The LCORL Locus Is under Selection in Large-Sized Pakistani Goat Breeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Selection
2.3. DNA Extraction and Identification of Selection Signatures
2.4. Fine Mapping
2.5. Gene Analysis
2.6. Sanger Sequencing
2.7. Association Analysis
3. Results
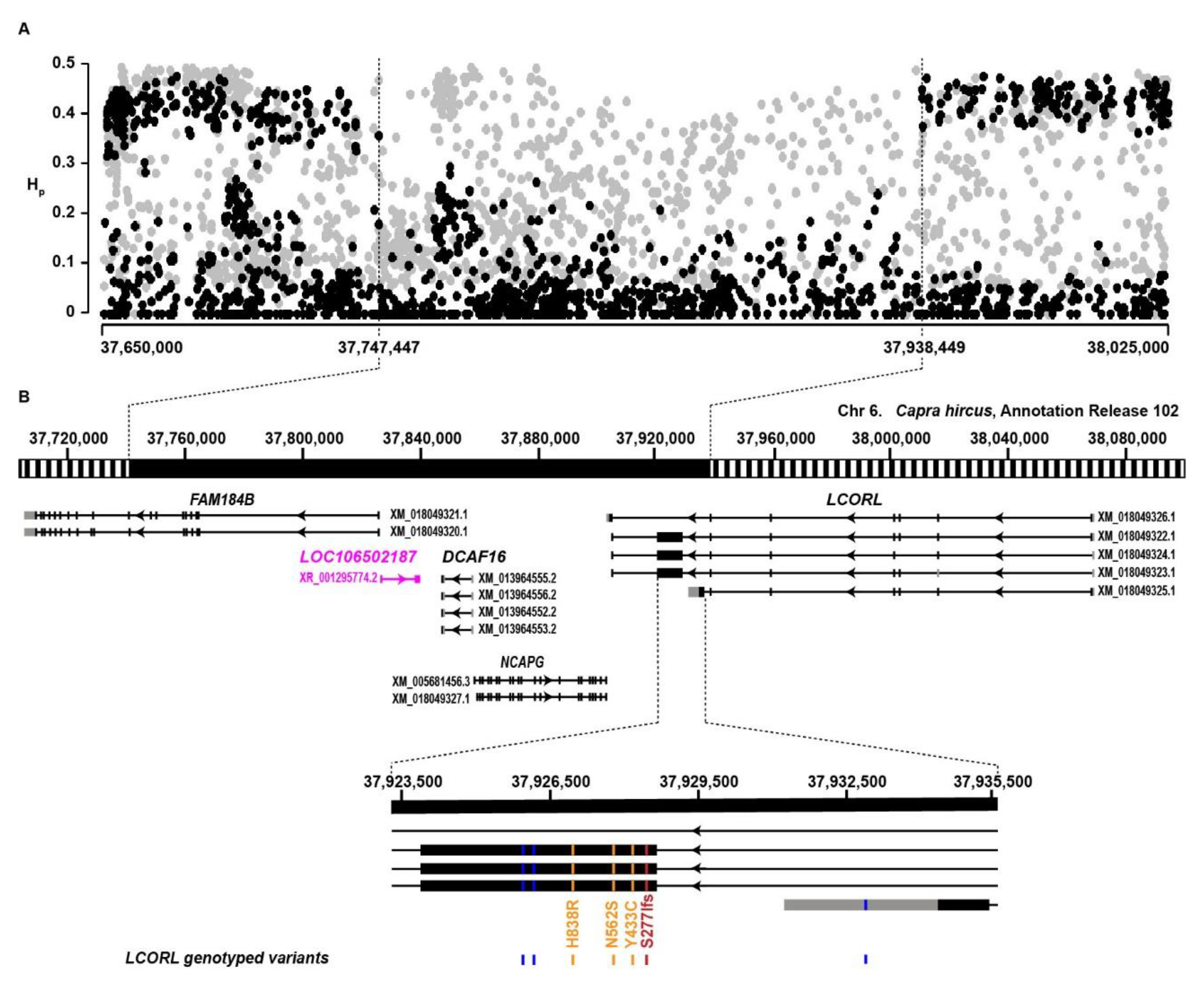
3.1. Selection Signatures in Large-Sized Goats
3.2. Fine-Mapping of the LCORL Selection Signature
3.3. Search for Candidate Causative Variants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Khan, M.S.; Khan, M.; Mahmood, S. Genetic resources and diversity in Pakistani goats. Int. J. Agric. Biol. 2008, 10, 227–231. [Google Scholar]
- Government of Pakistan. Pakistan Economic Survey 2018–2019. Available online: http://finance.gov.pk/survey/chapters_19/Economic_Survey_2018_19.pdf (accessed on 29 August 2019).
