The Reproductive Journey in the Genomic Era: From Preconception to Childhood
Abstract
:1. Introduction
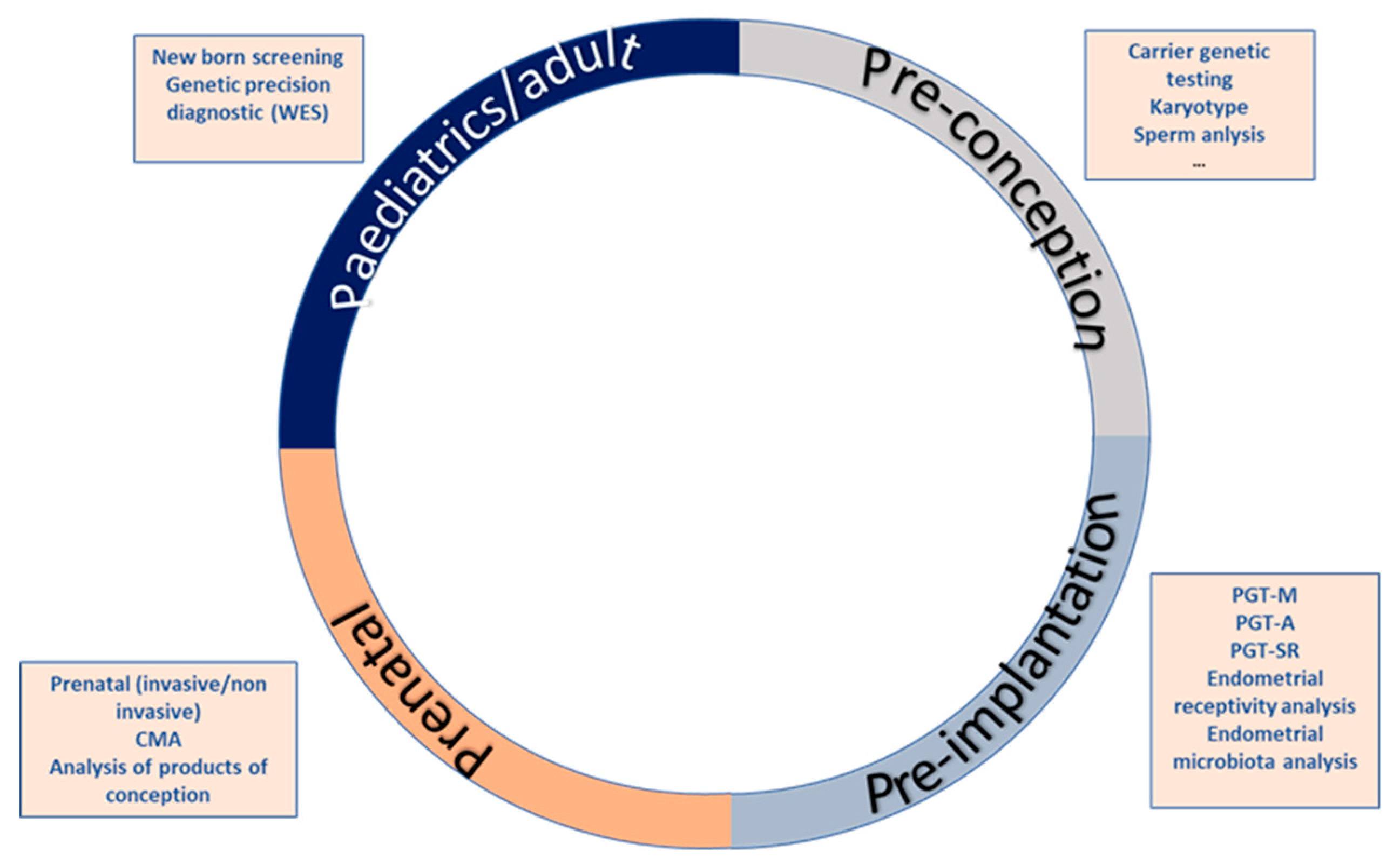
2. The Reproductive Journey
2.1. First Stage: Pre-Conceptional Care
- An evaluation of the overall well-being
- Medical history
- Surgical history
- Social and behavioral history
- Medication
- Occupational and education risks
2.2. Second Stage: Pre-Implantation Diagnosis
- Multiplex PCR (Polymerase Chain Reaction): Multiplex PCR uses targeted primers designed specifically for the mutation of interest combined with other markers for linked short tandem repeat (STR) markers.
