Cell Progeny in the Olfactory Bulb after Targeting Specific Progenitors with Different UbC-StarTrack Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Line
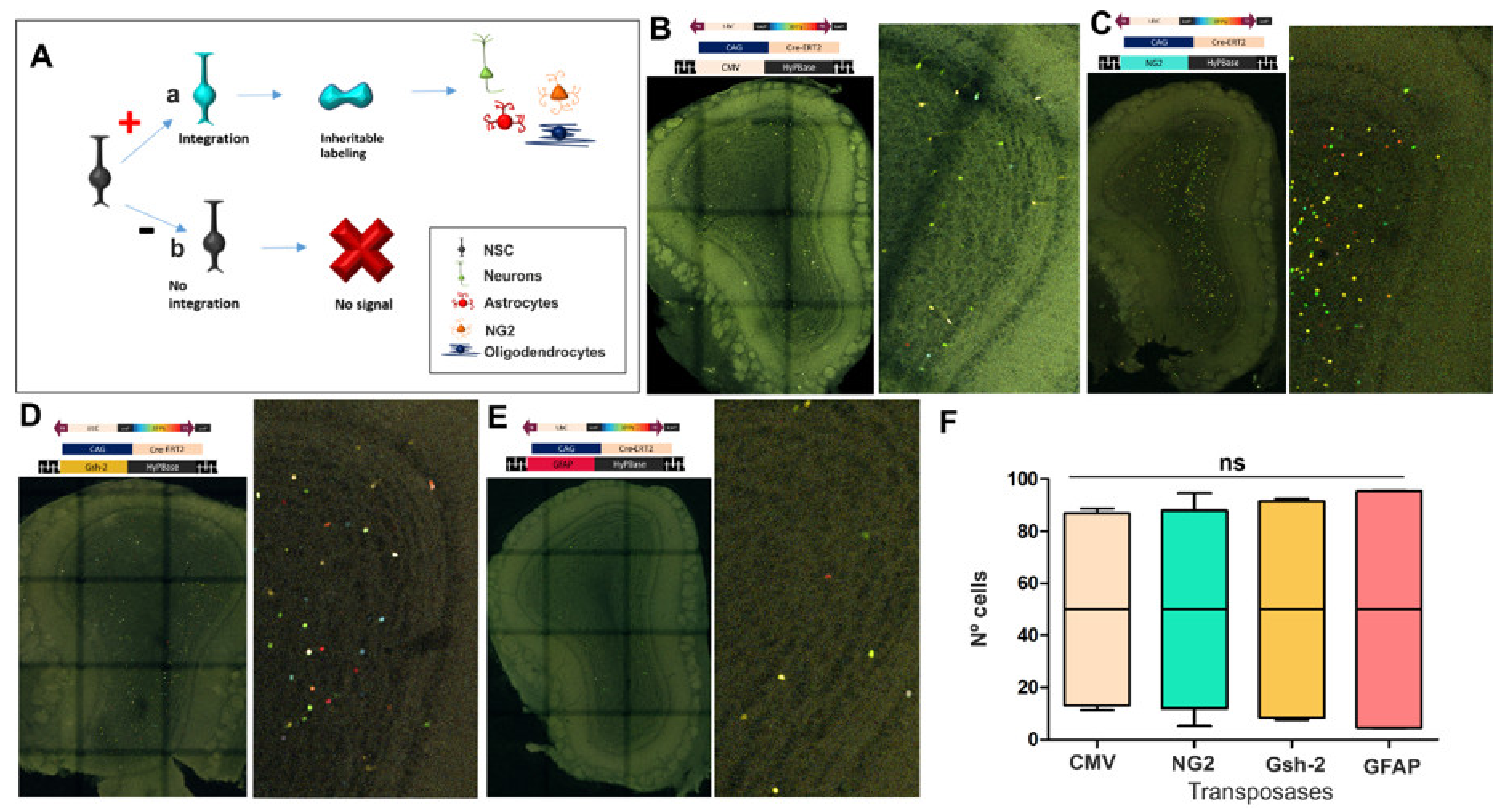
2.2. Vectors
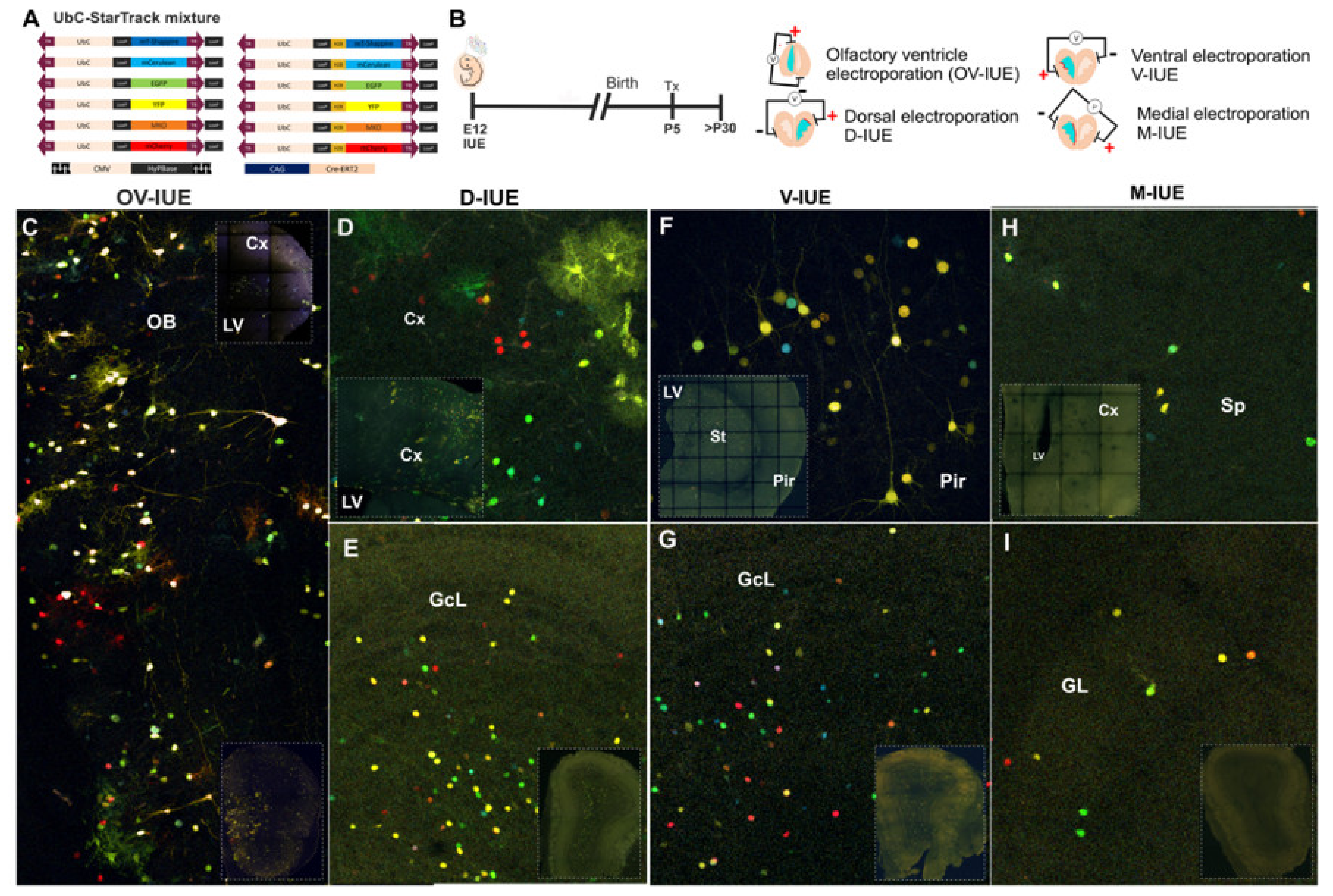