- Khan, M.F.U.; Ashfaq, F. Current status of dairy goat farming in Pakistan. In Proceedings of the 4th Asian-Australasian Dairy Goat Conference, Tra Vinh, Vietnam, 17–19 October 2018; Tra Vinh University Press: Tra Vinh, Vietnam, 2018; pp. 102–107, ISBN 978-604-60-2807-9. [Google Scholar]
- Mavrogenis, A.; Papachristoforou, C. Genetic and phenotypic relationships between milk production and body weight in Chios sheep and Damascus goats. Livest. Prod. Sci. 2000, 67, 81–87. [Google Scholar] [CrossRef]
- Dickerson, G. Animal size and efficiency: Basic concepts. Anim. Sci. 1978, 27, 367–379. [Google Scholar] [CrossRef]
- Blackmore, D.; McGilliard, L.; Lush, J. Relationships between body measurements, meat conformation, and milk production. J. Dairy Sci. 1958, 41, 1050–1056. [Google Scholar] [CrossRef]
- Soranzo, N.; Rivadeneira, F.; Chinappen-Horsley, U.; Malkina, I.; Richards, J.B.; Hammond, N.; Stolk, L.; Nica, A.; Inouye, M.; Hofman, A. Meta-analysis of genome-wide scans for human adult stature identifies novel Loci and associations with measures of skeletal frame size. PLoS Genet. 2009, 5, e1000445. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Medland, S.E.; Wright, M.J.; Henders, A.K.; Heath, A.C.; Madden, P.A.; Duncan, A.; Montgomery, G.W.; Martin, N.G.; McRae, A.F. Genome-wide association study of height and body mass index in Australian twin families. Twin Res. Hum. Genet. 2010, 13, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Lango Allen, H.; Estrada, K.; Lettre, G.; Berndt, S.I.; Weedon, M.N.; Rivadeneira, F.; Willer, C.J.; Jackson, A.U.; Vedantam, S.; Raychaudhuri, S.; et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 2010, 467, 832–838. [Google Scholar] [CrossRef] [Green Version]
- Horikoshi, M.; Yaghootkar, H.; Mook-Kanamori, D.O.; Sovio, U.; Taal, H.R.; Hennig, B.J.; Bradfield, J.P.; St Pourcain, B.; Evans, D.M.; Charoen, P.; et al. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat. Genet. 2013, 45, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Yengo, L.; Sidorenko, J.; Kemper, K.E.; Zheng, Z.; Wood, A.R.; Weedon, M.N.; Frayling, T.M.; Hirschhorn, J.; Yang, J.; Visscher, P.M. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum. Mol. Genet. 2018, 27, 3641–3649. [Google Scholar] [CrossRef]
- Carty, C.L.; Johnson, N.A.; Hutter, C.M.; Reiner, A.P.; Peters, U.; Tang, H.; Kooperberg, C. Genome-wide association study of body height in African Americans: The women’s health initiative SNP health association resource (share). Hum. Mol. Genet. 2011, 21, 711–720. [Google Scholar] [CrossRef] [Green Version]
- Kunieda, T.; Park, J.M.; Takeuchi, H.; Kubo, T. Identification and characterization of Mlr1,2: Two mouse homologues of Mblk-1, a transcription factor from the honeybee brain. FEBS Lett. 2003, 535, 61–65. [Google Scholar] [CrossRef] [Green Version]
- NCBI Genome Data Viewer. Homo Sapiens (Human) Annotation Release 109. Available online: https://www.ncbi.nlm.nih.gov/genome/gdv/ (accessed on 22 August 2019).