- Whole-genome amplification (WGA).
- Karyomapping: High-density SNP (Single Nucleotide Polymorphism) array that allows evaluation of DNA haplotypes).
- Sanger sequencing.
- Multiplex Ligation-dependent Probe Amplification (MLPA) [4].
2.3. Third Stage: Prenatal Diagnostis
- A variety of phenotypes.
- Multiple malformations.
- Congenital anomalies.
- Intellectual disabilities.
- Developmental delay.
- Epilepsy.
- Cerebral palsy.
- Neuropsychiatric disorders [23].
- Cell culture growth failure (the failure rate in POC samples cultured after curettage ranges between 5% and 42% [35]).
- Suboptimal chromosome preparations.
- Maternal cell contamination (MCC).
- Low-resolution limit that does not allow the detection of submicroscopic deletions and duplications that can cause miscarriages.
2.4. Fourth Step: Newborn Screening and Neonatal Care
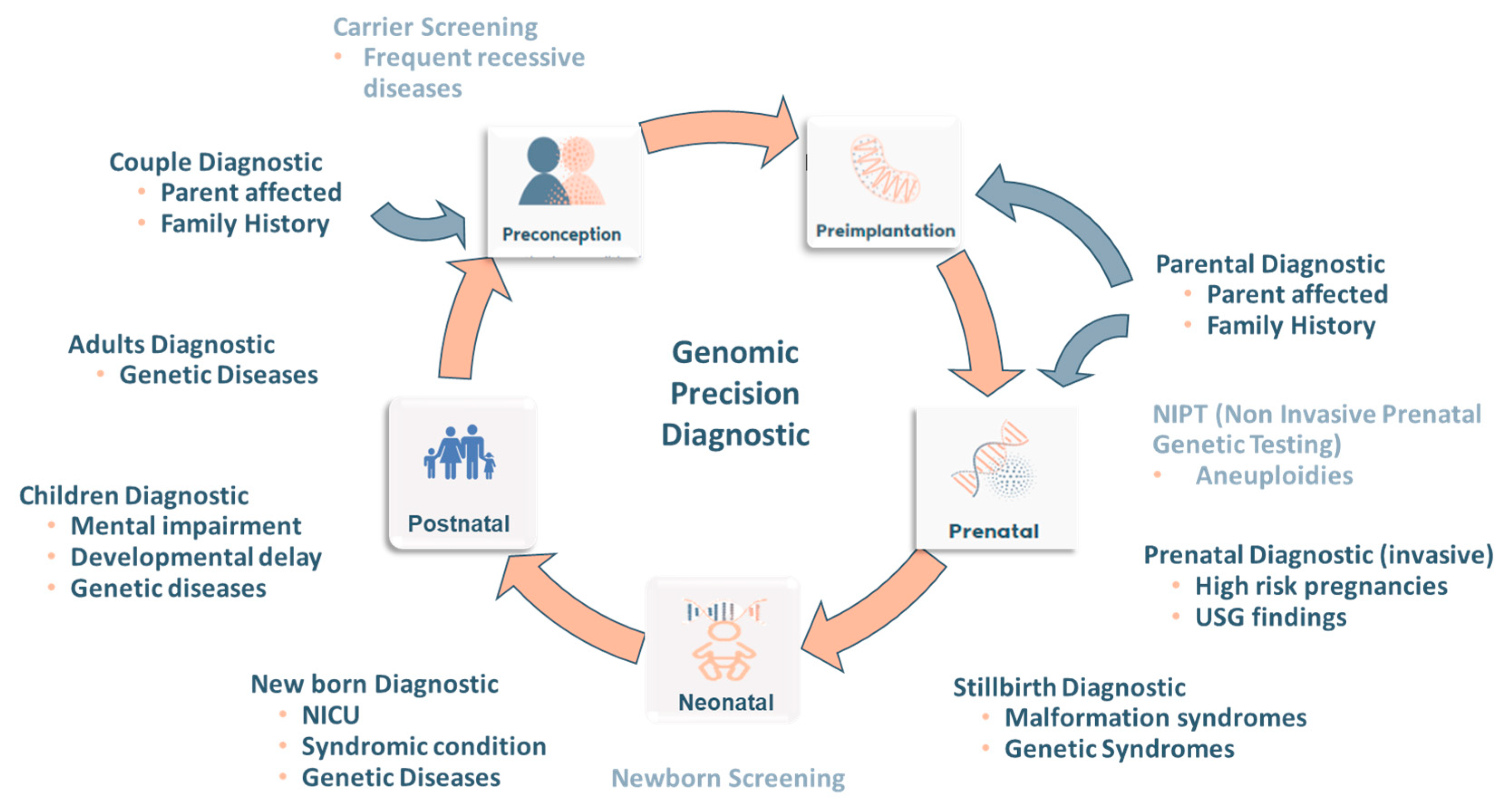
3. Genomic Precision Diagnostic
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fowler, J.R.; Mahdy, H.; Jack, B.W. Preconception Counseling; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Gao, Z.; Waggoner, D.; Stephens, M.; Ober, C.; Przeworski, M. An estimate of the average number of recessive lethal mutations carried by humans. Genetics 2015, 199, 1243–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henneman, L.; Borry, P.; Chokoshvili, D.; Cornel, M.C.; van El, C.G.; Forzano, F.; Hall, A.; Howard, H.C.; Janssens, S.; Kayserili, H.; et al. Responsible implementation of expanded carrier screening. Eur. J. Hum. Genet. 2017, 25, 1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overcoming Challenges in Reproductive Health Applications by Deploying More Sensitive and Accurate Molecular Technologies. EMJ Repro Health 2019, 5 (Suppl. 1), 2–12.
