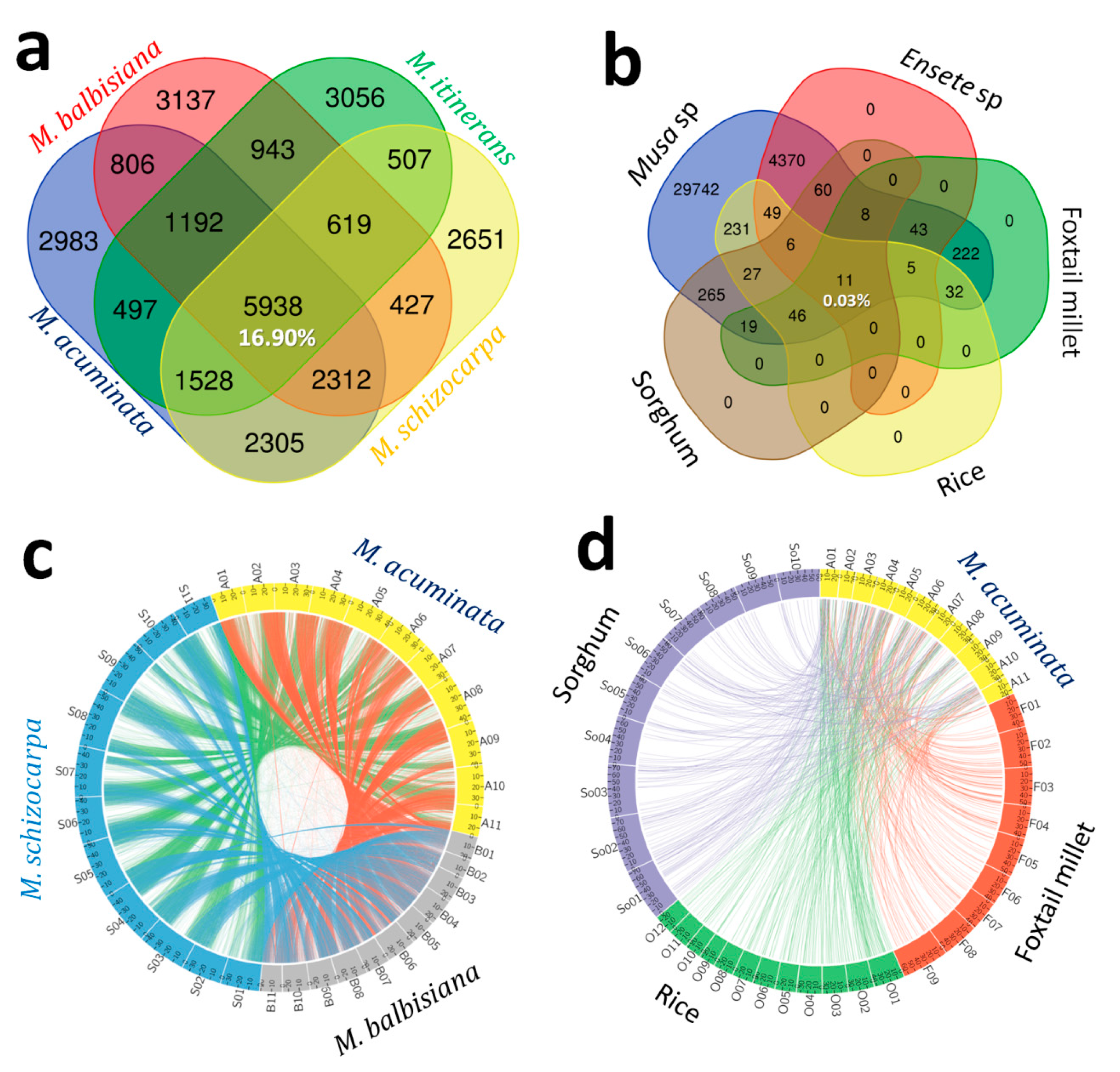
3.3. In-Silico Comparative Genomic Mapping between Musa and Non-Musa spp.
3.4. PCR Amplification Efficiency, Polymorphism, Transferability and Genetic Marker Potential
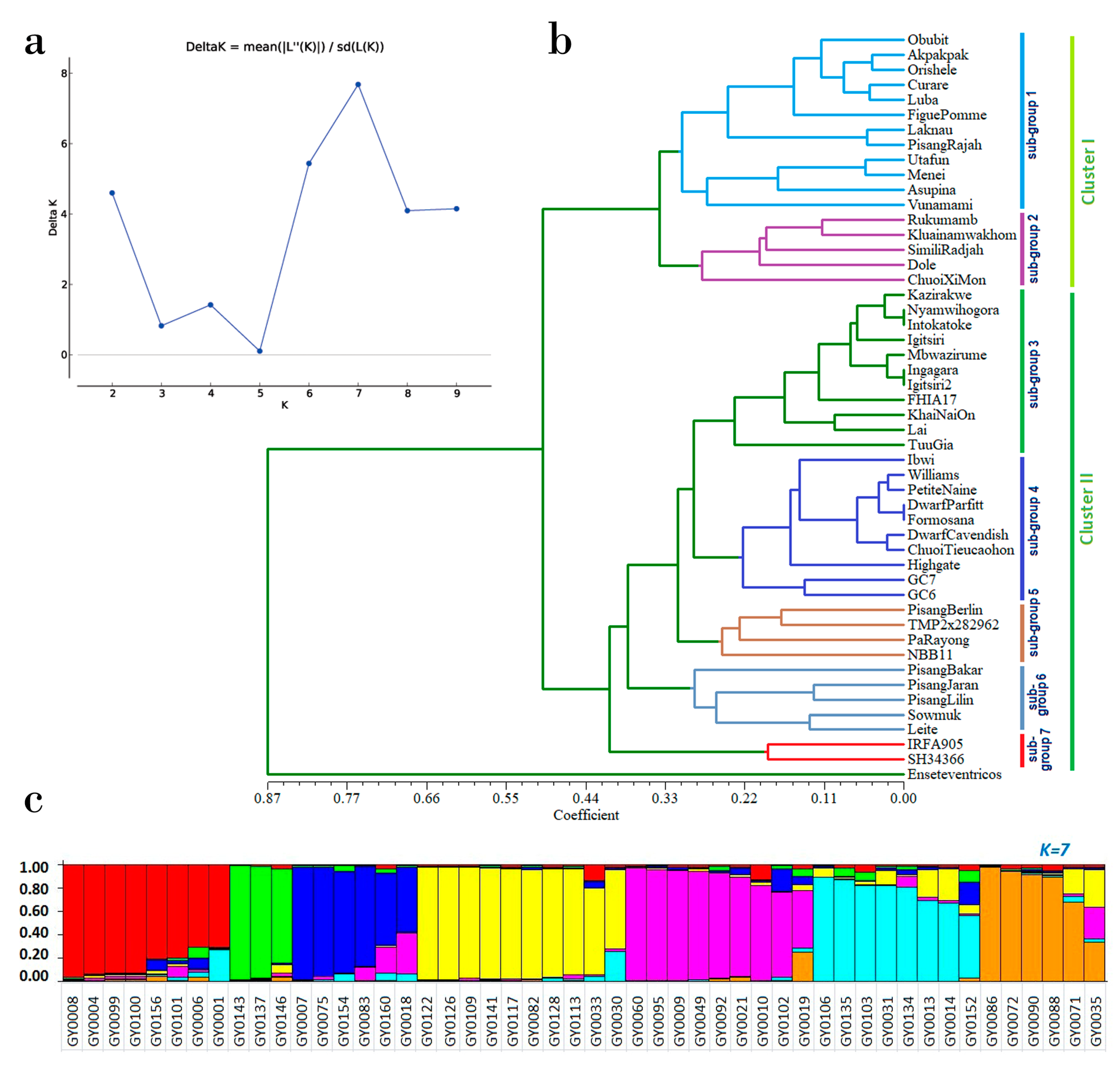
3.5. Assessment of Genetic Diversity and Population Structure
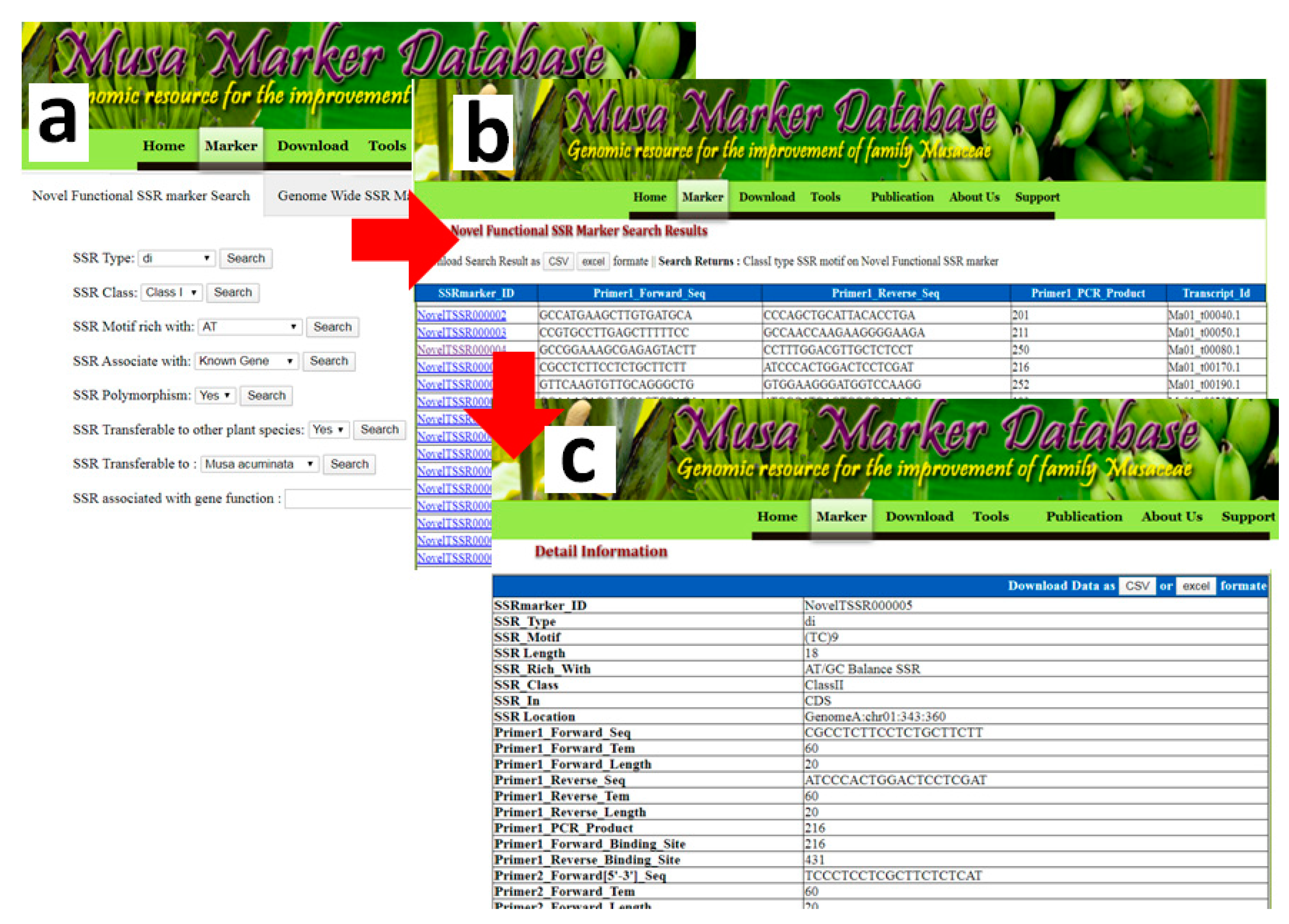
3.6. Novel Functional Musa SSR (NFMS) Marker Database Developments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Banana Market Review; FAO: Rome, Italy, 2020; p. 7. [Google Scholar]
- D’Hont, A.; Paget-Goy, A.; Escoute, J.; Carreel, F. The interspecific genome structure of cultivated banana, Musa spp. revealed by genomic DNA in situ hybridization. Theor. Appl. Genet. 2000, 100, 177–183. [Google Scholar] [CrossRef]
- Parida, S.; Pandit, A.; Gaikwad, K.; Sharma, T.; Srivastava, P.; Singh, N.; Mohapatra, T. Functionally relevant microsatellites in sugarcane unigenes. BMC Plant Biol. 2010, 10, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parida, S.; Dalal, V.; Singh, A.; Singh, N.; Mohapatra, T. Genic non-coding microsatellites in the rice genome: Characterization, marker design and use in assessing genetic and evolutionary relationships among domesticated groups. BMC Genom. 2009, 10, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varshney, R.K.; Graner, A.; Sorrells, M.E. Genic microsatellite markers in plants: Features and applications. Trends Biotechnol. 2005, 23, 48–55. [Google Scholar] [CrossRef]
- Li, Y.-C.; Korol, A.B.; Fahima, T.; Nevo, E. Microsatellites within genes: Structure, function, and evolution. Mol. Biol. Evol. 2004, 21, 991–1007. [Google Scholar] [CrossRef]
- Ramalingam, J.; Savitha, P.; Alagarasan, G.; Saraswathi, R.; Chandrababu, R. Functional marker assisted improvement of stable cytoplasmic male sterile lines of rice for bacterial blight resistance. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Gujaria, N.; Kumar, A.; Dauthal, P.; Dubey, A.; Hiremath, P.; Bhanu Prakash, A.; Farmer, A.; Bhide, M.; Shah, T.; Gaur, P.; et al. Development and use of genic molecular markers (GMMs) for construction of a transcript map of chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2011, 122, 1577–1589. [Google Scholar] [CrossRef] [Green Version]
- Backiyarani, S.; Uma, S.; Varatharj, P.; Saraswathi, M.S. Mining of EST-SSR markers of Musa and their transferability studies among the members of order the Zingiberales. Appl. Biochem. Biotechnol. 2013, 169, 228–238. [Google Scholar] [CrossRef]
- Passos, M.A.; de Oliveira Cruz, V.; Emediato, F.L.; de Camargo Teixeira, C.; Souza, M.T.; Matsumoto, T.; Rennó Azevedo, V.C.; Ferreira, C.F.; Amorim, E.P.; de Alencar Figueiredo, L.F.; et al. Development of expressed sequence tag and expressed sequence tag–simple sequence repeat marker resources for Musa acuminata. AoB Plants 2012, 2012, 1. [Google Scholar] [CrossRef]
