Abstract
Behavior is a readout of neural function. Therefore, any difference in behavior among different species is, in theory, an outcome of interspecies diversification in the structure and/or function of the nervous system. However, the neural diversity underlying the species-specificity in behavioral traits and its genetic basis have been poorly understood. In this article, we discuss potential neural substrates for species differences in the courtship pulse song frequency and mating partner choice in the Drosophila melanogaster subgroup. We also discuss possible neurogenetic mechanisms whereby a novel behavioral repertoire emerges based on the study of nuptial gift transfer, a trait unique to D. subobscura in the genus Drosophila. We found that the conserved central circuit composed primarily of fruitless-expressing neurons (the fru-circuit) serves for the execution of courtship behavior, whereas the sensory pathways impinging onto the fru-circuit or the motor pathways downstream of the fru-circuit are susceptible to changes associated with behavioral species differences.
1. Introduction
Every animal species displays a unique behavioral pattern, which often allows us to distinguish one species from others at a glance, even when the morphological characteristics of the species are highly similar. Such species-specific behavioral patterns may in turn lead to morphological changes as in Darwin’s finches, where diversified feeding preference likely drove the evolution of a variety of beak shapes among closely related species with distinct feeding habits [1]. As Konrad Lorenz inferred from the latent down-up courtship action in some species of goose, species-specific gain or loss of a discrete behavioral action could arise from a subtle change in gene expression [2], yet we know very little about the molecular genetic basis of this process. In Drosophila, cross-species comparative studies on mating behavior have highlighted remarkable diversities in courtship rituals among not only species belonging to distant clades but also those within the same species complex [3]. The best-studied element in the ritual is the male courtship song generated by wing vibration, the temporal structure of which varies depending on species [4]. However, some species produce songs by vibrating body parts other than wings [5] or by moving the substrates they sit on [6,7]. In yet another species, both females and males coordinately sing [4,8,9]; such coordinated singing typically occurs before copulation (premounting song), although there are also fly species in which individuals sing after copulation with or without a premounting song [10]. Therefore, both qualitative and quantitative differences in singing behavior account for a rich variety in courtship rituals.
Behavior represents an ensemble of motor outputs produced by dedicated neural circuits. Thus, any change in a behavioral pattern is a consequence of alterations in the structure (wiring) and/or function (physiology) of the neural circuit underlying the behavior [11]. Therefore, a deep understanding of the circuit basis for a given behavior is a prerequisite for gaining evolutionary insights into the mechanistic underpinnings of behavioral diversification. Drosophila melanogaster offers an unparalleled model system for untangling the complex wiring of neurons in the brain for anatomical and functional tracing of a circuit; its suitability is due to the availability of techniques and tools for labeling and manipulating single neurons, and to the large body of accumulated genetic resources specific to this species [12,13,14,15]. In particular, the architecture of circuits for courtship behavior has been revealed in some depth in this species, partly due to the discovery of the fruitless (fru) gene, which orchestrates the courtship circuit formation as a master regulator transcriptional factor gene [16,17,18,19]. Comparative studies of the fru-labeled courtship circuit have opened an avenue for exploring the latent neural evolution underlying diversification in courtship rituals of Drosophila species.
In this article, we focused on three aspects of courtship behavior: first, the neural basis for species differences in song characteristics among the members of the D. melanogaster subgroup; second, the neural basis for conspecific preference in mating partner choice between D. melanogaster and its sibling species D. simulans; third, the neural basis for nuptial gift transfer as a courtship ritual evolved exclusively in D. subobscura, a distant relative of D. melanogaster. Studies on these three traits represent exceptional cases in which neuronal groups responsible for the evolutionary remodeling of courtship rituals have been elucidated in some detail. Additionally, the species difference in these traits shows different levels of diversification, ranging from a quantitative difference in sensorimotor characteristics for a behavioral element shared by the sibling species to a qualitative difference due to the recruitment of a new repertoire to the courtship ritual only in select species.
2. D. melanogaster Male Courtship Ritual
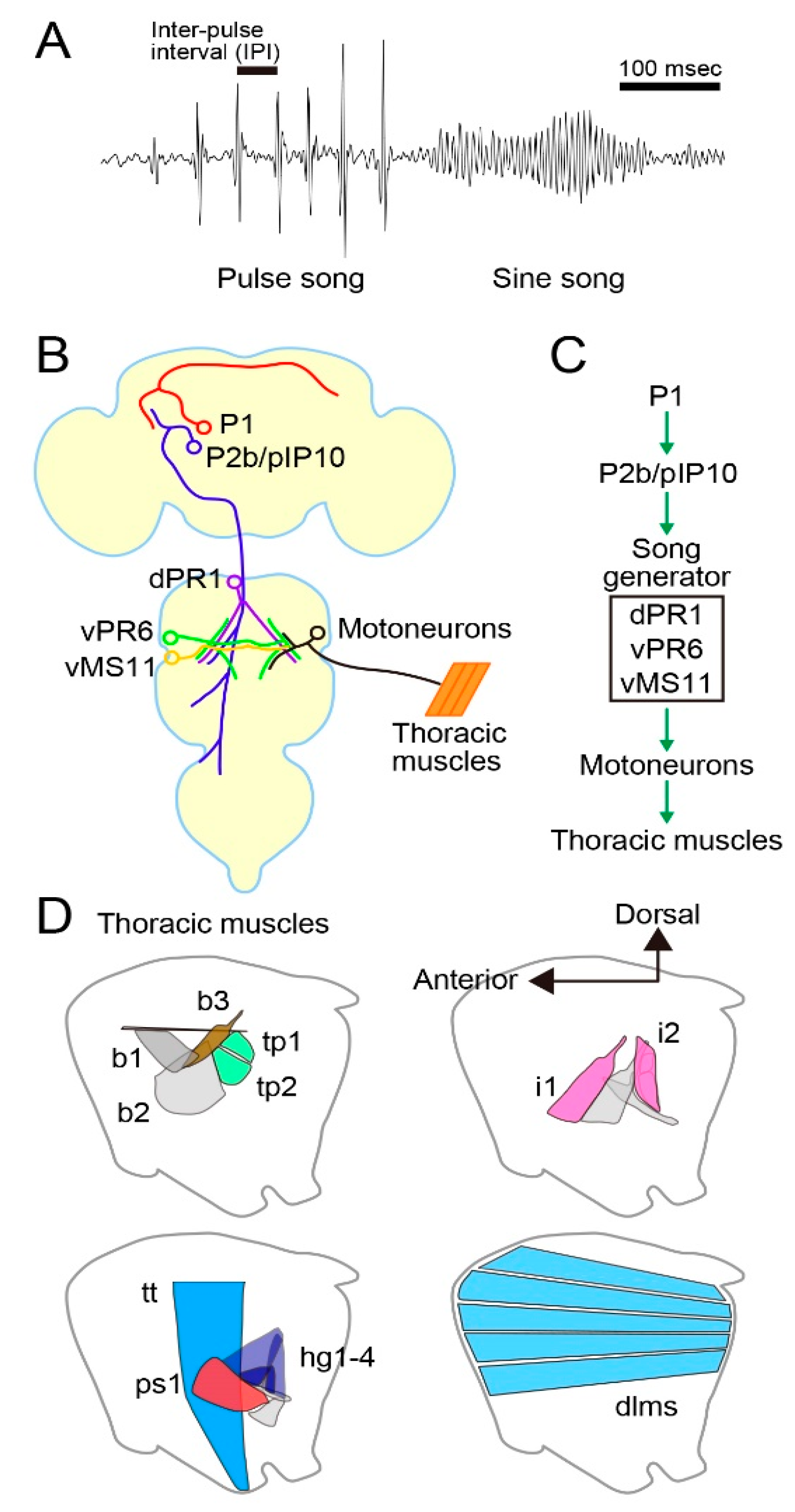
D. melanogaster male mating behavior comprises several discrete motor acts, including orientation of the body axis toward a target female, chasing her from behind, tapping her abdomen, circling around her, wing extension and vibration for courtship song generation, approaching and licking her genitalia, attempting to mount her with abdominal bending, mounting, and copulation [20,21]. Among these, courtship song has been intensively studied as it is a species-specific trait that is amenable to rigorous quantitative comparisons. Traditionally, the courtship song in D. melanogaster has been categorized into two types, i.e., pulse song and sine song: the pulse song is composed of a series of tone pulses with an average inter-pulse interval (IPI) of ~35 ms, whereas the sine song represents oscillatory tones with a mean peak frequency of ~230 Hz at room temperature [22] (Figure 1A). Both pulse and sine songs promote female acceptance of mating [22,23], and pulse song also elicits courtship activities in other males, presumably by acting as an anticipatory cue for the presence of the female [24,25]. The pulse song IPI varies from species to species [26,27], and flies respond to artificial pulse songs that have characteristics of conspecific songs [28], including the pulse song IPI [29]. Thus, the IPI of pulse song seems to convey the information of species identity of the singer. A recent intense analysis of courtship songs in D. melanogaster led to the distinction of two types of pulse song, fast pulse song (Pfast) and slow pulse song (Pslow), based on the differences in their time course and shape [30]. A male generates Pfast by fully extending a wing (~60°) when he is apart from a target female. In contrast, the male more often generates Pslow by weakly extending a wing (~20°) upon approaching and coming into proximity of the female [30].