2.3. In Utero Electroporation (IUE) and Tamoxifen Administration
2.4. Tissue Processing
2.5. Imaging
2.6. Data Analysis
3. Results
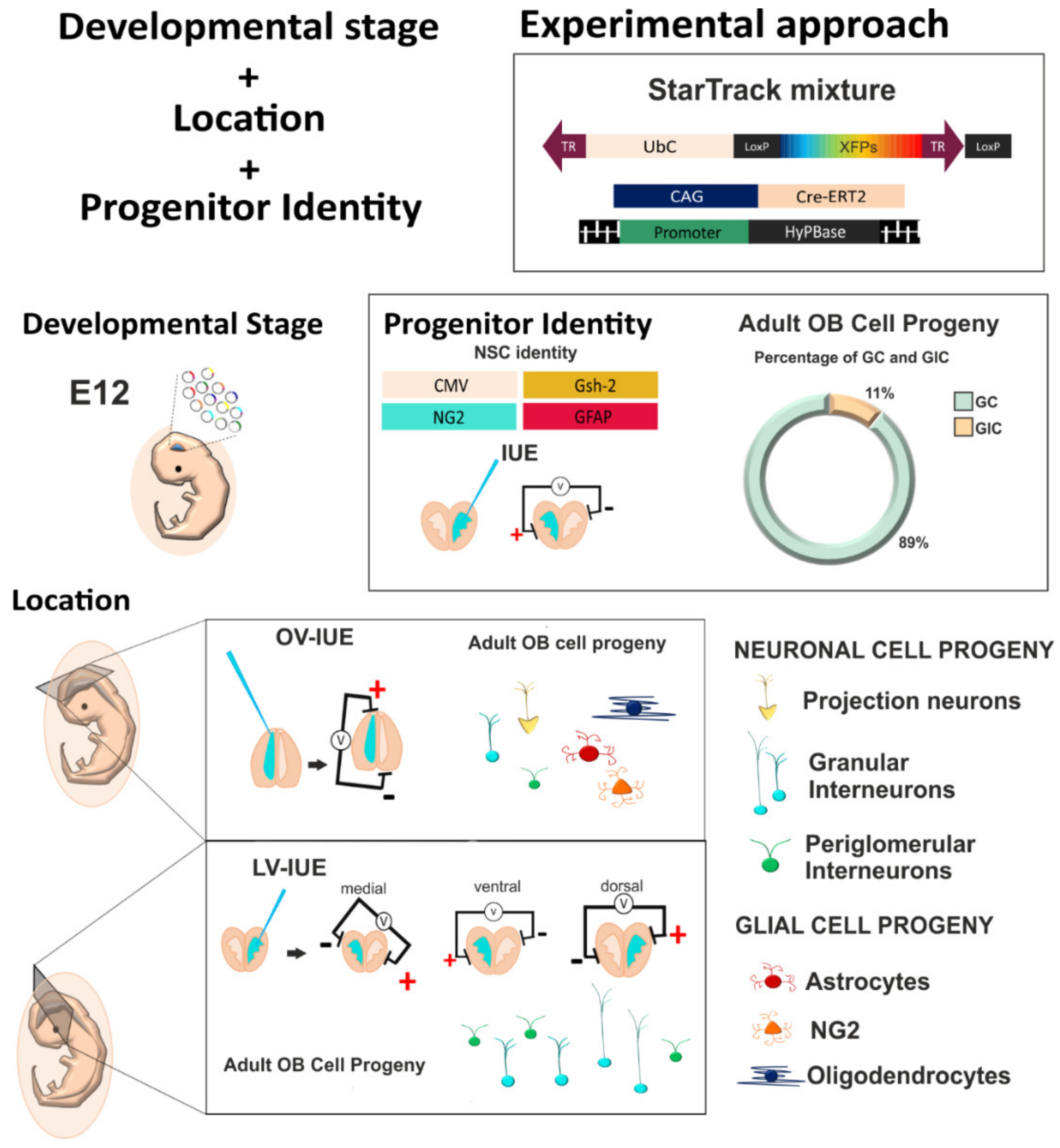
3.1. The Fate of OB Cells After Targeting Cell Progenitors at Distinct Ventricular Sites