- Li, W.J.; Ma, H.; Li, Z.H.; Wan, Y.M.; Liu, X.X.; Zhou, C.L. Thirty-four Musa (Musaceae) expressed sequence tag-derived microsatellite markers transferred to Musella lasiocarpa. Genet. Mol. Res. 2012, 11, 4. [Google Scholar] [CrossRef]
- Passos, M.A.N.; de Cruz, V.O.; Emediato, F.L.; Teixeira, C.C.D.; Miller, R.N. Analysis of the leaf transcriptome of Musa acuminata during interaction with Mycosphaerella musicola: Gene assembly, annotation and marker development. BMC Genom. 2013, 14, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, F.; Town, C.D. A BAC end view of the Musa acuminata genome. BMC Plant Biol. 2007, 7, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R.N.; Passos, M.A.; Menezes, N.N.; Souza, M.T.; do Carmo Costa, M.M.; Azevedo, V.C.R.; Amorim, E.P.; Pappas, G.J.; Ciampi, A.Y. Characterization of novel microsatellite markers in Musa acuminata subsp. burmannicoides, var. Calcutta 4. BMC Res. Notes 2010, 3, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, M.K.; Liu, Y.; Li, C.; Sheng, O.; Mayer, C.; Yi, G. Genome-wide computational analysis of Musa microsatellites: Classification, cross-taxon transferability, functional annotation, association with transposons & miRNAs, and genetic marker potential. PLoS ONE 2015, 10, e0131312. [Google Scholar]
- Christelová, P.; De Langhe, E.; Hřibová, E.; Čížková, J.; Sardos, J.; Hušáková, M.; Sutanto, A.; Kepler, A.K.; Swennen, R.; Roux, N. Molecular and cytological characterization of the global Musa germplasm collection provides insights into the treasure of banana diversity. Biodivers. Conserv. 2017, 26, 801–824. [Google Scholar] [CrossRef]
- Ray, T.; Dutta, I.; Saha, P.; Das, S.; Roy, S. Genetic stability of three economically important micropropagated banana (Musa spp.) cultivars of lower Indo-Gangetic plains, as assessed by RAPD and ISSR markers. Plant Cell Tissue Organ Cult. 2006, 85, 11–21. [Google Scholar] [CrossRef]
- Dhanapal, S.; Sekar, D.S.; Satheesh, P.M. Efficiency of RAPD, SSR and ISSR markers in evaluating the genetic fidelity for micropropagated Musa accuminata plant exposed to coal extracted humic acid and commercially available products. Int. J. Agric. Sci. Res. IJASR 2014, 4, 77–86. [Google Scholar]
- Bhat, K.; Jarret, R. Random amplified polymorphic DNA and genetic diversity in Indian Musa germplasm. Genet. Resour. Crop Evol. 1995, 42, 107–118. [Google Scholar] [CrossRef]
- Venkatachalam, L.; Sreedhar, R.; Bhagyalakshmi, N. Genetic analyses of micropropagated and regenerated plantlets of banana as assessed by RAPD and ISSR markers. Vitro Cell. Dev. Biol. Plant 2007, 43, 267–274. [Google Scholar] [CrossRef]
- Mou, H.; Lin, G.; Zhou, Y.; Li, X.; Li, C.; Wei, H.; Wu, D.; Zhang, J.; Wang, Q.; Pan, Y. Genetic diversity analysis of banana (Musa spp.) based on ISSR molecular marker. Southwest China J. Agric. Sci. 2010, 23, 1206–1210. [Google Scholar]