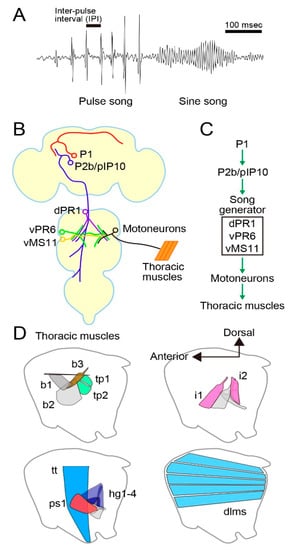
Figure 1.
Neural and motor systems for courtship song generation in Drosophila melanogaster. (A) Song structure. (B, C) Neural components for song production (B) and their hierarchical organization (C). (D) Major muscles for song generation.
3. Circuit Basis for Courtship Behavior in D. melanogaster
The P1 cluster, a male-specific interneuron group capable of initiating male courtship behavior (Figure 1B), was identified by clonal sexual transformation of these neurons in the brain of females: more than 80% of “females” carrying this 20 cell cluster (sexually mosaic flies harboring the male-specific P1 cluster) in their brain performed some male courtship actions to court other females, including orientation, following, and unilateral wing extension and vibration [31]. P1 neurons express fru and the other sex-determinant transcription factor gene, doublesex (dsx). P1 neurons are confined within the brain, densely innervating the lateral protocerebrum (lpr) of both hemispheres by forming a thick midline fiber bundle called the “arch” [31,32]. The ipsilateral branches contribute to the “ring” and “lateral crescent”, two prominent neurite fascicles, in which multiple fru-positive neurons intermingle [32]. A subsequent mosaic analysis with males used a warmth-sensitive dTrpA1 channel minigene to selectively activate, by heating, clonal neurons that express this minigene. This attempt successfully identified P1 neurons, along with an additional cluster called P2b (Figure 1B), as cells that can induce courtship in a solitary male upon an increase in ambient temperature beyond 30 °C [33]. The second experiment using males mosaic for dTrpA1 did identify P2b as a neural cluster with the ability to induce courtship behavior, but the first experiment with sexually mosaic “females” did not so identify P2b. What was the reason for this discrepancy? In the first experiment, natural stimuli derived from a target female ultimately activated neurons that make the decision to court a target. In the second experiment, in contrast, an experimenter activated the neurons by artificially increasing the temperature. We infer that P2b neurons are unable to make the decision to court, but rather must be activated by other neurons, e.g., P1 neurons, to trigger courtship behavior. Indeed, dendritic arbors of P2b neurons are superimposed on branches of P1 neurons in the lpr, suggesting that synaptic contacts exist between them. P2b neurons extend long, descending axons down to the thoracic ganglia, where presumptive motor pattern generators for courtship behavior exist. It is therefore likely that P1 neurons make the decision to court and the decision is conveyed to the motor center via P2b neurons.
Another study achieved activation of subsets of fru-expressing neurons by an intersectional approach with the combinatorial use of fru-neuron-specific flippase (fruFLP), flappable dTrpA1 (UAS>STOP>dTrpA1), and GAL4 driver transgenes [34]. This study also identified P1 and a pair of descending neurons named pIP10, likely a member of P2b, as neurons that can initiate male courtship singing (Figure 1B). Other studies identified the dsx-positive pC2l cluster [35] and the fru-positive aSP22 descending neurons [36] as neurons activating licking and attempted copulation, two later-phase components of mating behavior, in addition to following pursuits and singing.
Von Philipsborn [34] also identified a few intrinsic neurons important for song patterning in the thoracic ganglia (Figure 1B). For example, dTrpA1-mediated activation of vPR6 interneurons shortened the IPIs of pulse songs, and vMS11 interneurons induced, upon artificial activation, unilateral wing extension with no wing vibration, whereas, when silenced, pulse song (normally with a single peak, i.e., monocyclic pulse) was distorted to have multiple peaks (polycyclic pulse). A subsequent study employed Ca2+ imaging of muscle activities and neural silencing during courtship to characterize motor units composed of several motoneurons and their target muscles that contribute to song generation [37]. According to this work, overlapping motor units are activated during pulse and sine songs, yet pulse song production requires recruitments of additional motor units (for an alternative view, see [38]). Thus, the entire motor pathway for the generation of courtship song, from the courtship decision-making center to the muscles that mechanically move individual body parts for courtship actions, has been described in D. melanogaster (Figure 1C,D). These findings in D. melanogaster provide a solid basis for unraveling precisely where in the circuit homologous among different Drosophila species the divergence exists that may account for species differences in courtship behavior.