- Rubio, C.; Bellver, J.; Rodrigo, L.; Castillón, G.; Guillén, A.; Vidal, C.; Giles, J.; Ferrando, M.; Cabanillas, S.; Remohí, J.; et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: A randomized, controlled study. Fertil. Steril. 2017, 107, 1122–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Herrero, S.; Cervero, A.; Mateu, E.; Mir, P.; Póo, M.E.; Rodrigo, L.; Vera, M.; Rubio, C. Genetic Analysis of Human Preimplantation Embryos. Curr. Top. Dev. Biol. 2016, 120, 421–447. [Google Scholar] [PubMed]
- Vera-Rodriguez, M.; Rubio, C. Assessing the true incidence of mosaicism in preimplantation embryos. Fertil. Steril. 2017, 107, 1107–1112. [Google Scholar]
- Hardarson, T.; Hanson, C.; Lundin, K.; Hillensjö, T.; Nilsson, L.; Stevic, J.; Reismer, E.; Borg, K.; Wikland, M.; Bergh, C. Preimplantation genetic screening in women of advanced maternal age caused a decrease in clinical pregnancy rate: A randomized controlled trial. Hum. Reprod. 2008, 23, 2806–2812. [Google Scholar] [CrossRef] [Green Version]
- Staessen, C.; Verpoest, W.; Donoso, P.; Haentjens, P.; Van der Elst, J.; Liebaers, I.; Devroey, P. Preimplantation genetic screening does not improve delivery rate in women under the age of 36 following single-embryo transfer. Hum. Reprod. 2008, 23, 2818–2825. [Google Scholar] [CrossRef] [Green Version]
- Northrop, L.E.; Treff, N.R.; Levy, B.; Scott, R.T. SNP microarray-based 24 chromosome aneuploidy screening demonstrates that cleavage-stage FISH poorly predicts aneuploidy in embryos that develop to morphologically normal blastocysts. Mol. Hum. Reprod. 2010, 16, 590–600. [Google Scholar]
- Treff, N.R.; Levy, B.; Su, J.; Northrop, L.E.; Tao, X.; Scott, R.T. SNP microarray-based 24 chromosome aneuploidy screening is significantly more consistent than FISH. Mol. Hum. Reprod. 2010, 16, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Rubio, C.; Navarro-Sánchez, L.; García-Pascual, C.M.; Ocali, O.; Cimadomo, D.; Venier, W.; Barroso, G.; Kopcow, L.; Bahçeci, M.; Iuri Roos Kulmann, M.; et al. Multicenter prospective study of concordance between embryonic cell-free DNA and trophectoderm biopsies from 1301 human blastocysts. Am. J. Obstet. Gynecol. 2020, 223, 751.e1–751.e13. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.R.; Arrach, N.; Rhodes-Long, K.; Ahmady, A.; Ingles, S.; Chung, K.; Bendikson, K.A.; Paulson, R.J.; McGinnis, L.K. Pushing the limits of detection: Investigation of cell-free DNA for aneuploidy screening in embryos. Fertil. Steril. 2018, 110, 467–475.e2. [Google Scholar] [CrossRef] [PubMed]
- Simón, C.; Gómez, C.; Cabanillas, S.; Vladimirov, I.; Castillón, G.; Giles, J.; Boynukalin, K.; Findikli, N.; Bahçeci, M.; Ortega, I.; et al. A 5-year multicentre randomized controlled trial comparing personalized, frozen and fresh blastocyst transfer in IVF. Reprod. Biomed. Online 2020, 41, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Grau, I.; Perez-Villaroya, D.; Bau, D.; Gonzalez-Monfort, M.; Vilella, F.; Moreno, I.; Simón, C. Taxonomical and Functional Assessment of the Endometrial Microbiota in A Context of Recurrent Reproductive Failure: A Case Report. Pathogens 2019, 8, 205. [Google Scholar] [CrossRef] [Green Version]
- Generalitat de Catalunya Departamento de Salud. Protocolo de diagnóstico prenatal de anomalías congénitas fetales. Maternidad 2009, 24, 1–43.