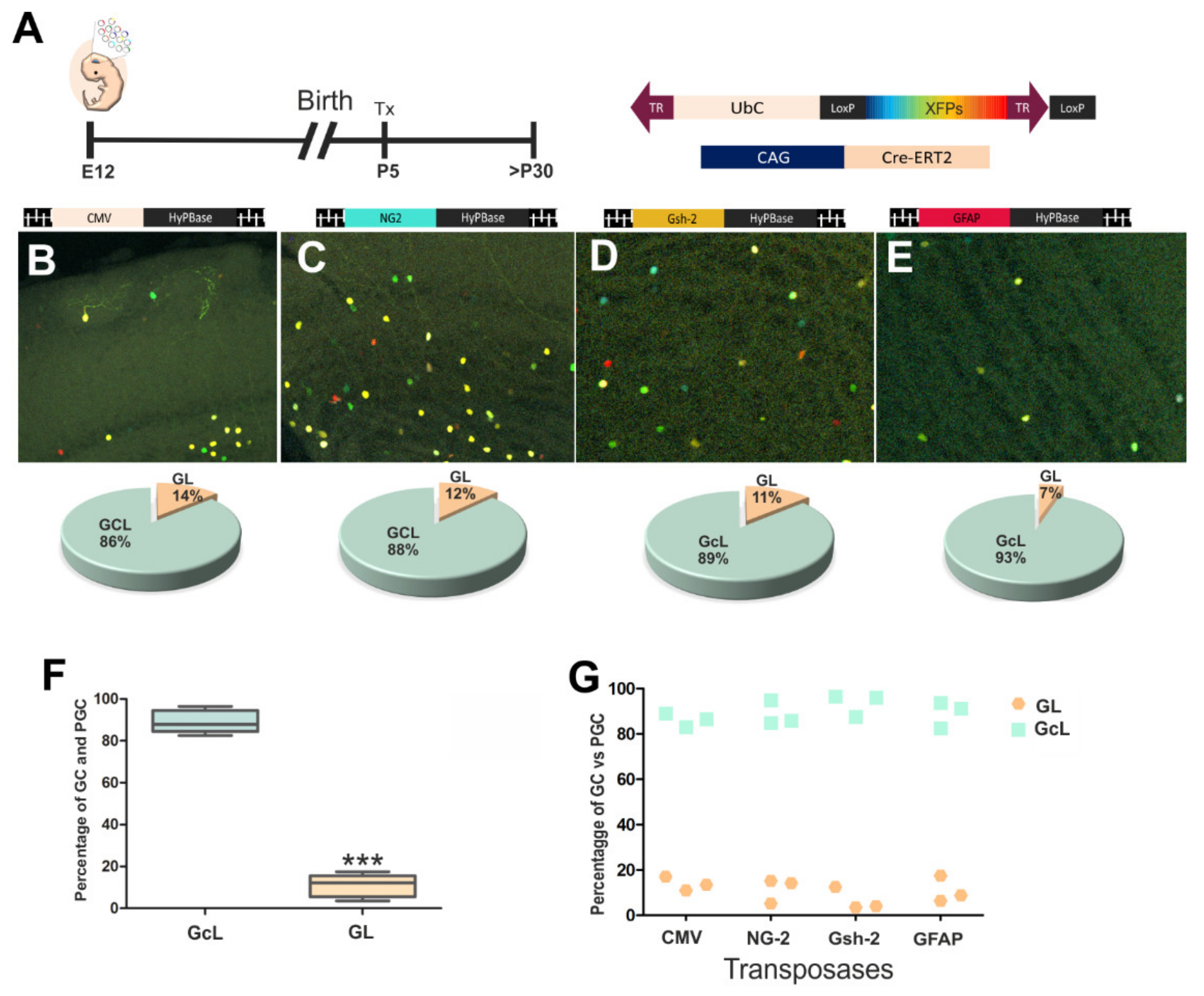
3.2. The Fate of Olfactory Bulb Cells After Targeting Specific Progenitors with StarTrack
3.3. Diversity of Olfactory Bulb Interneurons in Relation to Progenitor Cell Identity
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramón y Cajal, S. Origen y terminación de las fibras nerviosas olfatorias. Gaceta Sanitaria de Barcelona 1890, 3, 1–20. Available online: https://digital.csic.es/bitstream/10261/158702/1/Cap%C3%ADtulo43-T2-2%C2%AA%20parte.pdf (accessed on 25 December 2019).