4. Neural Basis for Species-Specific Song Characteristics
Although the overall pattern of courtship behaviors is conserved among the members of the D. melanogaster subgroup, the song characteristics vary widely. Among nine species examined, three species lacked sine song, and pulse song was absent from one species [39]. Some species had two forms of pulse and/or sine songs, whereas others had only one type of pulse and sine songs [39]. The amplitude (loudness) of songs and the shape and IPI of pulse songs are distinctly different from species to species. Ding et al. [39] attempted a comparative approach of the fru-labeled neural pathway to deduce which neural component is responsible for song differences between D. melanogaster and D. yakuba. D. yakuba males generate two forms of pulse song, called clack song and thud song, but do not generate sine song. The clack song is produced by simultaneous vibration of two wings and contains higher carrier frequency components over 400 Hz, in contrast to the thud song, which is generated by unilateral wing vibration and has a carrier frequency of ~200 Hz. Clack song is greater in amplitude than thud song, and more often generated when the target female is distantly located from the courting male [39]. This parallels the case of D. melanogaster, which also has two forms of pulse song: Pfast is louder than Pslow and is typically produced when the male is courting at a distance [30]. In D. melanogaster, selective activation of pIP10 neurons via a split-GAL4 intersection primarily evoked Pfast, and much less often Pslow and sine songs [30]. Ding et al. [39] introduced split-GAL4 constructs developed for D. melanogaster into the D. yakuba genome, and succeeded in selectively visualizing and manipulating a pIP10 homolog in this species. It turned out that while pIP10 in D. yakuba shared structural, electrical, and neurochemical properties with the D. melanogaster counterpart, optogenetic activation of D. yakuba pIP10 initiated predominantly (but not exclusively) clack song. This observation led them to propose that the command fiber role of pIP10 is conserved in two species: pIP10 preferentially triggers the large amplitude song type with a faster time course for long distance calls in both D. melanogaster and D. yakuba, i.e., pulse song (Pfast in particular) and clack song, respectively [39]. However, the structure and frequency of induced songs differ between the two species, suggesting that the pIP10 neuron connects with the motor center that specifies quantitative song characteristics inherent to each species. Which neurons in the motor center are responsible for the differences in quantitative parameters of courtship songs? This critical question remains unsolved.
5. Genetic Basis for Species-Specificity in Courtship Song Characteristics
Extensive searches for genes that potentially drove the song diversification among closely related species of Drosophila have been carried out by interspecific quantitative trait loci (QTL) analysis [40]. For example, the maleless (mle), fru, and croaker (cro) genes were implicated in the IPI difference between D. simulans and D. sechellia in the D. melanogaster species subgroup [41]. The Mle protein is an RNA helicase essential for doubling the transcriptional activity of the sole X-chromosome present in males so as to equalize the amounts of X-derived mRNAs in males with that in females with two X chromosomes, i.e., gene dosage compensation [42]. The no action potential (nap) allele of mle fails in nerve conduction at a restrictive temperature [43], accompanying prolongation of pulse song IPIs [44]. These behavioral phenotypes possibly result from disrupted splicing of the paralytic (para) Na+ channel gene [45], which may reduce fidelity in action potential firing in the courtship song circuit. The cro gene encodes the calmodulin-binding transcription factor (Camta) and displays multiple defects in mating behavior, including prolongation of pulse song IPIs [46,47]. The fru gene encodes a series of male-specific FruM transcription factor proteins that function as master regulatorw of male courtship circuit formation [19]. fru mutations affect a range of courtship behavioral elements, likely because the male courtship circuit forms through mutual connections among fru-expressing neurons. Indeed, fru mutant males devoid of functional FruM almost never sing courtship songs, and hypomorphic fru mutant males sing aberrant songs [48]. Nonetheless, cross-species transplantation of the intact genomic fru locus including the presumptive regulatory region as well as all exons and introns using BAC recombineering and P[acman] transgenesis did not affect species-specific song patterns: the host D. melanogaster male which received a non-melanogaster fru locus continued to produce courtship song characteristic of D. melanogaster, regardless of whether he was a mutant for fru and/or dsx [49]. This observation is not surprising, however, in view of the fact that even females can generate courtship-song-like sounds if fru-positive neurons in the ventral nerve cord [50] or the pC1 cluster, a female homolog of the male P1 cluster in the brain [51], are artificially activated. Because females have no FruM protein, these observations could suggest that FruM protein might be dispensable for fru-positive neurons to form a fundamental circuit required for courtship song generation. FruM might be important just for optimizing the male circuit to court conspecific females. There is an alternative explanation for these apparently conflicting observations, however. In the cross-species fru-locus transplantation experiment by Cande [49], males were required to sense female-derived stimuli to initiate courtship, whereas in the experiment where females were forced to sing, neurons were directly activated without any sensory involvement [50,51]. It would be of interest to examine, using Cande’s flies, whether the song characteristics would differ from those of the host song if the neurons were directly stimulated via the heterospecific fru promoter introduced into the host genome in the absence of a target female. Natural sensory stimuli could preferentially activate the endogenous fru song motor circuit, and the coexisting heterologous fru song motor circuit formed by an exogenous fru promoter transplanted from a different species could remain silent. In this case, the manipulated fly would produce a song inherent to the host species and not produce a song of the donor species.
A more recent study attempted to unravel the genetic basis for the quantitative difference in sine song between D. simulans and D. mauritiana [52]. QTL analysis and multiplexed shotgun genotyping of F1 hybrids and their backcrossed progeny with the D. simulans strain sim5 and the D. mauritiana strain mau29 were performed. The result pointed to a retrovirus insertion into the slowpoke (slo) locus of sim5, which appeared to impair splicing of slo transcripts and ultimately reduce the carrier frequency of sine song (as much as ~10Hz) in sim5 relative to that in mau29 [52]. The slo locus encodes a Ca2+ activated K+ channel also known as big K or KCa1.1 [53], the loss of which results in hyperexcitability of nerve and muscle [54]. However, subsequent examination of sine-song carrier frequencies in 12 independent isolates for each of D. simulans and D. mauritiana revealed that the carrier frequency is highly variable among individuals within both species, and the difference in this trait found between sim5 and mau29 fell into the range of intraspecies variations [52]. This study provided insights into the possible source of song variations among fly populations, yet the identification of genes responsible for species differences in song characteristics remains a major challenge for future studies.