- Nicolini, U.; Lalatta, F.; Natacci, F.; Curcio, C.; Bui, T.-H. The introduction of QF-PCR in prenatal diagnosis of fetal aneuploidies: Time for reconsideration. Hum. Reprod. Update 2004, 10, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Bayani, J.; Squire, J.A. Traditional banding of chromosomes for cytogenetic analysis. Curr. Protoc. Cell. Biol. 2004, 23, 22–23. [Google Scholar] [CrossRef]
- Midtrimester amniocentesis for prenatal diagnosis. Safety and accuracy. JAMA 1976, 236, 1471–1476. [Google Scholar] [CrossRef]
- Badeau, M.; Lindsay, C.; Blais, J.; Nshimyumukiza, L.; Takwoingi, Y.; Langlois, S.; Légaré, F.; Giguère, Y.; Turgeon, A.F.; Witteman, W.; et al. Genomics-based non-invasive prenatal testing for detection of fetal chromosomal aneuploidy in pregnant women. Cochrane Database Syst. Rev. 2017, 11, CD011767. [Google Scholar] [CrossRef]
- Rosenfeld, J.A.; Patel, A. Chromosomal Microarrays: Understanding Genetics of Neurodevelopmental Disorders and Congenital Anomalies. J. Pediatr. Genet. 2017, 6, 42–50. [Google Scholar]
- Stosic, M.; Levy, B.; Wapner, R. The Use of Chromosomal Microarray Analysis in Prenatal Diagnosis. Obstet. Gynecol. Clin. 2017, 45, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.T.; Adam, M.P.; Aradhya, S.; Biesecker, L.G.; Brothman, A.R.; Carter, N.P.; Church, D.M.; Crolla, J.A.; Eichler, E.E.; Epstein, C.J.; et al. Consensus statement: Chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am. J. Hum. Genet. 2010, 86, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.; Wapner, R. Prenatal diagnosis by chromosomal microarray analysis. Fertil. Steril. 2018, 109, 201–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, P.A.; Hassold, T.J. 4 The origin of numerical chromosome abnormalities. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 1995; Volume 33, pp. 101–133. [Google Scholar]
- Hassold, T.; Chen, N.; Funkhouser, J.; Jooss, T.; Manuel, B.; Matsuura, J.; Matsuyama, A.; Wilson, C.; Yamane, J.A.; Jacobs, P.A. A cytogenetic study of 1000 spontaneous abortions. Ann. Hum. Genet. 1980, 44, 151–178. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L. Incidence and timing of pregnancy losses: Relevance to evaluating safety of early prenatal diagnosis. Am. J. Med. Genet. 1990, 35, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Boué, J.; Bou, A.; Lazar, P. Retrospective and prospective epidemiological studies of 1500 karyotyped spontaneous human abortions. Teratology 1975, 12, 11–26. [Google Scholar] [CrossRef]
- Kajii, T.; Ferrier, A.; Niikawa, N.; Takahara, H.; Ohama, K.; Avirachan, S. Anatomic and chromosomal anomalies in 639 spontaneous abortuses. Hum. Genet. 1980, 55, 87–98. [Google Scholar] [CrossRef]
- Dimmick, J.E.; Kalousek, D.K. Developmental pathology of the embryo and fetus. Am. J. Clin. Pathol. 1992, 98. [Google Scholar] [CrossRef]
- Reddy, K.S. Double trisomy in spontaneous abortions. Hum. Genet. 1997, 101, 339–345. [Google Scholar] [CrossRef]
- Nagaishi, M.; Yamamoto, T.; Iinuma, K.; Shimomura, K.; Berend, S.A.; Knops, J. Chromosome abnormalities identified in 347 spontaneous abortions collected in Japan. J. Obstet. Gynaecol. Res. 2004, 30, 237–241. [Google Scholar] [CrossRef]