- Wang, Q.; Qin, Y.; Chen, H.; Hu, G. Establishment of an inter-simple sequence repeats reaction system (ISSR) for banana (Musa AAA). J. South China Agric. Univ. 2010, 31, 13–16. [Google Scholar]
- Khatri, A.; Bibi, S.; Dahot, M.U.; Khan, I.A.; Nizamani, G.S. In vitro mutagenesis in banana and variant screening through ISSR. Pak. J. Bot. 2011, 43, 2427–2431. [Google Scholar]
- Ude, G.; Pillay, M.; Nwakanma, D.; Tenkouano, A. Analysis of genetic diversity and sectional relationships in Musa using AFLP markers. Theor. Appl. Genet. 2002, 104, 6. [Google Scholar] [CrossRef] [PubMed]
- Risterucci, A.M.; Hippolyte, I.; Perrier, X.; Xia, L.; Caig, V.; Evers, M.; Huttner, E.; Kilian, A.; Glaszmann, J.C. Development and assessment of Diversity Arrays Technology for high-throughput DNA analyses in Musa. Theor. Appl. Genet. 2009, 119, 10. [Google Scholar] [CrossRef] [PubMed]
- Till, B.J.; Jankowicz-Cieslak, J.; Sági, L.; Huynh, O.A.; Utsushi, H.; Swennen, R.; Terauchi, R.; Mba, C. Discovery of nucleotide polymorphisms in the Musa gene pool by Ecotilling. Theor. Appl. Genet. 2010, 121, 10. [Google Scholar] [CrossRef] [Green Version]
- D’Hont, A.; Denoeud, F.; Aury, J.-M.; Baurens, F.-C.; Carreel, F.; Garsmeur, O.; Noel, B.; Bocs, S.; Droc, G.; Rouard, M. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 2012, 488, 213. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.; Baurens, F.C.; Droc, G.; Rouard, M.; Cenci, A.; Kilian, A.; Hastie, A.R.; Doležel, J.; Aury, J.; Alberti, A. Improvement of the banana “Musa acuminata” reference sequence using NGS data and semi-automated bioinformatics methods. BMC Genom. 2016, 17, 243. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Miao, H.; Liu, J.; Xu, B.; Yao, X.; Xu, C.; Zhao, S.; Fang, X.; Jia, C.; Wang, J. Musa balbisiana genome reveals subgenome evolution and functional divergence. Nat. Plants 2019, 5, 810–821. [Google Scholar] [CrossRef] [Green Version]
- Belser, C.; Istace, B.; Denis, E.; Dubarry, M.; Baurens, F.-C.; Falentin, C.; Genete, M.; Berrabah, W.; Chèvre, A.-M.; Delourme, R. Chromosome-scale assemblies of plant genomes using nanopore long reads and optical maps. Nat. Plants 2018, 4, 879. [Google Scholar] [CrossRef]
- Wu, W.; Yang, Y.-L.; He, W.-M.; Rouard, M.; Li, W.-M.; Xu, M.; Roux, N.; Ge, X.-J. Whole genome sequencing of a banana wild relative Musa itinerans provides insights into lineage-specific diversification of the Musa genus. Sci. Rep. 2016, 6, 31586. [Google Scholar] [CrossRef]
- Kujur, A.; Bajaj, D.; Saxena, M.S.; Tripathi, S.; Upadhyaya, H.D.; Gowda, C.L.; Singh, S.; Jain, M.; Tyagi, A.K.; Parida, S.K. Functionally relevant microsatellite markers from chickpea transcription factor genes for efficient genotyping applications and trait association mapping. DNA Res. 2013, 20, 355–374. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.-K.; Paik, H.; Choi, J.; Han, J.-H.; Choe, J.-K.; Hur, C.-G. Functional domain marker (FDM): An in silico demonstration in Solanaceae using simple sequence repeats (SSRs). Plant Mol. Biol. Rep. 2010, 28, 352–356. [Google Scholar] [CrossRef]