6. Conspecific Partner Preference Involves Species-Specific Neural Connections
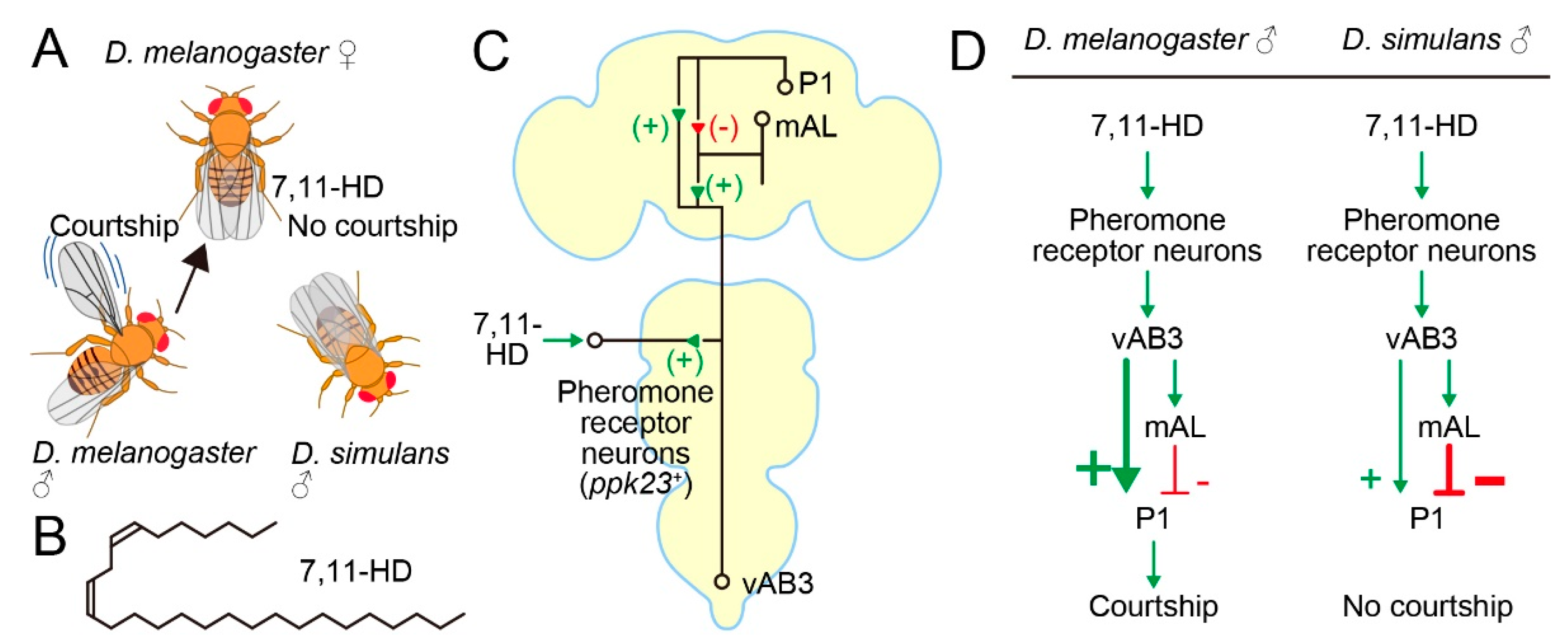
D. simulans males prefer D. simulans females over D. melanogaster females as mating partners (Figure 2A). This conspecific preference by D. simulans males is in part due to the melanogaster-female-specific pheromone 7,11-heptacosadiene (7,11-HD) (Figure 2B), which acts as an aphrodisiac for D. melanogaster males [55] and at the same time as a repellant for D. simulans males [56]. 7,11-HD is a hydrocarbon component of cuticle and is perceived by the tarsal gustatory receptor neurons that express pickpocket 23 (ppk23) and ppk25, two ENaC channel genes (Figure 2C) [57,58]. Kohatsu [33] demonstrated that the courtship decision-making P1 neurons in a test melanogaster male tethered on a treadmill were excited when he touched the abdomen of a conspecific female but not a conspecific male with his tarsus. Therefore, the contact-chemical pheromone information received at the tarsi is sent to P1 neurons. Kohatsu and Yamamoto [35] later showed that P1 neurons were recurrently activated throughout courtship pursuits by the male.
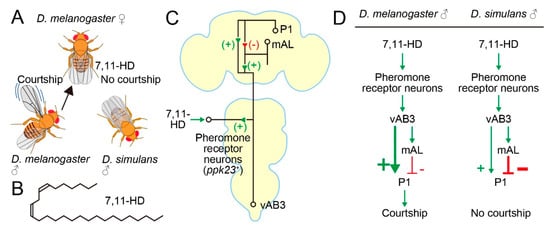
Figure 2.
Circuit basis for the conspecific preference in mate choice. (A) D. melanogaster female-specific pheromone 7,11-HD attracts and repels male D. melanogaster and male D. simulans, respectively. (B) Structure of 7,11-HD. (C) A pathway through which the 7,11-HD information reaches to P1 neurons. + and – denote excitatory and inhibitory connections, respectively. (D) Proposed species differences in the pheromone processing pathway. Thick lines indicate predominant pathways.
Subsequent studies showed that the 7,11-HD-induced excitatory input arrives at P1 across two synapses through an intervening ascending interneuron, i.e., fru-positive vAB3 [59] or fru-negative PPN1 [60] (Figure 2C). Notably, these studies identified an additional route for the 7,11-HD information to P1 neurons. This second pathway was mediated by fru-positive mAL interneurons [59,60], which were previously shown to be GABAergic [61] (Figure 2C). mAL neurons are also known to be postsynaptic to Gr32a gustatory neurons that respond to another pheromone substance, 7-tricosene (7-T), in males but presumably not females, because sexually dimorphic mAL dendritic trees [62] seem to contact the G32a sensory axon terminals only in males [61]. 7-T is more abundant in males than females of D. melanogaster, and in fact 7-T acts as a repellant for males [63]. In contrast, both females and males of D. simulans contain a large amount of 7-T. In line with their GABAergic nature, mAL neurons exert an inhibitory effect on P1 neurons when artificially activated or activated by an afferent pathway stimulation. Thus, sensing 7,11-HD at the periphery not only excited but also inhibited the courtship decision-making P1 neurons, although the ultimate outcome of the convergence of these two seemingly conflicting inputs was to induce excitation in P1 under the experimental conditions of these studies [59,60] (Figure 2C). mAL neurons might function as a gain adjuster for P1 activation, since mAL neurons receive both attractant and repellant stimuli from potential mating partners and thus are likely capable of evaluating the nature of the target. Notably, olfactory inputs initiated at the Or67d-expressing neurons that are responsive to the inhibitory male pheromone cis-Vaccenyl acetate cVA [64] are similarly sent through parallel excitatory and inhibitory pathways to P1 [59]. In addition to the processed sensory inputs from pheromone receptor neurons, internal states affect P1 activities via excitatory dopaminergic [65] and inhibitory NPFergic modulatory signals [66,67]: dopamine attenuates mAL-mediated inhibition to elevate P1 activities [68].
If the opposite responses to 7,11-HD are indeed the primary cause for the conspecific preference in mating partner selection in D. melanogaster and D. simulans, then the pathway connecting ppk23/25-expressing pheromone receptor neurons and P1 neurons is the most promising site in which to search for interspecies differences in the neural substrate for mating behavior. Indeed, Seeholzer [69] first confirmed, by generating ppk23 mutants in D. simulans, that males of this species lost the conspecific preference for a mate when they lost functional ppk23. They then introduced a P1-specific GAL4 construct commonly used in D. melanogaster into the D. simulans genome, making it possible to record P1 Ca2+ activities and to optogenetically stimulate P1 in D. simulans. When P1 neurons of D. simulans were irradiated with strong light stimuli, these males robustly displayed indiscriminative courtship toward both D. simulans and D. melanogaster females, as was expected if D. simulans P1 neurons had a courtship-initiating ability as do D. melanogaster P1 neurons [69]. However, neither D. simulans females nor D. melanogaster females induced increases in Ca2+ in D. simulans male P1 neurons when the test D. simulans male touched the female abdomen [69], in sharp contrast to the robust Ca2+ increases in D. melanogaster P1 neurons observed when the test melanogaster male touched a conspecific female abdomen [33,69]. Notably, peripheral ppk23 pheromone receptor neurons responded to 7,11-HD and induced activities in vAB3 even in D. simulans [69]. Based on these observations, Seeholzer [69] suggested that D. simulans males do not rely on pheromones in initiating courtship, although ppk23-responsive sensory neurons likely form functional synapses on the vAB3 neuron (see also Reference [70]). Although direct stimulation of vAB3 neurons by acetylcholine iontophoresis induced clear Ca2+ rises in these neurons in both D. simulans and D. melanogaster males, little response was elicited in P1 in D. simulans, unlike in D. melanogaster, in which obvious Ca2+ increases were evoked upon vAB3 stimulation, revealing a remarkable decline in the ability of vAB3 to activate P1 in D. simulans compared to D. melanogaster [69]. Seeholzer [69] proposed a model in which inhibition of P1 neuron activities imposed by input from mAL neurons are stronger in D. simulans than in D. melanogaster, such that vAB3 neurons are unable to trigger courtship even when vAB3 neurons become active by incoming 7,11-HD-induced afferent signals (Figure 2D).