- Yusuf, R.Z.; Naeem, R. Cytogenetic Abnormalities in Products of Conception: A Relationship Revisited. Am. J. Reprod. Immunol. 2004, 52, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Morales, C.; Sánchez, A.; Bruguera, J.; Margarit, E.; Borrell, A.; Borobio, V.; Soler, A. Cytogenetic study of spontaneous abortions using semi-direct analysis of chorionic villi samples detects the broadest spectrum of chromosome abnormalities. Am. J. Med. Genet. Part A 2008, 146A, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Robberecht, C.; Pexsters, A.; Deprest, J.; Fryns, J.P.; D’Hooghe, T.; Vermeesch, J.R. Cytogenetic and morphological analysis of early products of conception following hystero-embryoscopy from couples with recurrent pregnancy loss. Prenat. Diagn. 2012, 32, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, M.; Liu, Q.Y.; Hu, S.Q.; Li, L.R.; Li, J.; Ma, R.M. Detecting trisomy in products of conception from first-trimester spontaneous miscarriages by next-generation sequencing (NGS). Medicine 2020, 99, e18731. [Google Scholar] [CrossRef]
- Campos-Galindo, I.; García-Herrero, S.; Martínez-Conejero, J.A.; Ferro, J.; Simón, C.; Rubio, C. Molecular analysis of products of conception obtained by hysteroembryoscopy from infertile couples. J. Assist. Reprod. Genet. 2015, 32, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Kingsmore, S. Comprehensive carrier screening and molecular diagnostic testing for recessive childhood diseases. PLoS Curr. 2012, 4, e4f9877ab8ffa9. [Google Scholar] [CrossRef]
- Ceyhan-Birsoy, O.; Machini, K.; Lebo, M.S.; Yu, T.W.; Agrawal, P.B.; Parad, R.B.; Holm, I.A.; McGuire, A.; Green, R.C.; Beggs, A.H.; et al. A curated gene list for reporting results of newborn genomic sequencing. Genet. Med. 2017, 19, 809–818. [Google Scholar]
- Godino, L.; Turchetti, D.; Jackson, L.; Hennessy, C.; Skirton, H. Impact of presymptomatic genetic testing on young adults: A systematic review. Eur. J. Hum. Genet. 2016, 24, 496–503. [Google Scholar] [CrossRef] [Green Version]
- Van Campen, J.C.; Sollars, E.S.A.; Thomas, R.C.; Bartlett, C.M.; Milano, A.; Parker, M.D.; Dawe, J.; Winship, P.R.; Peck, G.; Grafham, D.; et al. Next Generation Sequencing in Newborn Screening in the United Kingdom National Health Service. Int. J. Neonatal Screen. 2019, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Beckmann, J.S.; Lew, D. Reconciling evidence-based medicine and precision medicine in the era of big data: Challenges and opportunities. Genome Med. 2016, 8, 134. [Google Scholar] [CrossRef] [Green Version]
- Mackley, M.P.; Fletcher, B.; Parker, M.; Watkins, H.; Ormondroyd, E. Stakeholder views on secondary findings in whole-genome and whole-exome sequencing: A systematic review of quantitative and qualitative studies. Genet. Med. 2017, 19, 283–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Marmiesse, A.; Gouveia, S.; Couce, M.L. NGS Technologies as a Turning Point in Rare Disease Research, Diagnosis and Treatment. Curr. Med. Chem. 2018, 25, 404–432. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Herrero, S.; Simon, B.; Garcia-Planells, J. The Reproductive Journey in the Genomic Era: From Preconception to Childhood. Genes 2020, 11, 1521. https://doi.org/10.3390/genes11121521
Garcia-Herrero S, Simon B, Garcia-Planells J. The Reproductive Journey in the Genomic Era: From Preconception to Childhood. Genes. 2020; 11(12):1521. https://doi.org/10.3390/genes11121521
Chicago/Turabian StyleGarcia-Herrero, Sandra, Blanca Simon, and Javier Garcia-Planells. 2020. "The Reproductive Journey in the Genomic Era: From Preconception to Childhood" Genes 11, no. 12: 1521. https://doi.org/10.3390/genes11121521
APA StyleGarcia-Herrero, S., Simon, B., & Garcia-Planells, J. (2020). The Reproductive Journey in the Genomic Era: From Preconception to Childhood. Genes, 11(12), 1521. https://doi.org/10.3390/genes11121521