- Gawel, N.J.; Jarret, R.L. A modified CTAB DNA extraction procedure for Musa and Ipomoea. Plant Mol. Biol. Rep. 1991, 9, 262–266. [Google Scholar] [CrossRef]
- Voorrips, R. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ness, R.W.; Siol, M.; Barrett, S.C. De novo sequence assembly and characterization of the floral transcriptome in cross- and self-fertilizing plants. BMC Genom. 2011, 12, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, C.Y.; Yang, H.; Wei, C.L.; Yu, O.; Zhang, Z.Z.; Jiang, C.J.; Sun, J.; Li, Y.Y.; Chen, Q.; Xia, T.; et al. Deep sequencing of the Camellia sinensis transcriptome revealed candidate genes for major metabolic pathways of tea-specific compounds. BMC Genom. 2011, 12, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.W.; Luan, J.B.; Li, J.M.; Bao, Y.Y.; Zhang, C.X.; Liu, S.S. De novo characterization of a whitefly transcriptome and analysis of its gene expression during development. BMC Genom. 2010, 11, 400. [Google Scholar] [CrossRef] [Green Version]
- Rohlf, F.J. NTSYSpc Numerical Taxonomy and Multivariate Analysis System Version 2.0 User Guide; Applied Biostatistics Inc.: New York, NY, USA, 1998. [Google Scholar]
- Yeh, F.C.; Yang, R.C.; Boyle, T. POPGENE Software Package Version 1.31 for Population Genetic Analysis; University of Alberta: Edmonton, AB, Canada, 1999. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar]
- Earl, D.A. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Rosenberg, N. Documentation for Distruct Software: Version 1.1; University of Michigan: Ann Arbor, MI, USA, 2007. [Google Scholar]
- Smouse, R.P.P.; Peakall, R. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar]
- Thiel, T.; Michalek, W.; Varshney, R.K.; Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Raji, A.; Anderson, J.; Kolade, O.; Ugwu, C.; Dixon, A.; Ingelbrecht, I. Gene-based microsatellites for cassava (Manihot esculenta Crantz): Prevalence, polymorphisms, and cross-taxa utility. BMC Plant Biol. 2009, 9, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poncet, V.; Rondeau, M.; Tranchant, C.; Cayrel, A.; Hamon, S.; de Kochko, A.; Hamon, P. SSR mining in coffee tree EST databases: Potential use of EST–SSRs as markers for the Coffea genus. Mol. Genet. Genom. 2006, 276, 436–449. [Google Scholar] [CrossRef]
- Kantety, R.V.; La Rota, M.; Matthews, D.E.; Sorrells, M.E. Data mining for simple sequence repeats in expressed sequence tags from barley, maize, rice, sorghum and wheat. Plant Mol. Biol. 2002, 48, 501–510. [Google Scholar] [CrossRef]
- Tang, S.; Okashah, R.A.; Cordonnier-Pratt, M.M.; Pratt, L.H.; Ed Johnson, V.; Taylor, C.A.; Arnold, M.L.; Knapp, S.J. EST and EST-SSR marker resources for Iris. BMC Plant Biol. 2009, 9, 72. [Google Scholar] [CrossRef] [Green Version]