- Blanes, T. Sobre algunos puntos dudosos de la estructura del bulbo olfatorio. Rev. Trimest. Microgr. 1898, 3, 99–127. [Google Scholar]
- Ramón y Cajal, S. Estudios sobre la corteza cerebral humana IV. Estructura de la corteza cerebral olfativa del hombre y mamíferos. Trab. Lab. Investig. Biol. 1901, 1, 1–140. [Google Scholar]
- Figueres-Oñate, M.; Gutiérrez, Y.; López-Mascaraque, L. Unraveling Cajal’s view of the olfactory system. Front. Neuroanat. 2014, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Blanchart, A.; de Carlos, J.A.; López-Mascaraque, L. Time frame of mitral cell development. J. Comp. Neurol. 2006, 543, 529–554. [Google Scholar] [CrossRef]
- Allison, A.C. The secondary olfactory areas in the human brain. J. Anat. 1954, 88, 481–488. [Google Scholar]
- Price, J.L. An autoradiographic study of complementary laminar patterns of termination of afferent fibers to the olfactory cortex. J. Comp. Neurol. 1973, 150, 87–108. [Google Scholar] [CrossRef]
- Kriegstein, A.; Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef] [Green Version]
- Fuentealba, L.C.; Rompani, S.B.; Parraguez, J.I.; Obernier, K.; Romero, R.; Cepko, C.L.; Alvarez-Buylla, A. Embryonic origin of postnatal neural stem cells. Cell 2015, 161, 1644–1655. [Google Scholar] [CrossRef] [Green Version]
- Ventura, R.E.; Goldman, J.E. Dorsal radial glia generates olfactory bulb interneurons in the postnatal murine brain. J. Neurosci. 2007, 27, 4297–4302. [Google Scholar] [CrossRef]
- Merkle, F.T.; Mirzadeh, Z.; Alvarez-Buylla, A. Mosaic organization of neural stem cells in the adult brain. Science 2007, 317, 381–384. [Google Scholar] [CrossRef] [Green Version]
- Batista-Brito, R.; Close, J.; Machold, R.; Fishell, G. The distinct temporal origins of olfactory bulb interneuron subtypes. J. Neurosci. 2008, 28, 3966–3975. [Google Scholar] [CrossRef]
- Fiorelli, R.; Azim, K.; Fischer, B.; Raineteau, O. Adding a spatial dimension to postnatal ventricular-subventricular zone neurogenesis. Development 2015, 142, 2109–2120. [Google Scholar] [CrossRef] [Green Version]
- De Marchis, S.; Bovetti, S.; Carletti, B.; Hsieh, Y.C.; Garzotto, D.; Peretto, P.; Fasolo, A.; Puche, A.C.; Rossi, F. Generation of distinct types of periglomerular olfactory bulb interneurons during development and in adult mice: Implication for intrinsic properties of the subventricular zone progenitor population. J. Neurosci. 2007, 27, 657–664. [Google Scholar] [CrossRef]
- García-Marqués, J.; López-Mascaraque, L. Clonal mapping of astrocytes in the olfactory bulb and rostral migratory stream. Cereb. Cortex 2017, 27, 2195–2209. [Google Scholar] [CrossRef] [Green Version]
- García-Marqués, J.; López-Mascaraque, L. Clonal identity determines astrocyte cortical heterogeneity. Cereb. Cortex 2013, 23, 1463–1472. [Google Scholar] [CrossRef] [Green Version]
- Figueres-Oñate, M.; García-Marqués, J.; López-Mascaraque, L. UbC-StarTrack, a clonal method to target the entire progeny of individual progenitors. Sci. Rep. 2016, 6, 33896. [Google Scholar] [CrossRef]
- Jiménez, D.; García, C.; de Castro, F.; Chédotal, A.; Sotelo, C.; De Carlos, J.A.; Valverde, F.; López-Mascaraque, L. Evidence for intrinsic development of olfactory structures in Pax-6 mutant mice. J. Comp. Neurol. 2000, 428, 511–526. [Google Scholar] [CrossRef]
- López-Mascaraque, L.; de Castro, F. The olfactory bulb as an independent developmental domain. Cell Death Differ. 2002, 9, 1279–1286. [Google Scholar] [CrossRef]
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin heterogeneity and regulation in the adult mammalian brain. Development 2019, 146, dev156059. [Google Scholar] [CrossRef] [Green Version]
- Figueres-Oñate, M.; Sánchez-Villalón, M.; Sánchez-González, R.; López-Mascaraque, L. Lineage tracing and clonal cell dynamics of postnatal progenitor cells in vivo. Stem Cell Rep. 2019, 13, 700–712. [Google Scholar] [CrossRef]