- Kemper, K.E.; Visscher, P.M.; Goddard, M.E. Genetic architecture of body size in mammals. Genome Biol. 2012, 13, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takasuga, A. PLAG1 and NCAPG-LCORL in livestock. Anim. Sci. J. 2016, 87, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plassais, J.; Kim, J.; Davis, B.W.; Karyadi, D.M.; Hogan, A.N.; Harris, A.C.; Decker, B.; Parker, H.G.; Ostrander, E.A. Whole genome sequencing of canids reveals genomic regions under selection and variants influencing morphology. Nat. Comm. 2019, 10, 1489. [Google Scholar] [CrossRef]
- Signer-Hasler, H.; Flury, C.; Haase, B.; Burger, D.; Simianer, H.; Leeb, T.; Rieder, S. A genome-wide association study reveals loci influencing height and other conformation traits in horses. PLoS ONE 2012, 7, e37282. [Google Scholar] [CrossRef] [Green Version]
- Makvandi-Nejad, S.; Hoffman, G.E.; Allen, J.J.; Chu, E.; Gu, E.; Chandler, A.M.; Loredo, A.I.; Bellone, R.R.; Mezey, J.G.; Brooks, S.A.; et al. Four loci explain 83% of size variation in the horse. PLoS ONE 2012, 7, e39929. [Google Scholar] [CrossRef] [Green Version]
- Tetens, J.; Widmann, P.; Kühn, C.; Thaller, G. A genome-wide association study indicates LCORL/NCAPG as a candidate locus for withers height in German Warmblood horses. Anim. Genet. 2013, 44, 467–471. [Google Scholar] [CrossRef]
- Rubin, C.-J.; Megens, H.-J.; Barrio, A.M.; Maqbool, K.; Sayyab, S.; Schwochow, D.; Wang, C.; Carlborg, Ö.; Jern, P.; Jørgensen, C.B. Strong signatures of selection in the domestic pig genome. Proc. Natl. Acad. Sci. USA 2012, 109, 19529–19536. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Larrañaga, O.; Langa, J.; Rendo, F.; Manzano, C.; Iriondo, M.; Estonba, A. Genomic selection signatures in sheep from the Western Pyrenees. Genet. Sel. Evol. 2018, 50, 9. [Google Scholar] [CrossRef] [Green Version]
- Signer-Hasler, H.; Burren, A.; Ammann, P.; Drögemüller, C.; Flury, C. Runs of homozygosity and signatures of selection: A comparison among eight local Swiss sheep breeds. Anim. Genet. 2019, 50, 512–525. [Google Scholar] [CrossRef]
- Setoguchi, K.; Furuta, M.; Hirano, T.; Nagao, T.; Watanabe, T.; Sugimoto, Y.; Takasuga, A. Cross-breed comparisons identified a critical 591-kb region for bovine carcass weight QTL (CW-2) on chromosome 6 and the Ile-442-Met substitution in NCAPG as a positional candidate. BMC Genet. 2009, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weikard, R.; Altmaier, E.; Suhre, K.; Weinberger, K.M.; Hammon, H.M.; Albrecht, E.; Setoguchi, K.; Takasuga, A.; Kühn, C. Metabolomic profiles indicate distinct physiological pathways affected by two loci with major divergent effect on Bos taurus growth and lipid deposition. Physiol. Genom. 2010, 42A, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randhawa, I.A.; Khatkar, M.S.; Thomson, P.C.; Raadsma, H.W. Composite selection signals for complex traits exemplified through bovine stature using multibreed cohorts of European and African Bos taurus. G3 (Bethesda) 2015, 5, 1391–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Li, J.; Guo, Y.; Zhang, L.; Xu, L.; Gao, X.; Zhu, B.; Gao, H.; Ni, H.; Chen, Y. Multi-strategy genome-wide association studies identify the DCAF16-NCAPG region as a susceptibility locus for average daily gain in cattle. Sci. Rep. 2016, 6, 38073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouwman, A.C.; Daetwyler, H.D.; Chamberlain, A.J.; Ponce, C.H.; Sargolzaei, M.; Schenkel, F.S.; Sahana, G.; Govignon-Gion, A.; Boitard, S.; Dolezal, M.; et al. Meta-analysis of genome-wide association studies for cattle stature identifies common genes that regulate body size in mammals. Nat. Genet. 2018, 50, 362–367. [Google Scholar] [CrossRef]