- Varshney, R.K.; Thiel, T.; Stein, N.; Langridge, P.; Graner, A. In silico analysis on frequency and distribution of microsatellites in ESTs of some cereal species. Cell Mol. Biol. Lett. 2002, 7, 537–546. [Google Scholar]
- Gao, L.; Tang, J.; Li, H.; Jia, J. Analysis of microsatellites in major crops assessed by computational and experimental approaches. Mol. Breed. 2003, 12, 245–261. [Google Scholar] [CrossRef]
- Wang, J.Y.; Chen, Y.Y.; Liu, W.L.; Wu, Y.T.; Abdullah, M.O. Development and application of EST-derived SSR markers for bananas (Musa nana Lour.). Hereditas 2008, 30. [Google Scholar] [CrossRef]
- Gupta, S.; Kumari, K.; Sahu, P.P.; Vidapu, S.; Prasad, M. Sequence-based novel genomic microsatellite markers for robust genotyping purposes in foxtail millet [Setaria italica (L.) P. Beauv]. Plant Cell Rep. 2012, 31, 323–337. [Google Scholar] [CrossRef]
- Parida, S.K.; Anand Raj Kumar, K.; Dalal, V.; Singh, N.K.; Mohapatra, T. Unigene derived microsatellite markers for the cereal genomes. Theor. Appl. Genet. 2006, 112, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.K.; Chai, L.; Mayer, C.; Xu, Q.; Guo, W.; Deng, X. Exploiting BAC-end sequences for the mining, characterization and utility of new short sequences repeat (SSR) markers in Citrus. Mol. Biol. Rep. 2012, 39, 5373–5386. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.K.; Xu, Q.; Mayer, C.; Deng, X. Genome wide characterization of short tandem repeat markers in sweet orange (Citrus sinensis). PLoS ONE 2014, 9, e104182. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Chen, L.L.; Ruan, X.; Chen, D.; Zhu, A.; Chen, C.; Bertrand, D.; Jiao, W.B.; Hao, B.H.; Lyon, M.P.; et al. The draft genome of sweet orange (Citrus sinensis). Nat. Genet. 2013, 45, 59–66. [Google Scholar] [CrossRef]
- Kalia, R.; Rai, M.; Kalia, S.; Singh, R.; Dhawan, A.K. Microsatellite markers: An overview of the recent progress in plants. Euphytica 2011, 177, 309–334. [Google Scholar] [CrossRef]
- Eujayl, I.; Sledge, M.K.; Wang, L.; May, G.D.; Chekhovskiy, K.; Zwonitzer, J.C.; Mian, M.A.R. Medicago truncatula EST-SSRs reveal cross-species genetic markers for Medicago spp. Theor. Appl. Genet. 2004, 108, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.K.; Chai, L.; Qiang, X.; Deng, X. Generation, functional analysis and utility of Citrus grandis EST from a flower-derived cDNA library. Mol. Biol. Rep. 2012, 39, 7221–7235. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Misra, G.; Kumari, K.; Gupta, S.; Parida, S.K.; Chattopadhyay, D.; Prasad, M. Genome-wide development and use of microsatellite markers for large-scale genotyping applications in foxtail millet [Setaria italica (L.)]. DNA Res. 2013, 20, 197–207. [Google Scholar] [CrossRef]
- Kraemer, L.; Beszteri, B.; Gäbler-Schwarz, S.; Held, C.; Leese, F.; Mayer, C.; Pöhlmann, K.; Frickenhaus, S. S TAMP: Extensions to the S TADEN sequence analysis package for high throughput interactive microsatellite marker design. BMC Bioinform. 2009, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Scott, K.D.; Eggler, P.; Seaton, G.; Rossetto, M.; Ablett, E.M.; Lee, L.S.; Henry, R.J. Analysis of SSRs derived from grape ESTs. Theor. Appl. Genet. 2000, 100, 723–726. [Google Scholar] [CrossRef]