To our knowledge, the present work is the sole successful case of a species-difference in behavioral responses being traced down to the functional divergence of a neural circuit at the identified neuron level. It remains an open question how the mAL neurons of D. simulans achieve more efficient inhibition of P1 neuron activities than their D. melanogaster counterparts. Unraveling the molecular entities contributing to the species difference in vAB3-P1 signaling efficacy may provide clues to the identity of the causative genes that drove neural divergence in the mate choice circuits between D. simulans and D. melanogaster.
7. Nuptial Gift Transfer as a Novel Component of the Courtship Ritual
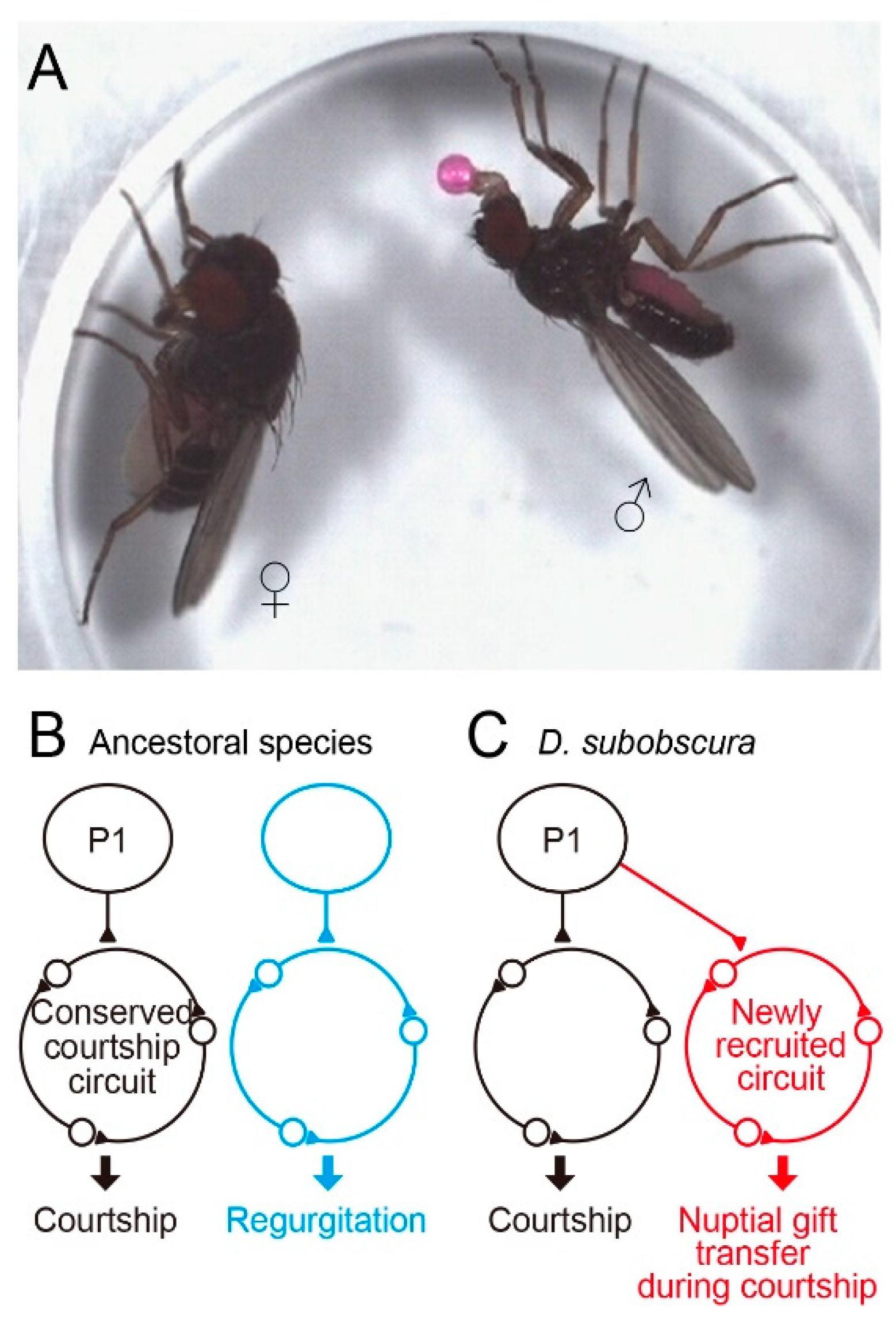
In the preceding sections, we overviewed studies exploring the neural divergence underlying quantitative differences in mating behavior among members of the D. melanogaster subgroup in the genus Drosophila. Here, we consider the neural bases for divergent behavior among Drosophila species which are distantly related to each other and thus unable to produce interspecies hybrids. Since an ancestral species of the obscura species group and the melanogaster group diverged 25 million years ago, distinct mating behavior has evolved in these two clades. D. subobscura is particularly interesting, because nuptial gift transfer in this species is indispensable for acceptance of a male by a female as a mating partner [71,72] (Figure 3A). Other members of the obscura species group rarely display nuptial gift transfer [3,7]. When a male fly of D. subobscura finds a female, he extends a foreleg toward her to tap her abdomen (tapping), and then extends his proboscis toward her. The male may rapidly open and close two wings several times (scissoring) and slowly swing his bilateral midlegs (midleg swing). Each of these courtship acts is triggered by the distinct motion pattern of a visual target [73]. After repeating these displays, the male positions himself in front of the female with a quick motion, then fully opens both wings, culminating in nuptial gift transfer when the male is successful: the male extends his proboscis with a regurgitated droplet on its tip, i.e., the labellum, and the female responds to the gift by protruding her proboscis, so that the labellar surfaces of the male and female come into contact [3]. The two proboscises move back and forth while maintaining the labellar contact for seconds and the female swallows the droplet provided by the male. The male then quickly circles to the rear and attempts to mount the female. Despite the overall difference in mating behavior, the fru gene could play a master regulator role for courtship circuit formation in D. subobscura, as it does in D. melanogaster. Assuming that was the case, Tanaka [74] produced fru variants in D. subobscura, with the aid of CRISPR/Cas9 genome editing and piggyBac- and ø31-mediated transgenesis [75]. Venus-tagged channelrhodopsin was integrated into exon-2 of the fru locus, allowing visualization and artificial activation of the fru-labeled circuit in D. subobscura. The fru-labeled circuit thus visualized with Venus in D. subobscura was very similar to that in D. melanogaster at the gross anatomy level, and some of the key neural elements of the fru-labeled circuit, such as P1 and mAL neurons, were identifiable in D. subobscura by their characteristic structures common to the D. melanogaster counterparts [74]. Optogenetic activation of the fru-labeled circuit in D. subobscura induced robust behavioral responses such as abdominal bending, which resembles the motor act for copulation. Remarkably, in addition to the copulation-like abdominal bending, some male flies exhibited regurgitation, an essential component of nuptial gift transfer, upon fru-circuit activation [74]. These observations suggest that, although the fru-labeled circuit is conserved across species in terms of its overall anatomy and courtship-inducing ability, this circuit also triggers a species-specific courtship element, i.e., nuptial gift transfer. After eating to satiety, Dipterans often regurgitate and reingest their crop contents [76]. Regurgitation after feeding is suggested to be a strategy by which flies eliminate their crop water load to concentrate crop solute [77]. One interesting evolutionary scenario is that regurgitation as a component of feeding behavior may have been incorporated into the courtship ritual in D. subobscura. This idea further invites supposition that, during evolution, a neural module for feeding-associated regurgitation in toto was placed downstream of the courtship command system in an ancestral species of D. subobscura (Figure 3B,C). It might be that the fru gene of the subobscura ancestor happened to acquire cis elements that drive expression in neurons composing the regurgitation module and, as a consequence, nuptial gift transfer became possible as an innate repertoire of D. subobscura courtship behavior. Identification of D. subobscura neurons involved in nuptial gift transfer would be an important next step toward understanding of the neural bases for the evolution of novel behavioral traits.