- Henkel, J.; Saif, R.; Jagannathan, V.; Schmocker, C.; Zeindler, F.; Bangerter, E.; Herren, U.; Posantzis, D.; Bulut, Z.; Ammann, P.; et al. Selection signatures in goats reveal copy number variants underlying breed-defining coat color phenotypes. PLoS Genet. 2019, 15, e1008536. [Google Scholar] [CrossRef]
- Rubin, C.-J.; Zody, M.C.; Eriksson, J.; Meadows, J.R.; Sherwood, E.; Webster, M.T.; Jiang, L.; Ingman, M.; Sharpe, T.; Ka, S.; et al. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature 2010, 464, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Bickhart, D.M.; Rosen, B.D.; Koren, S.; Sayre, B.L.; Hastie, A.R.; Chan, S.; Lee, J.; Lam, E.T.; Liachko, I.; Sullivan, S.T.; et al. Single-molecule sequencing and chromatin conformation capture enable de novo reference assembly of the domestic goat genome. Nat. Genet. 2017, 49, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.; Daly, M.J. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Sohaib, M.; Jamil, F. An insight of meat industry in Pakistan with special reference to halal meat: A comprehensive review. Korean J. Food Sci. Anim. Resour. 2017, 37, 329–341. [Google Scholar] [CrossRef]
- Skapetas, B.; Bampidis, V. Goat production in the World: Present situation and trends. Livest. Res. Rural Dev. 2016, 28, 200. [Google Scholar]
- Escareño, L.; Salinas-González, H.; Wurzinger, M.; Iñiguez, L.; Sölkner, J.; Meza-Herrera, C. Dairy goat production systems. Trop. Anim. Health Prod. 2012, 45, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, F.; Servin, B.; Talenti, A.; Rochat, E.; Kim, E.S.; Oget, C.; Palhière, I.; Crisà, A.; Catillo, G.; Steri, R. Signatures of selection and environmental adaptation across the goat genome post-domestication. Genet. Sel. Evol. 2018, 50, 57. [Google Scholar] [CrossRef]
- Stella, A.; Nicolazzi, E.L.; Van Tassell, C.P.; Rothschild, M.F.; Colli, L.; Rosen, B.D.; Sonstegard, T.S.; Crepaldi, P.; Tosser-Klopp, G.; Joost, S. AdaptMap: Exploring goat diversity and adaptation. Genet. Sel. Evol. 2018, 50, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talenti, A.; Bertolini, F.; Williams, J.; Moaeen-ud-Din, M.; Frattini, S.; Coizet, B.; Pagnacco, G.; Reecy, J.; Rothschild, M.F.; Crepaldi, P. Genomic analysis suggests KITLG is responsible for a roan pattern in two Pakistani goat breeds. J. Hered. 2017, 109, 315–319. [Google Scholar] [CrossRef]
- Shungin, D.; Winkler, T.W.; Croteau-Chonka, D.C.; Ferreira, T.; Locke, A.E.; Mägi, R.; Strawbridge, R.J.; Pers, T.H.; Fischer, K.; Justice, A.E.; et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015, 518, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norgard, E.A.; Lawson, H.A.; Pletscher, L.S.; Wang, B.; Brooks, V.R.; Wolf, J.B.; Cheverud, J.M. Genetic factors and diet affect long-bone length in the F 34 LG, SM advanced intercross. Mamm. Genome 2011, 22, 178–196. [Google Scholar] [CrossRef] [Green Version]