- Codega, P.; Silva-Vargas, V.; Paul, A.; Maldonado-Soto, A.R.; DeLeo, A.M.; Pastrana, E.; Doetsch, F. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron 2014, 82, 545–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, N.; Pataskar, A.; Péron, S.; Thakurela, S.; Sahu, S.K.; Figueres-Oñate, M.; Berninger, B. Stage-specific transcription factors drive Astrogliogenesis by remodeling gene regulatory landscapes. Cell Stem Cell 2018, 23, 557–571.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzolari, F.; Michel, J.; Baumgart, E.V.; Theis, F.; Gotz, M.; Ninkovic, J. Fast clonal expansion and limited neural stem cell self-renewal in the adult subependymal zone. Nat. Neurosci. 2015, 18, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Bribián, A.; Figueres-Oñate, M.; Martín-López, E.; López-Mascaraque, L. Decoding astrocyte heterogeneity: New tools for clonal analysis. Neuroscience 2016, 323, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shen, Z.; Yu, Y.C.; Shi, S.H. Neural lineage tracing in the mammalian brain. Curr. Opin. Neurobiol. 2018, 50, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Figueres-Oñate, M.; García-Marqués, J.; Pedraza, M.; De Carlos, J.A.; López-Mascaraque, L. Spatiotemporal analyses of neural lineages after embryonic and postnatal progenitor targeting combining different reporters. Front. Neurosci. 2015, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-López, E.; García-Marques, J.; Núñez-Llaves, R.; López- Mascaraque, L. Clonal astrocytic response to cortical injury. PLoS ONE 2013, 8, e74039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmigiani, E.; Leto, K.; Rolando, C.; Figueres-Oñate, M.; López-Mascaraque, L.; Buffo, A.; Rossi, F. Heterogeneity and bipotency of astroglial-like cerebellar progenitors along the interneuron and glial lineages. J. Neurosci. 2015, 35, 7388–7402. [Google Scholar] [CrossRef] [Green Version]
- Cerrato, V.; Parmigiani, E.; Betizeau, M.; Aprato, J.; Nanavaty, I.; Berchialla, P.; Luzzati, F.; de’Sperati, C.; López-Mascaraque, L.; Buffo, A. Multiple origins and modularity in the spatiotemporal emergence of cerebellar astrocyte heterogeneity. PLoS Biol. 2018, 16, e2005513. [Google Scholar] [CrossRef]
- Bribián, A.; Pérez-Cerda, F.; Matute, C.; López-Mascaraque, L. Clonal glial response in a multiple sclerosis mouse model. Front. Cell. Neurosci. 2018, 23, 375. [Google Scholar] [CrossRef] [Green Version]
- Redmond, S.A.; Figueres-Oñate, M.; Obernier, K.; Nascimento, M.A.; Parraguez, J.I.; López-Mascaraque, L.; Fuentealba, L.C.; Alvarez-Buylla, A. Development of ependymal and postnatal neural stem cells and their origin from a common embryonic progenitor. Cell. Rep. 2019, 27, 429–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, Y.; García-Marqués, J.; Liu, X.; Fortes-Marco, L.; Sánchez-González, R.; Giaume, C.; López-Mascaraque, L. Sibling astrocytes share preferential coupling via gap junctions. Glia 2019, 67, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Komitova, M.; Suzuki, R.; Zhu, X. Polydendrocytes (NG2 cells): Multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 2009, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhao, N.; Bai, X.; Karram, K.; Trotter, J.; Goebbels, S.; Scheller, A.; Kirchhoff, F. Novel NG2-CreERT2 knock-in mice demonstrate heterogeneous differentiation potential of NG2 glia during development. Glia 2014, 62, 896–913. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Guo, Q.; Bai, X.; Scheller, A.; Kirchhoff, F. Early embryonic NG2 glia are exclusively gliogenic and do not generate neurons in the brain. Glia 2019, 67, 1094–1103. [Google Scholar] [CrossRef]
- Shimogori, T.; Ogawa, M. Gene application with in utero electroporation in mouse embryonic brain. Develop. Growth Differ. 2008, 50, 499–506. [Google Scholar] [CrossRef]
- Dimou, L.; Götz, M. Glial Cells as Progenitors and stem cells: New roles in the healthy and diseased brain. Physiol. Rev. 2014, 94, 709–737. [Google Scholar] [CrossRef] [Green Version]
- Llorca, A.; Ciceri, G.; Beattie, R.; Wong, F.K.; Diana, G.; Serafeimidou-Pouliou, E.; Fernández-Otero, M.; Streicher, C.; Arnold, S.J.; Meyer, M.; et al. A stochastic framework of neurogenesis underlies the assembly of neocortical cytoarchitecture. eLife 2019, 8, e51381. [Google Scholar] [CrossRef]