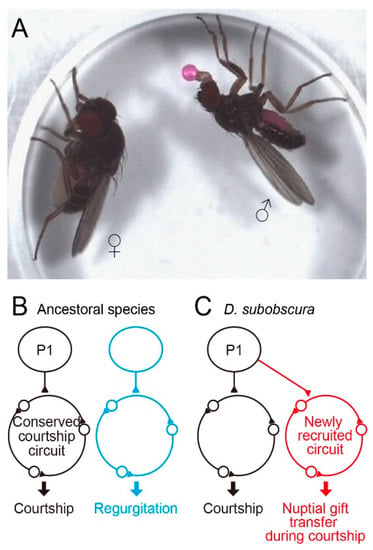
Figure 3.
A possible circuit mechanism whereby D. subobscura acquired the novel courtship action element nuptial gift. (A) Nuptial gift transfer. (B) The regurgitation circuit operating independent of the courtship circuit in the ancestral state. (C) The regurgitation circuit neurons in toto happened to express the courtship master gene fruitless, resulting in the incorporation of this circuit into the courtship neural network in D. subobscura.
8. Perspectives
The current state of knowledge regarding the species differences in neural circuitry remains scanty, making it impossible to draw a generalized framework to illuminate causal links among the genes, circuits, and behaviors underlying the diversification of courtship rituals. Nonetheless, a few pioneering works have explored the neural underpinnings of behavioral divergence, and their findings augur explosive development in this field in the coming decade. In the very near future, exhaustive identification of neurons will be accomplished in D. melanogaster, accompanying databases for GAL4 lines that are highly specific for every neuron thus identified. Recent advances in single-cell or single-nucleus RNA sequencing techniques will open an avenue for deciphering the molecular landscape within seemingly similar Fru neurons across species. As we learned in the published works reviewed in this article, these GAL4s are easily introduced into non-model Drosophila species, where they can drive the expression of any gene of interest in homologous neurons in different species. This allows us to compare the structure and function of homologous neurons and the circuits they form across species, making it possible to determine which circuit element is different and how that change contributes to behavioral differences between species. Indeed, the CRISPR/Cas9 technique opened an avenue for the manipulation of any gene potentially involved in such behavioral diversity across species. The combinatorial uses of traditional genetics and state-of-the-art technologies in data science will provide a key to understanding how the neural circuit evolves to elaborate existing behavior and to create a novel behavioral repertoire.
Author Contributions
Conceptualization: D.Y.; Writing—original draft: K.S., D.Y.; Writing—review & editing: Y.I., R.T., D.Y.; Visualization: K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (16H06371 and 19H04923 to D.Y.; 17K07040 and 19H04766 to K.S.; 19K16186 to R.T.; 18H02488, 18H04819 and 19K22453 to Y.I.).
Acknowledgments
We thank Y. Takamura for secretarial assistance.
Conflicts of Interest
The authors declare no competing or financial interests.
References
- Abzhanov, A.; Kuo, P.W.; Hartmann, C.; R-Grant, B.; Grant, P.R.; Tabin, C.J. The calmodulin pathway and evolution of elongated beak morphology in Darwin’s finches. Nature 2006, 442, 563–567. [Google Scholar] [CrossRef]
- Lorenz, K. Evolution and Modification of Behavior. The University of Chicago Press: Chicago, IL, USA, 1965; p. 121. [Google Scholar]
- Spieth, H.T. Mating behavior within the genus Drosophila (Diptera). Bull. Am. Mus. Nat. Hist. 1952, 99, 399–474. [Google Scholar]
- Ewing, A.W.; B-Clark, H.C. The courtship songs of Drosophila. Behaviour 1968, 31, 288–301. [Google Scholar] [CrossRef]
- Hoy, R.R.; Hoikkala, A.; Kaneshiro, K.Y. Hawaiian courtship songs; evolutionary innovation in communication signals in Drosophila. Science 1988, 240, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Fabre, C.C.; Hedwig, B.; Conduit, G.; Lawrence, P.A.; Goodwin, S.F.; Casal, J. Substrate-borne vibratory communication during courtship in Drosophila melanogaster. Curr. Biol. 2012, 22, 2180–2185. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.V.; Fabre, C.C. The elaborate postural display of courting Drosophila persimilis flies produces substrate-borne vibratory signals. J. Insect Behav. 2016, 29, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Satokangas, P.; Liimatainen, J.O.; Hoikkala, A. Songs produced by the females of the Drosophila virilis group of species. Behav. Genet. 1994, 24, 263–272. [Google Scholar] [CrossRef] [PubMed]
- LaRue, K.M.; Clemens, J.; Berman, G.J.; Murthy, M. Acoustic duetting in Drosophila virilis relies on the integration of auditory and tactile signals. eLife 2015, 4, e07277. [Google Scholar] [CrossRef]
- Chen, A.L.; Chen, C.C.; Katoh, T.; Katoh, T.K.; Watada, M.; Toda, M.J.; Ritchie, M.G.; Wen, S.Y. Evolution and diversity of the courtship repertoire in the Drosophila montium species group (Diptera: Drosophilidae). J. Evol. Biol. 2019, 32, 1124–1140. [Google Scholar] [CrossRef]
- Yamamoto, D.; Ishikawa, Y. Genetic and neural bases for species-specific behavior in Drosophila species. J. Neurogenet. 2013, 27, 130–142. [Google Scholar] [CrossRef]
- Davie, K.; Janssens, J.; Koldere, D.; De Waegeneer, M.; Pech, U.; Kreft, Ł.; Aibar, S.; Makhzami, S.; Christiaens, V.; Bravo González-Blas, C.; et al. A single-cell transcriptome atlas of the aging Drosophila brain. Cell 2018, 174, 982–998. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Asano, S.M.; Upadhyayula, S.; Pisarev, I.; Milkie, D.E.; Liu, T.L.; Singh, V.; Graves, A.; Huynh, G.H.; Zhao, Y.; et al. Cortical column and whole-brain imaging with molecular contrast and nanoscale resolution. Science 2019, 363, 6424. [Google Scholar] [CrossRef] [PubMed]
- Robie, A.A.; Hirokawa, J.; Edwards, A.W.; Umayam, L.A.; Lee, A.; Phillips, M.L.; Card, G.M.; Korff, W.; Rubin, G.M.; Simpson, J.H.; et al. Mapping the neural substrates of behavior. Cell 2017, 170, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Lauritzen, J.S.; Perlman, E.; Robinson, C.G.; Nichols, M.; Milkie, D.; Torrens, O.; Price, J.; Fisher, C.B.; Sharifi, N.; et al. A complete electron microscopy volume of the brain of adult Drosophila melanogaster. Cell 2018, 174, 730–743. [Google Scholar] [CrossRef]
- Dickson, B.J. Wired for sex: The neurobiology of Drosophila mating decisions. Science 2008, 322, 904–909. [Google Scholar] [CrossRef]