- Dida, M.; Gale , M.; Devos, K. Comparative analyses reveal high levels of conserved colinearity between the finger millet and rice genomes. Theor. Appl. Genet. 2007, 115, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.; Muthamilarasan, M.; Misra, G.; Gupta, S.; Subramanian, A.; Parida, S.K.; Chattopadhyay, D.; Prasad, M. Development of eSSR-markers in Setaria italica and their applicability in studying genetic diversity, cross-transferability and comparative mapping in millet and non-millet species. PLoS ONE 2013, 8, e67742. [Google Scholar] [CrossRef] [PubMed]
- Devos, K.M.; Gale, M.D. Genome relationships: The grass model in current research. Plant Cell 2000, 12, 637–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wei, L.; Miao, H.; Zhang, T.; Wang, C. Development and validation of genic-SSR markers in sesame by RNA-seq. BMC Genomics 2012, 13, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahu, J.; Das Talukdar, A.; Devi, K.; Choudhury, M.D.; Barooah, M.; Modi, M.K.; Sen, P. E-microsatellite markers for Centella asiatica (Gotu Kola) genome: Validation and cross-transferability in Apiaceae family for plant omics research and development. OMICS 2015, 19, 52–65. [Google Scholar] [CrossRef]
- de Jesus, O.N.; Silva Sde, O.; Amorim, E.P.; Ferreira, C.F.; de Campos, J.M.; Silva Gde, G.; Figueira, A. Genetic diversity and population structure of Musa accessions in ex situ conservation. BMC Plant Biol. 2013, 13, 41. [Google Scholar] [CrossRef]
- Nei, M.; Takezaki, N. Estimation of genetic distances and phylogenetic trees from DNA analysis. In Proceedings of the 5th World Congress on Genetics Applied to Livestock Production, University of Guelph, Guelph, ON, Canada, 7–12 August 1983; Volume 21, pp. 405–412. [Google Scholar]
- Ford, M.J. Applications of selective neutrality tests to molecular ecology. Mol. Ecol. 2002, 11, 1245–1262. [Google Scholar] [CrossRef]
- Kim, K.S.; Ratcliffe, S.T.; French, B.W.; Liu, L.; Sappington, T.W. Utility of EST-derived SSRs as population genetics markers in a beetle. J. Hered. 2008, 99, 112–124. [Google Scholar] [CrossRef] [Green Version]
- Ning, S.P.; Xu, L.B.; Lu, Y.; Huang, B.Z.; Ge, X.J. Genome composition and genetic diversity of Musa germplasm from China revealed by PCR-RFLP and SSR markers. Sci. Hortic. Amst. 2007, 114, 281–288. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- Jayashree, B.; Punna, R.; Prasad, P.; Bantte, K.; Hash, C.T.; Chandra, S.; Hoisington, D.A.; Varshney, R.K. A database of simple sequence repeats from cereal and legume expressed sequence tags mined in silico: Survey and evaluation. Silico Biol. 2006, 6, 607–620. [Google Scholar]