| Breed Name | Abbr. | WH (cm) | BW (kg) | Main Purposes | Geographic Distribution | Population Size (2006) a |
|---|---|---|---|---|---|---|
| Angora | ANG | 75 | 47 | Meat, hair | Punjab | NA |
| Barbari (Bari) | BAR | 68 | 30 | Meat, milk | Punjab, Sindh | 2306 |
| Beetal | BEE | NA | NA | Meat, milk | Punjab | 4214 |
| Dera Din Panah | DDP | 81 | 61.7 | Meat, milk, hair | Punjab | 1424 |
| Kamori | KAM | 90 | 55 | Meat, milk | Sindh | 5294 |
| Nachi (Bikaneri) | NAC | 71 | 35 | Meat, milk, hair | Punjab | 1135 |
| Pahari (Kajli) | PAH | 77 | 67 | Meat, milk, hair | Punjab, Balochistan | NA |
| Teddy | TED | 64 | 33.9 | Meat, milk | Punjab, Azad Jammu and Kashmir | 1342 |
| Primer Name | Primer Sequence | Product (bp) | Amplified Variants |
|---|---|---|---|
| LCORL_1F | CTTTCACCCAAGTCAGTGTCA | 332 | c.3480C>T |
| LCORL_1R | CCCCAGGTTGTGAAACAGAT | c.3360G>A | |
| LCORL_2F | TTGGATGCTTTATACCCTTCTGA | 213 | c.2513A>G |
| LCORL_2R | AAAATCCCCTAAGGC CAAAA | ||
| LCORL_3F | CATGTTGACTCAGCAATTCCA | 226 | c.1685A>G |
| LCORL_3R | ACAAATCAT GAAAAGGGTGAAAC | ||
| LCORL_4F | TGCTGGTGTCAGAGATGGAG | 215 | c.1298A>G |
| LCORL_4R | CAGGCTTTCAGAGTCCTCGT | ||
| LCORL_5F | AACAGCAAAGAGAAGCAGCA | 495 | c.828_829insA |
| LCORL_5R | TCCTTCTGAAGCACTTTCCA | ||
| LCORL_6F | GGGTTCAGTATAGATCTGAGAGACC | 479 | c.777-4235T>C |
| LCORL_6R | TGGGCAGTGCATTTTAACTTT |
| Breed | Animals per Pool | Windows under Selection | Selection Signatures | Genes in Selection Signatures |
|---|---|---|---|---|
| ANG | 10 | 140 | 61 | 144 |
| BAR | 12 | 95 | 51 | 109 |
| BEE | 12 | 126 | 58 | 142 |
| DDP | 12 | 132 | 66 | 178 |
| KAM | 12 | 1244 | 341 | 889 |
| NAC | 12 | 130 | 79 | 137 |
| PAH | 12 | 61 | 44 | 92 |
| TED | 12 | 136 | 49 | 120 |
| Total | 2064 | 749 | 1811 |
| Chr. | Position | cDNA Variant XM_018049322.1 | Protein Variant XP_017904811.1 | Alternative Allele Frequency in Breeds with the Selection Signature a | Alternative Allele Frequency in Breeds without the Selection Signature b | p-Value |
|---|---|---|---|---|---|---|
| 6 | 37,925,990 | c.3480C>T | p.= | 0.05 | 0.26 | 3.0 × 10−04 |
| 6 | 37,926,110 | c.3360G>A | p.= | 0.93 | 0.35 | 2.1 × 10−13 |
| 6 | 37,926,957 | c.2513A>G | p.His838Arg | 1.00 | 1.00 | NA |
| 6 | 37,927,785 | c.1685A>G | p.Asn562Ser | 0.01 | 0.26 | 1.8 × 10−07 |
| 6 | 37,928,172 | c.1298A>G | p.Tyr433Cys | 0.96 | 0.53 | 3.9 × 10−10 |
| 6 | 37,928,645 | c.828_829insA | p.Ser277Ilefs*38 | 0.95 | 0.35 | 8.0 × 10−15 |
| 6 | 37,932,928 | c.777-4235T>C | intronic/3′-UTR of short isoform | 0.01 | 0.09 | 1.0 × 10−02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saif, R.; Henkel, J.; Jagannathan, V.; Drögemüller, C.; Flury, C.; Leeb, T. The LCORL Locus Is under Selection in Large-Sized Pakistani Goat Breeds. Genes 2020, 11, 168. https://doi.org/10.3390/genes11020168
Saif R, Henkel J, Jagannathan V, Drögemüller C, Flury C, Leeb T. The LCORL Locus Is under Selection in Large-Sized Pakistani Goat Breeds. Genes. 2020; 11(2):168. https://doi.org/10.3390/genes11020168
Chicago/Turabian StyleSaif, Rashid, Jan Henkel, Vidhya Jagannathan, Cord Drögemüller, Christine Flury, and Tosso Leeb. 2020. "The LCORL Locus Is under Selection in Large-Sized Pakistani Goat Breeds" Genes 11, no. 2: 168. https://doi.org/10.3390/genes11020168