- Ito, H.; Fujitani, K.; Usui, K.; Shimizu-Nishikawa, K.; Tanaka, S.; Yamamoto, D. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc. Natl. Acad. Sci. USA 1996, 93, 9687–9692. [Google Scholar] [CrossRef]
- Ryner, L.C.; Goodwin, S.F.; Castrillon, D.H.; Anand, A.; Villella, A.; Baker, B.S.; Hall, J.C.; Taylor, B.J.; Wasserman, S.A. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 1996, 87, 1079–1089. [Google Scholar] [CrossRef]
- Yamamoto, D.; Koganezawa, M. Genes and circuits of courtship behaviour in Drosophila males. Nat. Rev. Neurosci. 2013, 14, 681–692. [Google Scholar] [CrossRef]
- Bastock, M.; Manning, A. The courtship of Drosophila melanogaster. Behaviour 1955, 8, 85–111. [Google Scholar] [CrossRef]
- Hall, J.C. The mating of a fly. Science 1994, 264, 1702–1714. [Google Scholar] [CrossRef]
- von Schilcher, F. The function of pulse song and sine song in the courtship of Drosophila melanogaster. Anim. Behav. 1976, 24, 622–625. [Google Scholar] [CrossRef]
- Kyriacou, C.P.; Hall, J.C. Learning and memory mutations impair acoustic priming of mating behaviour in Drosophila. Nature 1984, 308, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Okamoto, N.; Yoneyama, Y.; Maeda, N.; Kamikouchi, A. A single male auditory response test to quantify auditory behavioral responses in Drosophila melanogaster. J. Neurogenet. 2019, 33, 64–74. [Google Scholar] [CrossRef] [PubMed]
- von Schilcher, F. The role of auditory stimuli in the courtship of Drosophila melanogaster. Anim. Behav. 1976, 24, 18–26. [Google Scholar] [CrossRef]
- Cowling, D.E.; Burnet, B. Courtship songs and genetic control of their acoustic characteristics in sibling species of the Drosophila melanogaster. Anim. Behav. 1981, 29, 924–935. [Google Scholar] [CrossRef]
- Tomaru, M.; Oguma, Y. Differences in courtship song in the species of the Drosophila auraria complex. Anim. Behav. 1994, 47, 133–140. [Google Scholar] [CrossRef]
- Riabinina, O.; Dai, M.; Duke, T.; Albert, J.T. Active process mediates species-specific tuning of Drosophila ears. Curr. Biol. 2011, 21, 658–664. [Google Scholar] [CrossRef]
- Saarikettu, M.; Liimatainen, J.O.; Hoikkala, A. The role of male courtship song in species recognition in Drosophila montana. Behav. Genet. 2005, 35, 257–263. [Google Scholar] [CrossRef]
- Clemens, J.; Coen, P.; Roemschied, F.A.; Pereira, T.D.; Mazumder, D.; Aldarondo, D.E.; Pacheco, D.A.; Murthy, M. Discovery of a new song mode in Drosophila reveals hidden structure in the sensory and neural drivers of behavior. Curr. Biol. 2018, 28, 2400–2412. [Google Scholar] [CrossRef]
- Kimura, K.-I.; Hachiya, T.; Koganezawa, M.; Tazawa, T.; Yamamoto, D. Fruitless and Doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron 2008, 59, 759–769. [Google Scholar] [CrossRef]
- Yu, J.Y.; Kanai, M.I.; Demir, E.; Jefferis, G.S.; Dickson, B.J. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr. Biol. 2010, 20, 1602–1614. [Google Scholar] [CrossRef] [PubMed]
- Kohatsu, S.; Koganezawa, M.; Yamamoto, D. Female contact activates male-specific interneurons that trigger stereopypic courtship behavior in Drosophila. Neuron 2011, 69, 498–508. [Google Scholar] [CrossRef] [PubMed]
- von Philipsborn, A.; Liu, T.; Yu, J.Y.; Masser, C.; Bidaye, S.S.; Dickson, B.J. Neural control of Drosophila courtship song. Neuron 2011, 69, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Kohatsu, S.; Yamamoto, D. Visually induced initiation of Drosophila innate courtship-like pursuit is mediated by central excitatory state. Nat. Commun. 2015, 6, 6457. [Google Scholar] [CrossRef]
- McKellar, C.E.; Lilvis, J.L.; Bath, D.E.; Fitsgerald, J.E.; Cannon, J.G.; Simpson, J.H.; Dickson, B.J. Threshold-based ordering of sequential actions during Drosophila courtship. Curr. Biol. 2019, 29, 426–434. [Google Scholar] [CrossRef]
- O’Sullivan, A.; Lindsay, T.; Prudrikova, A.; Erdi, B.; Dickinson, M.; von Philipsborn, A.C. Multifunctional wing motor control of song and flight. Curr. Biol. 2018, 28, 2705–2717. [Google Scholar] [CrossRef]
- Shirangi, T.R.; Stern, D.L.; Truman, J.W. Motor control of Drosophila courtship song. Cell Rep. 2013, 5, 678–686. [Google Scholar] [CrossRef]
- Ding, Y.; Lillvis, J.L.; Cande, J.; Berman, G.J.; Arthur, B.J.; Long, X.; Xu, M.; Dickson, B.J.; Stern, D.L. Neural evolution of context-dependent fly song. Curr. Biol. 2019, 29, 1089–1099. [Google Scholar] [CrossRef]
- Cande, J.; Andolfatto, P.; Prud’homme, B.; Stern, D.; Gompel, N. Evolution of multiple additive loci caused divergence between Drosophila yakuba and D. santomea in wing rowing during male courtship. PLoS ONE 2012, 7, e43888. [Google Scholar] [CrossRef]
- Gleason, J.M.; Ritchie, M.G. Do quantitative trait loci (QTL) for a courtship song difference between Drosophila simulans and D. sechellia coincide with candidate genes and intraspecific QTL? Genetics 2004, 166, 1303–1311. [Google Scholar] [CrossRef]
- Belote, J.M.; Lucchesi, J.C. Control of X chromosome transcription by the maleless gene in Drosophila. Nature 1980, 285, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Ganetzky, B.; Jan, L.Y.; Benzer, S. A Drosophila mutant with a temperature-sensitive block in nerve conduction. Proc. Natl. Acad. Sci. USA 1978, 75, 4047–4051. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.A.; Hall, J.C. Analysis of temperature-sensitive mutants reveals new genes involved in the courtship song of Drosophila. Genetics. 1998, 148, 827–838. [Google Scholar]
- Reenan, R.A.; Hanrahan, C.J.; Ganetzky, B. The mle (napts) RNA helicase mutation in Drosophila results in a splicing catastrophe of the para Na+ channel transcript in a region of RNA editing. Neuron 2000, 25, 139–149. [Google Scholar] [CrossRef]
- Sato, K.; Ahsan, M.T.; Ote, M.; Koganezawa, M.; Yamamoto, D. Calmodulin-binding transcription factor shapes the male courtship song in Drosophila. PLoS Genet. 2019, 15, e1008309. [Google Scholar] [CrossRef]
- Yokokura, T.; Ueda, R.; Yamamoto, D. Phenotypic and molecular characterization of croaker, a new mating behavior mutant of Drosophila melanogaster. Jpn. J. Genet. 1995, 70, 103–117. [Google Scholar] [CrossRef]