- Zhao, Z.; Guo, C.; Sutharzan, S.; Li, P.; Echt, C.S.; Zhang, J.; Liang, C. Genome-wide analysis of tandem repeats in plants and green algae. G3 Genes Genomes Genet. 2014, 4, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iquebal, M.A.; Arora, V.; Verma, N.; Rai, A.; Kumar, D. First whole genome based microsatellite DNA marker database of tomato for mapping and variety identification. BMC Plant Biol. 2013, 13, 197. [Google Scholar] [CrossRef] [Green Version]
- Doddamani, D.; Katta, M.A.; Khan, A.W.; Agarwal, G.; Shah, T.M.; Varshney, R.K. CicArMiSatDB: The chickpea microsatellite database. BMC Bioinform. 2014, 15, 212. [Google Scholar] [CrossRef] [Green Version]
- Arora, V.; Iquebal, M.; Rai, A.; Kumar, D. PIPEMicroDB: Microsatellite database and primer generation tool for pigeonpea genome. Database 2013, 2013, bas054. [Google Scholar] [CrossRef]
- Arora, V.; Kapoor, N.; Fatma, S.; Jaiswal, S.; Iquebal, M.A.; Rai, A.; Kumar, D. BanSatDB, a whole-genome-based database of putative and experimentally validated microsatellite markers of three Musa species. Crop J. 2018, 6, 642–650. [Google Scholar] [CrossRef]

| Characters under Study | Unigenes | % |
|---|---|---|
| Total number of sequences examined | 52,453 | |
| Total size of examined sequences (bp) | 359,005,912 | |
| Total number of identified SSRs | 166,503 | |
| Number of SSR-containing sequences | 51,814 | |
| Number of sequences containing > 1 SSR | 30,780 | |
| Number of SSRs present in compound form | 47,796 | |
| SSR density (1 SSR per bp) | 2156 | |
| Class II SSRs (12–20 nucleotides) | 99,231 | 59.60 |
| Class I SSRs (>20 nucleotides) | 67,272 | 40.40 |
| AT rich SSRs | 112,814 | 67.75 |
| GC rich SSRs | 17,073 | 10.25 |
| AT/GC balance | 36,616 | 21.99 |
| Mononucleotide repeats | 52,440 | 31.49 |
| Dinucleotide repeats | 85,090 | 51.10 |
| Trinucleotide repeats | 24,330 | 14.61 |
| Tetranucleotide repeats | 2884 | 1.73 |
| Pentanucleotide repeats | 413 | 0.25 |
| Hexanucleotide repeats | 763 | 0.46 |
| Heptanucleotide repeats | 180 | 0.11 |
| Octanucleotide repeats | 238 | 0.14 |
| Nanonucleotide repeats | 28 | 0.02 |
| Decanucleotide repeats | 137 | 0.08 |
| No. of primers designed | 80,899 | 71.29 |
| Non-redundant primers | 66,246 | 81.89 |
| Novel SSR primer identified | 35,136 | 43.43 |
| TF-associated SSRs | 1104 | 1.67 |
| Transferable to other Musa sp. | 18,209 | 51.82 |
| Genome specific | 16,927 | 48.18 |
| In-silico polymorphic | 8605 | 24.49 |
| No. of SSRs in CDS | 13,223 | 37.63 |
| No. of SSRs in 5′ UTR | 11,718 | 33.35 |
| No. of SSRs in 3′ UTR | 10,099 | 28.74 |
| Genome | No of Markers Mapped | Density (1 Primer/Mb) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A | (%) | B | (%) | S | (%) | A | B | S | |
| Chr1 | 1176 | 3.3 | 1227 | 3.5 | 1098 | 3.1 | 24.72 | 17.96 | 32.80 |
| Chr2 | 1215 | 3.5 | 1276 | 3.6 | 1151 | 3.3 | 24.29 | 13.60 | 32.35 |
| Chr3 | 1731 | 4.9 | 1155 | 3.3 | 1639 | 4.7 | 20.23 | 20.92 | 27.33 |