- Gebara, E.; Bonaguidi, M.A.; Beckervordersandforth, R.; Sultan, S.; Udry, F.; Gijs, P.J.; Lie, D.C.; Ming, G.-L.; Song, H.; Toni, N. Heterogeneity of radial glia-like cells in the adult hippocampus. Stem Cells 2016, 34, 997–1010. [Google Scholar] [CrossRef] [Green Version]
- Merkle, F.T.; Fuentealba, L.C.; Sanders, T.A.; Magno, L.; Kessaris, N.; Alvarez-Buylla, A. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat. Neurosci. 2014, 17, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Guardado, L.; Lois, C. Lineage does not regulate the sensory synaptic input of projection neurons in the mouse olfactory bulb. eLife 2019, 27, 8. [Google Scholar] [CrossRef]
- Rushing, G.V.; Bollig, M.K.; Ihrie, R.A. Heterogeneity of neural stem cells in the ventricular–subventricular zone. Adv. Exp. Med. Biol. 2019, 1169, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Marcy, G.; Raineteau, O. Contributions of single-cell approaches for probing heterogeneity and dynamics of neural progenitors throughout life. Stem Cells 2019, 37, 1381–1388. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Postiglione, M.P.; Krieger, T.G.; Hernandez, L.; Wang, C.; Han, Z.; Streicher, C.; Papusheva, E.; Insolera, R.; Chugh, K.; et al. Deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell 2014, 159, 775–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Moreno, F.; Vasistha, N.A.; Begbie, J.; Molnár, Z. CLoNe is a new method to target single progenitors and study their progeny in mouse and chick. Development 2014, 141, 1589–1598. [Google Scholar] [CrossRef] [Green Version]
- Gil-Sanz, C.; Espinosa, A.; Fregoso, S.P.; Bluske, K.K.; Cunningham, C.L.; Martinez-Garay, I.; Zeng, H.; Franco, S.J.; Müller, U. Lineage tracing using Cux2-Cre and Cux2-CreERT2 mice. Neuron 2015, 86, 1091–1099. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, A.A.; Gallo, V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J. Neurosci. 2004, 24, 10530–10541. [Google Scholar] [CrossRef]
- Suzuki, S.O.; Goldman, J.E. Multiple cell populations in the early postnatal subventricular zone take distinct migratory pathways: A dynamic study of glial and neuronal progenitor migration. J. Neurosci. 2003, 23, 4240–4250. [Google Scholar] [CrossRef] [Green Version]
- Blanchart, A.; Martín-López, E.; De Carlos, J.A.; López- Mascaraque, L. Peripheral contributions to olfactory bulb cell populations (migrations towards the olfactory bulb). Glia 2011, 59, 278–292. [Google Scholar] [CrossRef]

| Vectors | Promoter | Source | Abbreviation |
|---|---|---|---|
| PiggyBac plasmid | Ubiquitin C | Prof. Bradley | UbC-StarTrack |
| PiggyBac Transposase | CMV | Prof. Bradley | CMV-hyPBase |
| NG2 | Kirchoff | NG2-hyPBase | |
| GFAP | Dr Lundberg | GFAP-hyPBase | |
| Gsx2 | Dr K. Campbell | Gsx2-hyPBase | |
| Cre-recombinase | CAG | Dr C. Cepko | Cre-ERT2 |
| Wavelength (nm) | YFP | mKO | mCerulean | mCherry | mTSapphire | EGFP |
|---|---|---|---|---|---|---|
| Excitation | 514 | 458 | 561 | 405 | 488 | |
| Emission | 520–535 | 560–580 | 468–480 | 601–620 | 520–535 | 498–514 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-González, R.; Figueres-Oñate, M.; Ojalvo-Sanz, A.C.; López-Mascaraque, L. Cell Progeny in the Olfactory Bulb after Targeting Specific Progenitors with Different UbC-StarTrack Approaches. Genes 2020, 11, 305. https://doi.org/10.3390/genes11030305
Sánchez-González R, Figueres-Oñate M, Ojalvo-Sanz AC, López-Mascaraque L. Cell Progeny in the Olfactory Bulb after Targeting Specific Progenitors with Different UbC-StarTrack Approaches. Genes. 2020; 11(3):305. https://doi.org/10.3390/genes11030305
Chicago/Turabian StyleSánchez-González, Rebeca, María Figueres-Oñate, Ana Cristina Ojalvo-Sanz, and Laura López-Mascaraque. 2020. "Cell Progeny in the Olfactory Bulb after Targeting Specific Progenitors with Different UbC-StarTrack Approaches" Genes 11, no. 3: 305. https://doi.org/10.3390/genes11030305