- Villella, A.; Gailey, D.A.; Berwald, B.; Ohshima, S.; Barnes, P.T.; Hall, J.C. Extended reproductive roles of the fruitless gene in Drosophila melanogaster revealed by behavioral analysis of new fru mutants. Genetics 1997, 147, 1107–1130. [Google Scholar]
- Cande, J.; Stern, D.L.; Morita, T.; Prud’homme, B.; Gompel, N. Looking under the lamp post: Neither fruitless nor doublesex has evolved to generate divergent male courtship in Drosophila. Cell Rep. 2014, 8, 363–370. [Google Scholar] [CrossRef]
- Clyne, J.D.; Miesenbock, G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell 2008, 133, 354–363. [Google Scholar] [CrossRef]
- Rezaval, C.; Pattnaik, S.; Pavlou, H.J.; Nojima, T.; Brüggemeier, B.; D’Souza, L.A.D.; Dweck, H.K.M.; Goodwin, S.F. Activation of latent courtship circuitry in the brain of Drosophila females induces male-like behaviors. Curr. Biol. 2016, 26, 2508–2515. [Google Scholar] [CrossRef]
- Ding, Y.; Berrocal, A.; Morita, T.; Longden, K.D.; Stern, D.L. Natural courtship song variation caused by an intronic retroelement in an ion channel gene. Nature 2016, 536, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Frolov, R.V.; Bagati, A.; Casino, B.; Singh, S. Potassium channels in Drosophila: Historical breakthroughs, significance, and perspectives. J. Neurogenet. 2012, 26, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Elkins, T.; Ganetzky, B.; Wu, C.F. A Drosophila mutation that eliminates a calcium-dependent potassium current. Proc. Natl. Acad. Sci. USA 1986, 83, 8415–8419. [Google Scholar] [CrossRef] [PubMed]
- Jallon, J.-M. A few chemical words exchanged by Drosophila during courtship and mating. Behav. Genet. 1984, 14, 441–478. [Google Scholar] [CrossRef] [PubMed]
- Billeter, J.-C.; Atallah, J.; Krupp, J.J.; Millar, J.G.; Levine, J.D. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 2009, 461, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Thistle, R.; Cameron, P.; Ghorayshi, A.; Dennison, L.; Scott, K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell 2012, 149, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Toda, H.; Zhao, X.; Dickson, B.J. The Drosophila female aphrodisiac pheromone activates ppk23(+) sensory neurons to elicit male courtship behavior. Cell. Rep. 2012, 1, 599–607. [Google Scholar] [CrossRef]
- Clowney, E.J.; Iguchi, S.; Bussell, J.J.; Scheer, E.; Ruta, V. Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron 2015, 87, 1036–1049. [Google Scholar] [CrossRef]
- Kallman, B.R.; Kim, H.; Scott, K. Excitation and inhibition onto central courtship neurons biases Drosophila mate choice. eLife 2015, 4, e11188. [Google Scholar] [CrossRef]
- Koganezawa, M.; Haba, D.; Matsuo, T.; Yamamoto, D. The shaping of male courtship posture by lateralized gustatory inputs to male-specific interneurons. Curr. Biol. 2010, 20, 1–8. [Google Scholar] [CrossRef]
- Kimura, K.-I.; Ote, M.; Tazawa, T.; Yamamoto, D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature 2005, 438, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Grillet, M.; Everaerts, C.; Houot, B.; Ritchie, M.G.; Cobb, M.; Ferveur, J.-F. Incipient speciation in Drosophila melanogaster involves chemical signals. Sci. Rep. 2012, 2, 224. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, F.M. Lipids of Drosophila: A newly detected lipid in the male. Science 1969, 163, 1356–1357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Rogulja, D.; Crickmore, M.A. Dopaminergic circuitry underlying mating drive. Neuron 2016, 91, 168–181. [Google Scholar] [CrossRef]
- Liu, W.; Ganguly, A.; Huang, J.; Wang, Y.; Ni, J.D.; Gurav, A.S.; Aguilar, M.A.; Montell, C. Neuropeptide F regulates courtship in Drosophila through a male-specific neuronal circuit. eLife 2019, 8, e49574. [Google Scholar] [CrossRef]
- Zhang, S.X.; Rogulja, D.; Crickmore, M.A. Recurrent circuitry sustains Drosophila courtship drive while priming itself for satiety. Curr. Biol. 2019, 29, 3216–3228. [Google Scholar] [CrossRef]
- Zhang, S.X.; Miner, L.E.; Boutros, C.L.; Rogulja, D.; Crickmore, M.A. Motivation, perception, and chance converge to make a binary decision. Neuron 2018, 99, 376–388. [Google Scholar] [CrossRef]
- Seeholzer, L.F.; Seppo, M.; Stern, D.L.; Ruta, V. Evolution of a central neural circuit underlies Drosophila mate preferences. Nature 2018, 559, 564–569. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Avila-Herrera, A.; Tun, K.M.; Serpa, P.H.; Peng, J.; Parthasarathy, S.; Knapp, J.-M.; Stern, D.L.; Davis, G.W.; Pollard, K.S.; et al. Evolution of mechanisms that control mating in Drosophila males. Cell Rep. 2019, 27, 2527–2536. [Google Scholar] [CrossRef]
- Immonen, E.; Hoikkala, A.; Kozem, A.J.N.; Ritchie, M.G. When are vomiting males attractive? Sexual selection on condition-dependint nuptial feeding in Drosophila subobscura. Behaiv. Ecol. 2009, 20, 289–295. [Google Scholar] [CrossRef]
- Steele, R.H. Courtship feeding in Drosophila subobscura. Anim. Behav. 1986, 34, 1087–1098. [Google Scholar] [CrossRef]
- Higuchi, T.; Kohatsu, S.; Yamamoto, D. Quantitative analysis of visually induced courtship elements in Drosophila subobscura. J. Neurogenet. 2017, 31, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Higuchi, T.; Kohatsu, S.; Sato, K.; Yamamoto, D. Optogenetic activation of the fruitless-labeled circuitry in Drosophila subobscura males induces mating motor zcts. J. Neurosci. 2017, 37, 11662–11674. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Murakami, H.; Ote, M.; Yamamoto, D. Clustered regulatory interspaced short palindromic repeats (CRISPR)-mediated mutagenesis and phenotype rescue by piggyBac transgenesis in a nonmodel Drosophila species. Insect. Mol. Biol. 2016, 25, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Dacks, A.M.; Nickel, T.; Mictcell, B.K. An examination of serotonin and feeding in the flesh fly Neobellieria bullata (Sarcophagidae: Diptera). J. Insect Behav. 2003, 16, 1–21. [Google Scholar] [CrossRef]
- Stoffolano, J.G.; Acaron, A.; Conway, M. “Bubbling” or droplet regurgitation in both sexes of adult Phormia regina (Diptera: Calliphoridae) fed various concentrations of sugar and protein solutions. Ann. Entomol. Soc. Am. 2008, 101, 964–970. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).