| Chr4 | 2203 | 6.3 | 1001 | 2.8 | 2080 | 5.9 | 16.84 | 24.63 | 23.67 |
| Chr5 | 1566 | 4.5 | 1512 | 4.3 | 1478 | 4.2 | 26.73 | 15.64 | 35.19 |
| Chr6 | 1982 | 5.6 | 1547 | 4.4 | 1649 | 4.7 | 18.97 | 17.99 | 26.06 |
| Chr7 | 1464 | 4.2 | 1276 | 3.6 | 1377 | 3.9 | 23.93 | 17.41 | 34.13 |
| Chr8 | 1824 | 5.2 | 1567 | 4.5 | 1696 | 4.8 | 24.61 | 17.66 | 32.35 |
| Chr9 | 1486 | 4.2 | 1232 | 3.5 | 1428 | 4.1 | 27.80 | 21.02 | 36.93 |
| Chr10 | 1498 | 4.3 | 1564 | 4.5 | 1443 | 4.1 | 25.15 | 16.13 | 30.01 |
| Chr11 | 1258 | 3.6 | 1204 | 3.4 | 1170 | 3.3 | 22.22 | 17.21 | 31.42 |
| ChrUn | 158 | 0.4 | 812 | 2.3 | 77 | 0.2 | |||
| Total Mapped | 17,561 | 50.0 | 15,373 | 43.8 | 16,286 | 46.4 | |||
| Unmapped | 17,575 | 50.0 | 19,763 | 56.2 | 18,850 | 53.6 | |||
| Musa Chromosomes | Foxtail Millet Chromosomes | Rice Chromosomes | Sorghum Chromosomes |
|---|---|---|---|
| Chr 01 | F03 (10; 42%) | R01 (13; 42%) | S01 (11; 41%) |
| Chr 02 | F04 (3; 23%) | R01 (5; 25%) | S01 (6; 29%) |
| Chr 03 | F09 (4; 16%) | R01 (5; 16%) | S02 (9; 27%) |
| Chr 04 | F03 (9; 27%) | R01 (13; 46%) | S02 (7; 21%) |
| Chr 05 | F02 (6; 24%) | R01 (5; 25%) | S06 (4; 20%) |
| Chr 06 | F08 (5; 22%) | R03 (6; 20%) | S02 (6; 21%) |
| Chr 07 | F02 (5; 28%) | R01 (6; 30%) | S03 (5; 24%) |
| Chr 08 | F04 (6; 19%) | R01 (8; 27%) | S01 (6; 23%) |
| Chr 09 | F04 (3; 21%) | R07 (4; 16%) | S01 (3; 18%) |
| Chr 10 | F04 (6; 33%) | R02 (4; 24%) | S01 (5; 19%) |
| Chr 11 | F09 (5; 33%) | R01 (3; 25%) | S04 (4; 27%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, M.K.; Bagchi, M.; Biswas, D.; Harikrishna, J.A.; Liu, Y.; Li, C.; Sheng, O.; Mayer, C.; Yi, G.; Deng, G. Genome-Wide Novel Genic Microsatellite Marker Resource Development and Validation for Genetic Diversity and Population Structure Analysis of Banana. Genes 2020, 11, 1479. https://doi.org/10.3390/genes11121479
Biswas MK, Bagchi M, Biswas D, Harikrishna JA, Liu Y, Li C, Sheng O, Mayer C, Yi G, Deng G. Genome-Wide Novel Genic Microsatellite Marker Resource Development and Validation for Genetic Diversity and Population Structure Analysis of Banana. Genes. 2020; 11(12):1479. https://doi.org/10.3390/genes11121479
Chicago/Turabian StyleBiswas, Manosh Kumar, Mita Bagchi, Dhiman Biswas, Jennifer Ann Harikrishna, Yuxuan Liu, Chunyu Li, Ou Sheng, Christoph Mayer, Ganjun Yi, and Guiming Deng. 2020. "Genome-Wide Novel Genic Microsatellite Marker Resource Development and Validation for Genetic Diversity and Population Structure Analysis of Banana" Genes 11, no. 12: 1479. https://doi.org/10.3390/genes11121479
APA StyleBiswas, M. K., Bagchi, M., Biswas, D., Harikrishna, J. A., Liu, Y., Li, C., Sheng, O., Mayer, C., Yi, G., & Deng, G. (2020). Genome-Wide Novel Genic Microsatellite Marker Resource Development and Validation for Genetic Diversity and Population Structure Analysis of Banana. Genes, 11(12), 1479. https://doi.org/10.3390/genes11121479







